The current SARS-CoV-2 pandemic poses numerous health challenges, including the adequate use and proper interpretation of the different available tests in different clinical settings. As any diagnostic test, those of SARS-CoV-2 have methodological limitations of sensitivity (S) and specificity (E), which eventually determine their positive (PPV) and negative (NPV) predictive value. Furthermore, their diagnostic performance depends on the clinical context in which these tests are used, that is, on the pretest probability. This article: (1) reviews the main methodological aspects that influence the S, E, PPV and NPV of the most common SARS-CoV-2 diagnostic tests; and, (2) discusses its diagnostic interpretation in different clinical settings.
La actual pandemia de SARS-CoV-2 plantea numerosos retos sanitarios, entre los que destaca el uso adecuado e interpretación correcta de las pruebas diagnósticas disponibles en diferentes contextos clínicos. Como cualquier prueba diagnóstica, las de SARS-CoV-2 tienen limitaciones metodológicas de sensibilidad (S) y especificidad (E) que determinan su valor predictivo positivo (VPP) y negativo (VPN). Además, su rendimiento diagnóstico depende del contexto clínico en el que se evalúen, es decir de la probabilidad pretest. Este artículo revisa los principales aspectos metodológicos que influyen sobre la S, E, VPP y VPN de las pruebas diagnósticas de SARS-CoV-2 más habituales y discute su interpretación diagnóstica en diferentes escenarios clínicos.
The current SARS-CoV-2 pandemic poses enormous health, social and economic challenges. A correct and rapid diagnosis of SARS-CoV-2 infection is critical, both epidemiologically (as many infected persons are asymptomatic) and clinically (to identify and treat patients as early as possible).
There are two main types of SARS-CoV-2 diagnostic tests: those that report the presence of current infection and those that confirm previous infection. Alongside these, other diagnostic tests, such as imaging tests and certain biochemical markers, can help to complement the diagnosis of SARS-CoV-2 disease (COVID-19). As with any other diagnostic test, COVID-19 tests have methodological sensitivity (Se) and specificity (Sp) limitations that determine their positive predictive value (PPV) and negative predictive value (NPV). Furthermore, their diagnostic yield depends on the clinical context in which they are evaluated, that is, on the pretest probability. This article: 1) reviews the main methodological aspects that influence the Se, Sp, PPV and NPV of the most common diagnostic tests for COVID-19 and 2) discusses their diagnostic utility in different clinical settings.
Diagnostic tests for COVID-19Real-time polymerase chain reactionReal-time polymerase chain reaction (RT-PCR) identifies the presence of SARS-CoV-2 RNA in various biological samples and is considered the gold standard diagnostic test.1 In general, lower respiratory tract samples provide a higher diagnostic yield than upper respiratory tract samples, but their collection is more invasive and increases the risk of transmission to healthcare workers.2 As discussed below, various methodological constraints must also be considered depending on the type of sample analysed.3
Oropharyngeal/nasopharyngeal swabsThey are the most frequently used biological samples for the diagnosis of COVID-19.4 They must be obtained by trained personnel, following a protocolised methodology that includes the availability of the necessary material, the correct labelling of the tubes and the appropriate use of Personal Protective Equipment (PPE), instructions to the patient, the procedure for obtaining the sample and its correct handling and transfer to the laboratory.5 Poor specimen collection can lead to a false negative result.5 On the other hand, although the Sp of this technique is close to 100%, its Se is more variable and depends on the course of the infectious process (the viral load is higher in the initial stages) and the site where the sample is taken.6 Therefore, as discussed below, a negative RT-PCR result should be evaluated considering the prevalence and pre-test probability of the disease in the population tested. The NPV, which decreases with increasing prevalence, should be interpreted with caution, and self-isolation is indicated for any patient with typical COVID-19 symptoms. A second test may be indicated for the patient who has several typical symptoms, who would have a prior probability of 40–50%.7
SputumSputum samples are difficult to obtain because few COVID-19 patients produce spontaneous sputum8 and sputum induced by the risk of viral shedding is not indicated. However, it has been observed that the viral load is generally higher in sputum samples than in those obtained with nasopharyngeal and oropharyngeal swabs.9
Salivary samplesSalivary glands express the angiotensin II-converting enzyme (ACE2) surface receptor, which facilitates SARS-CoV-2 cell entry.10 Saliva sampling for the diagnosis of SARS-CoV-2 infection has certain advantages such as ease of non-invasive collection, lower risk of infection for healthcare workers, the possibility of self-sampling and lower risk of bleeding for patients with coagulation disorders, among others.11 However, the PCR Se of saliva samples is lower than that obtained in nasopharyngeal samples. Moreover, in the convalescent phase the viral load decreases earlier in saliva samples than in nasopharyngeal samples, in which dead virus could persist and lead to a “false positive”.12 Finally, saliva plays an important role in the transmission of SARS-CoV-2 in the population, so salivary samples could be an alternative for monitoring the epidemiological burden of SARS-CoV-2 in the general population.13
Tracheobronchial aspirateThis type of sampling is only possible in mechanically ventilated or tracheostomy patients. Although the viral load detected is high, this procedure can pose a significant risk to the healthcare professional performing it.14
Bronchoalveolar lavageSARS-COV-2 can be detected in broncho-alveolar lavage (BAL) specimens in critically ill patients with COVID-19 pneumonia, even in the absence of positivity in upper respiratory tract specimens.6 As in the case of tracheobronchial aspirate, this procedure may pose a significant risk to the healthcare professional performing it and is therefore not routinely indicated for the diagnosis of COVID-19.2,15
Rectal swabs and stool samplesSome COVID-19 patients have digestive symptoms. SARS-CoV-2 can be detected in rectal samples, especially in the advanced stages of infection.16 Since patients with early or mild disease may have a low viral load in nasal and pharyngeal swabs, obtaining stool samples may be an alternative diagnostic strategy, even in the absence of gastrointestinal symptoms.17
Rapid antigen detection at the point of careSince RT-PCR results are not immediate (generally not available for a few hours, which tends to increase to 24−48 h, depending on the delay in transport from the health centre to the central laboratory, and in situations of health care collapse, the result may even take seven to 10 days), that they must be processed in a central laboratory with biosafety level 2 or higher, and that it is an expensive technique, fast (cheaper) antigen tests have been developed which, by diffusion immunochromatography (lateral-flow), can give results on oropharyngeal samples in 15–30 min.18 They do not require a biosafety laboratory and can be performed at the usual patient point of care. Its main limitation is that its Se is low (around 50%) in asymptomatic individuals, so it can lead to false negatives. In addition, Se decreases if test is delayed from sample collection (it has to be done within less than two hours from sample collection). On the other hand, Se increases significantly in symptomatic patients (98.2%).19 In any case, their Sp is remarkably high (99.5%).20 These tests can only be performed on nasopharyngeal swabs and, as they are qualitative, cannot quantify the amount of antigen present. Point of care testing (POCT) offers results in just a few minutes, allowing rapid diagnostic decisions to be made, facilitating access to diagnostic tests for remote communities and populations that cannot easily access healthcare.
Humoral immunityAntibody levels (IgM, IgG, and IgA) specific for SARS-CoV-221 can be detected in blood samples. However, the body does not produce these antibodies immediately. SARS-CoV-2 S specific IgG and IgM antibodies are not detectable in the first three days of infection. From day four post-infection, SARS-CoV-2 specific IgM antibodies can be detected, which peaked at about day 20 and then declined. IgG antibodies take longer to appear but remain elevated for several months.22 The Sp of this type of test is high (98.7%) and its Se varies over time: 72.2% between eight and 14 days and 100% between 15 and 39 days of the process.23,24 On the other hand, it should be noted that human antibodies are more stable than viral RNA, so serological samples are less prone to deterioration during sample collection, preparation, transport, storage, and analysis compared to RT-PCR samples. Furthermore, serological samples have less variability compared to nasopharyngeal specimens, because the antibodies are homogeneously dispersed in the blood sample. Finally, serological samples can be easily collected with minimal discomfort to the patient during phlebotomy.
Secretory IgA plays an important role in protecting mucosal surfaces against pathogens by neutralizing viruses or hindering their adhesion to epithelial cells.25 In an experimental murine model of SARS-CoV-2 infection, intranasal instillation of SARS-CoV-2 proteins induces virus-specific IgA responses (localized and systemic) and provides better protection against exposure to SARS-CoV-2 compared to intramuscular administration.26 It has been suggested that IgA-mediated mucosal immunity may also reduce the infectivity of human secretions and thus population viral transmission. These observations may inform the development of vaccines that induce specific respiratory IgA responses to SARS-CoV-2.27
Cellular immunityIt has recently been shown that some patients can mount a cellular immune response (CD4+ and CD8+T-cells), with or without simultaneous humoral response.28 Although this knowledge may be important for the better understanding of the pathogenesis of SARS-CoV-2 infection and may help in the design and evaluation of potential vaccines,29 there are currently no diagnostic tests based on this type of immune response.
Complementary diagnostic techniquesVarious tests can help complement the diagnosis of COVID-19. These include imaging tests and some biochemical markers.
Imaging testsCOVID-19 is initially and primarily a respiratory infection (pneumonia). Therefore, chest imaging tests are essential to define the clinical context in which to interpret the diagnostic tests performed in the acute phase of infection, although they do not by themselves establish the diagnosis of COVID-19. They are used to support diagnosis, establish the severity of lung disease, guide treatment and assess therapeutic response.30 There are various chest imaging tests to consider in this setting.
Plain chest X-rayPlain chest X-ray (CXR) is an essential ancillary examination to identify the presence of SARS-CoV-2 (COVID-19) pneumonia. The most common radiological images in these patients include irregular, patchy, hazy, reticular, and bilateral ground-glass opacities, although CXR may be normal in patients with symptoms and positive RT-PCR.31 In general, radiological images on CXR usually improve after two weeks of satisfactory progression of disease symptoms, but the majority of patients (98%) continue to show abnormalities on CXR 28 days after the onset of symptoms.32 Some studies suggest that RT findings may vary according to the age of the patients, with consolidations being more common in older patients, while ground-glass images tend to be more consistent in younger patients.33
Chest CT scanComputed tomography (CT) images in patients with COVID-19 are diverse and depend on the stage of infection after symptom onset. Bilateral and peripheral ground-glass opacities31 and lung consolidation34 are common. These alterations are common (56%) in the early stages of the disease (0–2 days)35 and peak about 10 days after the onset of symptoms.36 CT has a Se of 86–98%37 but its Sp is low (25%), since these images can be observed in other respiratory diseases.37 CT cannot be used as a diagnostic test in patients with a negative RT-PCR.38 Other logistical issues to consider include infection control problems related to the transfer of patients to CT rooms, possible inefficiencies in the decontamination of the CT room and the lack of availability of CTs in some areas. For all these reasons, portable chest X-ray is the recommended modality for the identification and follow-up of pulmonary anomalies.39
Lung ultrasoundIn the hands of qualified personnel, lung ultrasound (LUS) is a useful technique in patients with suspected COVID-19.40 Its advantages include the fact that it is a technique that can be performed at the point of care, that it is safe (it does not radiate) and reproducible, that it provides rapid information on the state of the lung parenchyma, that it is low cost and that only the health professional performing the examination is needed, which reduces the risk of infection. Among its limitations are that experience with the technique is essential and that LUS does not detect lesions that are not in contact with the pleura due to the air between the probe and the lesion (in these cases it is necessary to complement the examination with CXR or CT).41 LUS findings in patients with COVID-19 depend on the stage of the disease, the severity of lung damage and possible co-morbidities. In general, varying degrees of interstitial involvement and alveolar consolidation are seen in COVID-19 patients, especially in the most severe patients. LUS can also be useful to assess the possible presence of SARS-CoV-2 acute respiratory distress syndrome (ARDS), to monitor the progression of the disease in response to established treatment, the effect of lung recruitment manoeuvres, the response to prone positioning, and to make decisions related to weaning from mechanical ventilation.
Biochemical markersPatients with COVID-19 can have multiple biochemical abnormalities including: 1. leukocytosis or leukopenia, usually accompanied by lymphopenia, eosinopenia, and neutrophilia,42 2. elevated levels of acute-phase inflammatory reactants, such as C-reactive protein, ferritin, interleukin-6, serum amyloid, and procalcitonin,8,43 3. markers of liver (ALT, AST, LDH, bilirubin) and kidney (creatinine) dysfunction,8,21 4. clotting disorders, including thrombocytopenia or thrombocytosis42 and elevation of D-dimer levels,44 5. increased troponin,45 6. decreased serum albumin levels and 7. elevation of blood glucose. Many of these abnormalities have prognostic value. For example, elevated blood glucose, AST, LDH, and creatinine are predictors of in-hospital mortality. Elevated D-dimer values indicate the possibility of thromboembolic events,46 troponin values indicate the risk of acute myocardial infarction, myocarditis, heart failure, arrhythmias and sudden death47 and liver dysfunction values indicate the possible presence of hepatitis.48 Finally, inflammatory biomarkers such as IL-6, C-reactive protein, lymphocyte counts, and fibrinogen levels have been associated with the need for mechanical ventilation in patients with SARS-CoV-2 infection.49
Clinical context and interpretation of COVID-19 diagnostic testsThe principles of Bayesian statistics state that the value of any diagnostic test depends on the pretest probability of having the disease.50 Therefore, the interpretation of its results must always consider the clinical context in which the test is performed. Some contextual aspects that can complement the algorithms recommended by the Public Health Commission of the Interterritorial Council of the National Health System in different clinical situations are discussed below (https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf).20
Asymptomatic person/population-based screeningIn this context, both RT-PCR and serological tests can play an important diagnostic role. First, RT-PCR, which detects the presence of viral RNA in biological samples regardless of the individual's symptoms, is useful in detecting contacts and, therefore, in isolating them in order to stop the chain of community disease transmission. However, if an asymptomatic patient has recovered from the initial SARS-CoV-2 infection, PCR will not identify this previous infection. In any case, they should continue to apply the standard epidemiological control measures (distance, face mask, hand washing).51 Rapid antigen tests are also being performed in population screening or risk groups, although sensitivity is lower in asymptomatic individuals.
Serological tests provide important (but not immediate) epidemiological information, as the detection of antibodies (especially IgG) allows to determine the role of asymptomatic infections in the community and especially in healthcare professionals and at-risk populations (nursing homes).
Acute patientFirst consider whether the patient's clinical condition is compatible with a diagnosis of COVID-19 (acute, febrile, anosmia, ageusia, etc.). In this context, active infection detection tests (RT-PCR or rapid antigen detection test) are indicated in the first hours of onset of symptoms. Whether one or the other is done depends on the setting, their availability, and the number of days of symptom progression. If this test is negative and there is high clinical suspicion of COVID-19, the test will be repeated. If a rapid antigen detection was carried out initially, a RT-PCR should be performed afterwards. If a PCR was performed initially, a PCR will be repeated after 48 h. If they continue to be negative and several days (at least seven) have elapsed since the onset of symptoms, the detection of IgM or IgA by means of an ELISA-type serological test or other high-throughput immunoassay could be considered. The response kinetics of both immunoglobulins are similar, reaching their maximum value between days eight to 14 after the onset of the first symptoms.52
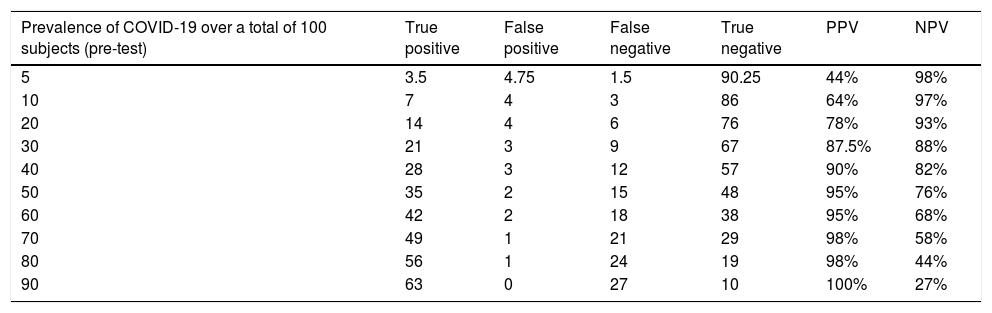
The PPV and NPV of these tests vary according to the pretest probability. Thus, in a context with a low prevalence of infection, the NPV will be high and the PPV low, while, in an epidemiological situation with a high pretest probability of infection, the NPV will be low and the PPV high (Table 1).53 Although, as discussed above, lower respiratory tract specimens, especially BAL, tend to provide a higher diagnostic yield than upper respiratory tract specimens in patients with pneumonia, their collection also increases the risk of transmission to healthcare workers.2,15
Influence of COVID-19 prevalence on the diagnostic performance of a test having a sensitivity of 70% and a specificity of 95%.53
| Prevalence of COVID-19 over a total of 100 subjects (pre-test) | True positive | False positive | False negative | True negative | PPV | NPV |
|---|---|---|---|---|---|---|
| 5 | 3.5 | 4.75 | 1.5 | 90.25 | 44% | 98% |
| 10 | 7 | 4 | 3 | 86 | 64% | 97% |
| 20 | 14 | 4 | 6 | 76 | 78% | 93% |
| 30 | 21 | 3 | 9 | 67 | 87.5% | 88% |
| 40 | 28 | 3 | 12 | 57 | 90% | 82% |
| 50 | 35 | 2 | 15 | 48 | 95% | 76% |
| 60 | 42 | 2 | 18 | 38 | 95% | 68% |
| 70 | 49 | 1 | 21 | 29 | 98% | 58% |
| 80 | 56 | 1 | 24 | 19 | 98% | 44% |
| 90 | 63 | 0 | 27 | 10 | 100% | 27% |
NPV: negative predictive value. PPV: positive predictive value.
NB: sensitivity of 70% and specificity of 95% are assumed.
In most patients who have suffered from COVID-19, specific antibodies (of one or more isotypes) can be detected in the first 15 days after the onset of symptoms, regardless of the nature of the serological test used.54
Some convalescent patients who have been positive for SARS-CoV-2 by RT-PCR or rapid antigen detection test in the acute period of COVID-19, may remain so for a long time (Long COVID-1955). It is generally considered that viral RNA can be detected for approximately two to four weeks from the onset of the disease but that the infectious capacity of the virus decreases after seven to 10 days.56 However, it is not clear whether the persistence of a positive RT-PCR in COVID-19 patients, with persistence of clinical symptoms, indicates that the patient continues to be a potential source of infection.57 In these cases, it may be useful to consider the cycle threshold (CT) value of the RT-PCR. Since the CT value is inversely related to viral load, its consideration can help in the decision-making process (shorter isolation, etc.).58 It has been suggested that a CT value > 30 indicates that the viral load is low.3,59 If the patient is also antibody positive, he/she may not be at risk of developing COVID-19 or infecting close contacts.60
ConclusionsRT-PCR and rapid antigen detection tests are useful for diagnosis of acute SARS-CoV-2 infection. Serological tests identify previous exposure to the virus (with a corresponding humoral immune response). Along with them, there are a series of ancillary diagnostic tests such as imaging tests (chest X-ray and CT, lung ultrasound) and some biochemical markers that are useful to assess the severity of the disease and help establish its prognosis. Like any other diagnostic test, however, all of them have to be interpreted considering both their methodological characteristics (Sp, Se, PPV and NPV) as well as the clinical context (pretest probability) in which they are interpreted (acute patient, convalescent patient, asymptomatic person, or population screening).
FundingThe authors declare that no funding bodies or institutions have provided financial support for the conduct of the research and/or the preparation of the article, nor have they been involved in any of the following: in the design of the study, review in the analysis and interpretation of the data, the writing of the article or the decision to submit the article for publication.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that may have influenced the work reported in this paper.
Please cite this article as: Vila Muntadas M, Agustí Sunyer I, Agustí Garcia-Navarro A. Pruebas diagnósticas COVID-19: importancia del contexto clínico. Med Clin (Barc). 2021. https://doi.org/10.1016/j.medcli.2021.03.007