Journal Information
Vol. 157. Issue 4.
Pages 191-198 (August 2021)
Share
Download PDF
More article options
Vol. 157. Issue 4.
Pages 191-198 (August 2021)
Special article
Guidelines of the Spanish ITP Group for the diagnosis, treatment and follow-up of patients with immune thrombocytopenia
Recomendaciones del Grupo Español de PTI para el diagnóstico, tratamiento y seguimiento de pacientes con trombocitopenia inmune
Visits
6
María L. Lozanoa,
, Miguel A. Sanzb, Vicente Vicentea, Spanish ITP Group - GEPTI ◊
Corresponding author
a Grupo de Investigación CB15/00055, Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Instituto de Salud Carlos III (ISCIII), Hospital General Universitario Morales Meseguer, Universidad de Murcia, IMIB-Arrixaca, Murcia, Spain
b Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Instituto Carlos III, Hospital Universitari i Politècnic La Fe, Valencia, Spain
This item has received
Article information
Full Text
Bibliography
Download PDF
Statistics
Tables (5)
Table 1. Key points of the guidelines for the diagnosis, treatment and follow-up of primary immune thrombocytopenia concerning general concepts, diagnosis and quality of life, and complementary and alternative treatments.
Table 2. Key points of the guidelines for the diagnosis, treatment, and follow-up of primary immune thrombocytopenia regarding the treatment of first-line, second-line and multidrug-resistant patients.
Table 3. Key points of the guidelines for the diagnosis, treatment, and follow-up of primary immune thrombocytopenia about patient follow-up and vaccination, surgery preparation and treatment discontinuation.
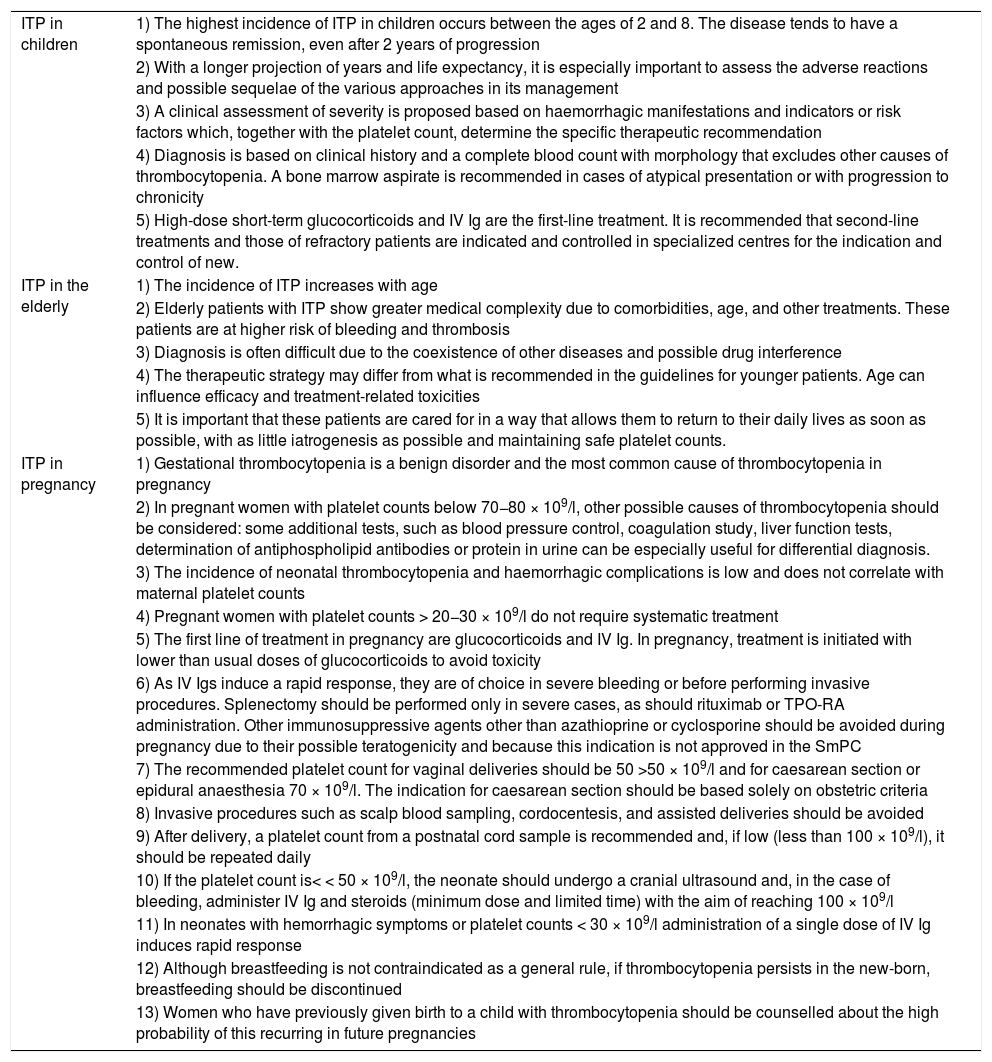
Table 4. Key points of the guidelines for the diagnosis, treatment, and follow-up of primary immune thrombocytopenia regarding ITP in children, the elderly and pregnant women.
Table 5. Key Points of the primary immune thrombocytopenia diagnosis, treatment, and follow-up guidelines concerning special situations: thrombosis, other immune thrombocytopenia and ITP, and COVID-19.
Show moreShow less
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail