Modulation of the immune system to prevent lung injury is being widely used against the new coronavirus disease (COVID-19). The primary endpoint was mortality at 7 days after tocilizumab administration. Secondary endpoints were admission to the intensive care unit, development of ARDS and respiratory insufficiency among others.
MethodsWe report the preliminary results from the Vall d’Hebron cohort study at Vall d’Hebron University Hospital, in Barcelona (Spain), including all consecutive patients who had a confirmed SARS-CoV-2 infection and who were treated with tocilizumab until March 25th.
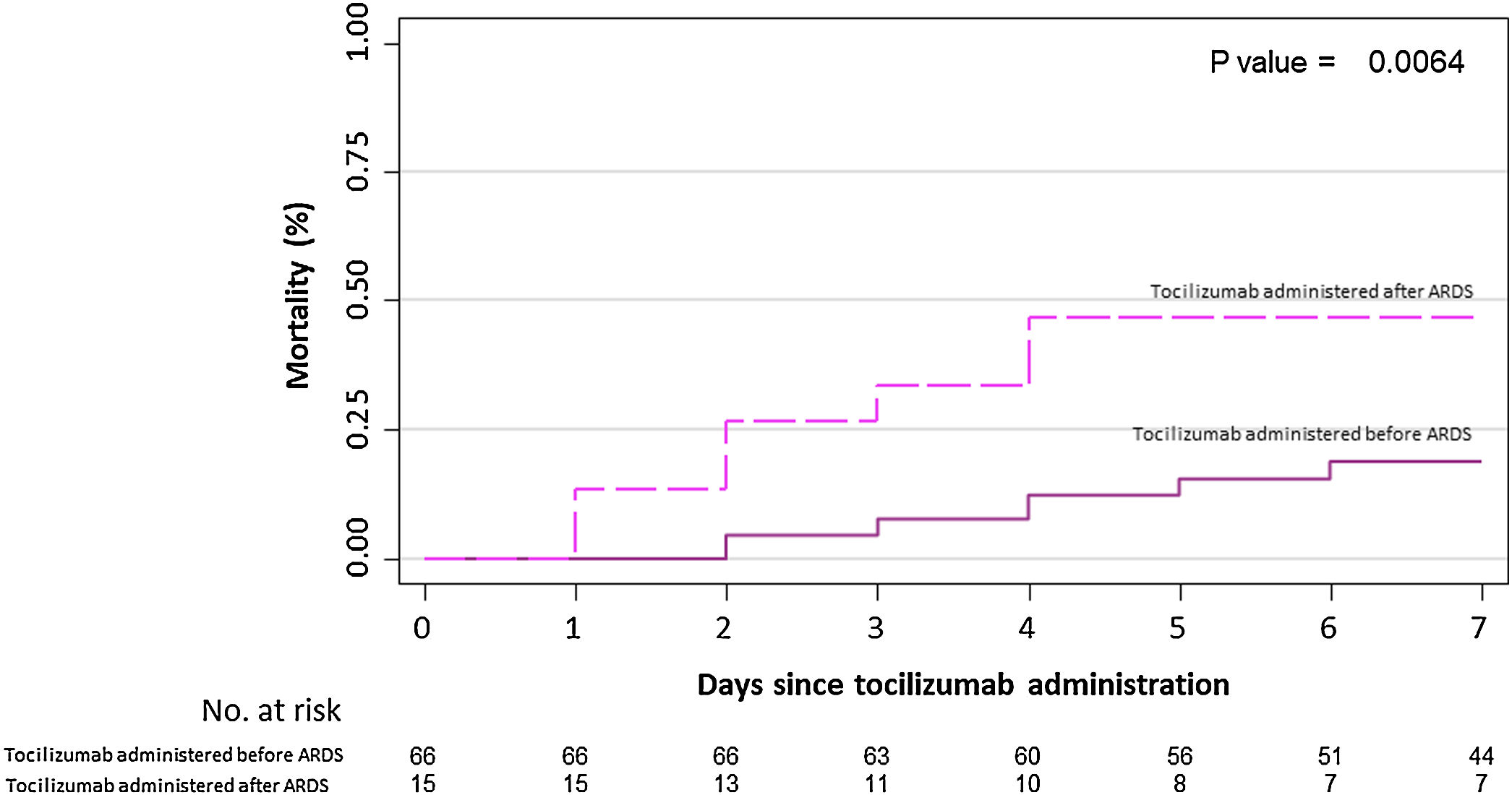
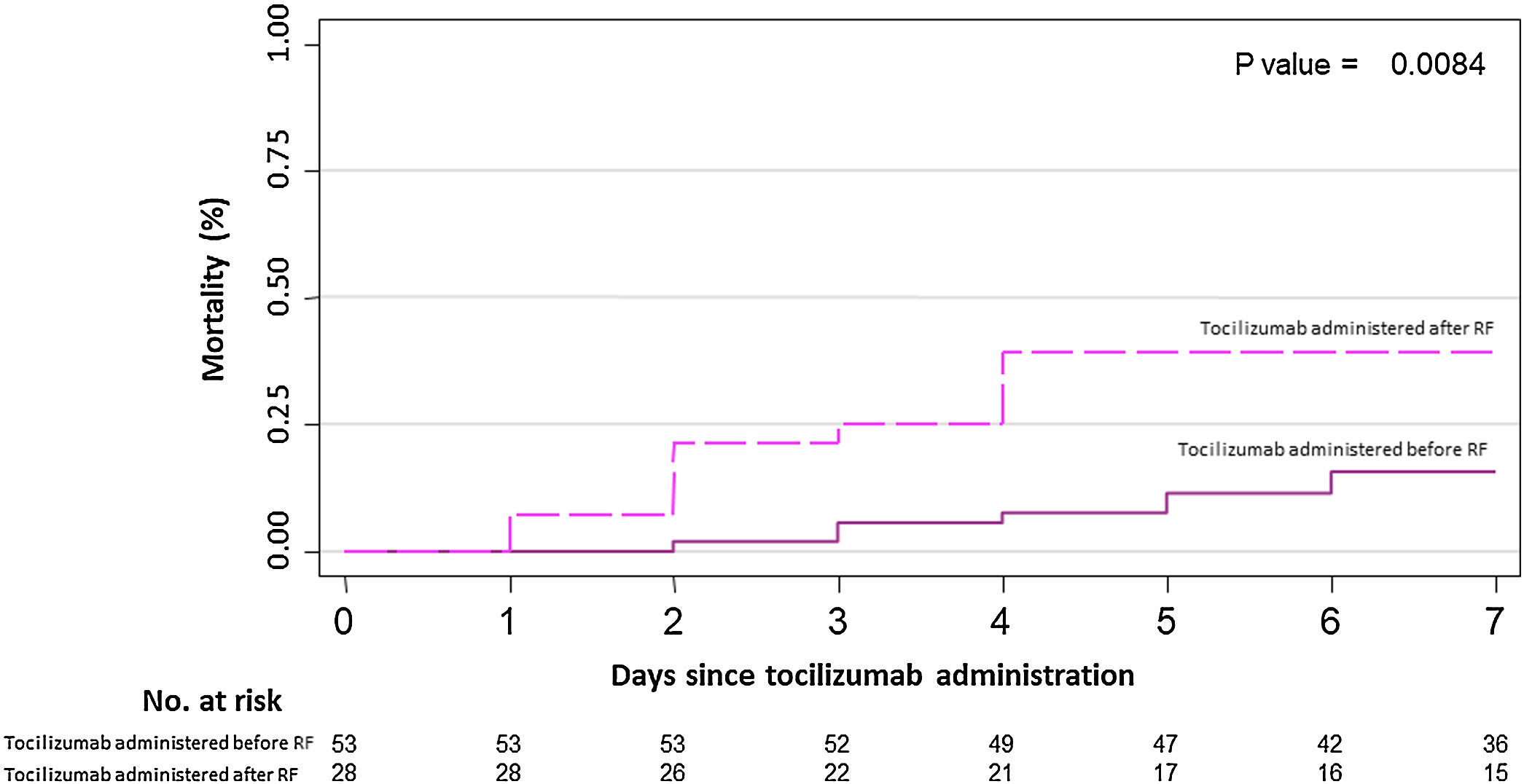
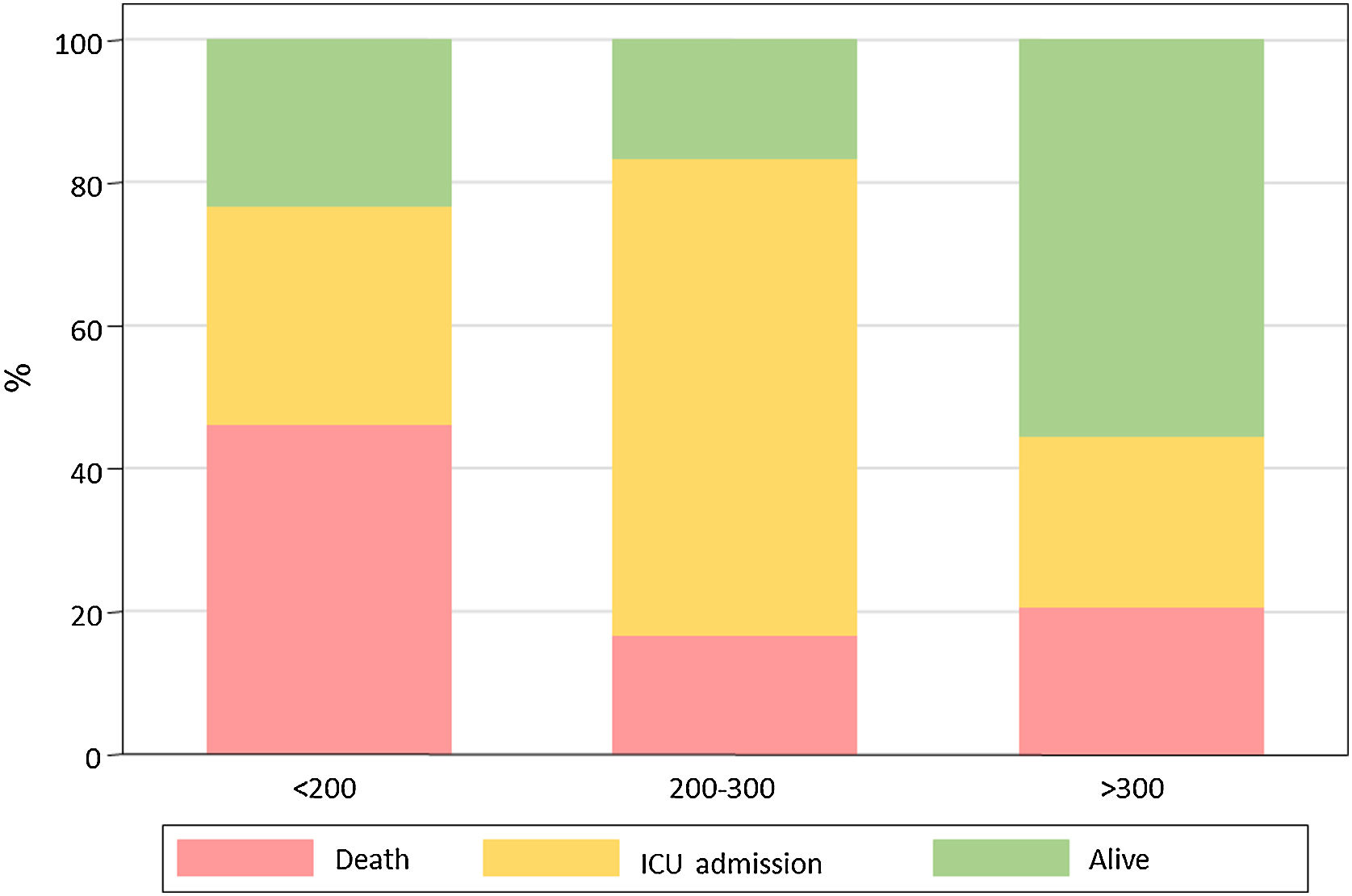
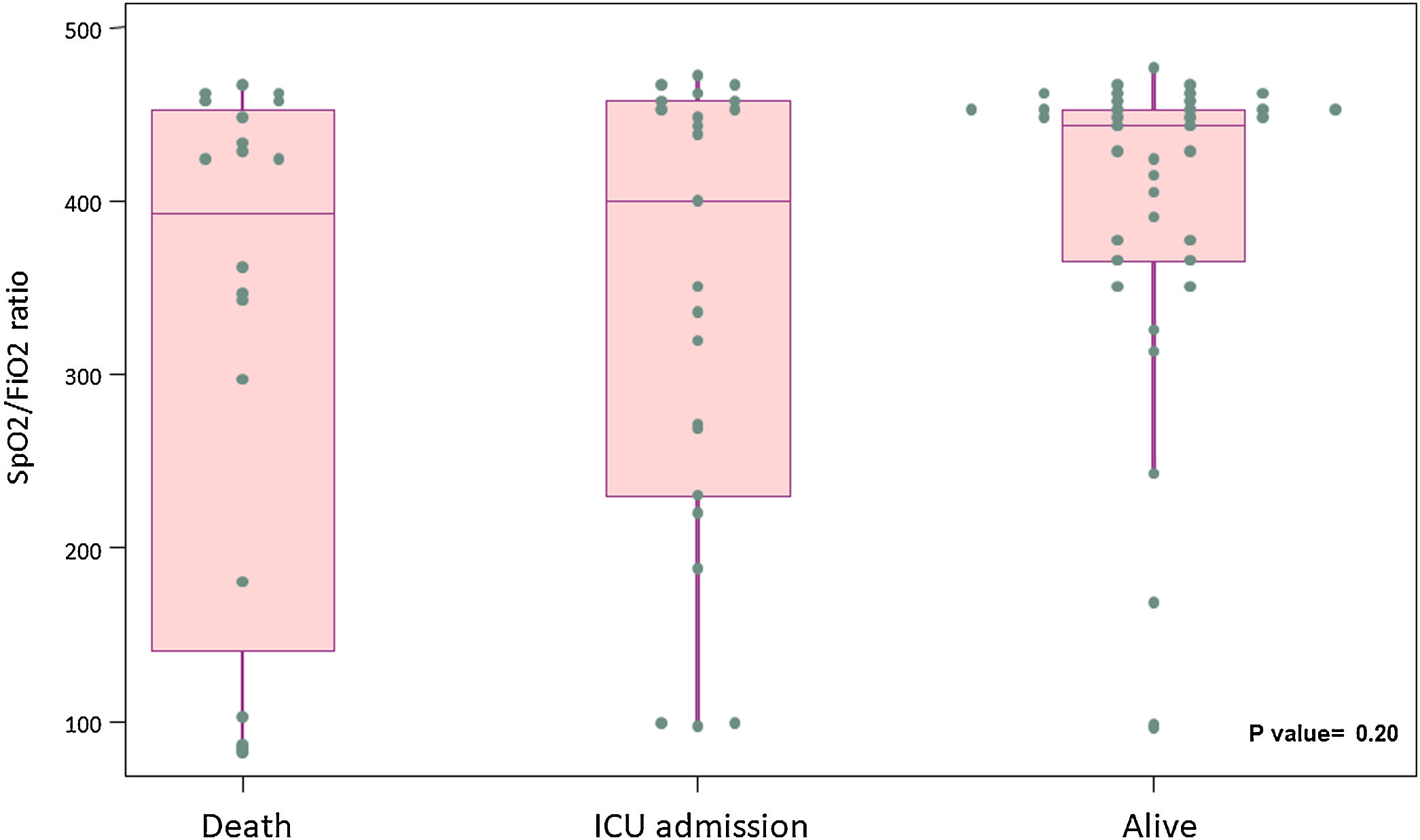
Results82 patients with COVID-19 received at least one dose of tocilizumab. The mean (± SD) age was 59.1 (19.8) years, 63% were male, 22% were of non-Spanish ancestry, and the median (IQR) age-adjusted Charlson index at baseline was 3 (1–4) points. Respiratory failure and ARDS developed in 62 (75.6%) and 45 (54.9%) patients, respectively. Median time from symptom onset to ARDS development was 8 (5–11) days. Mortality at 7 days was 26.8%. Hazard ratio for mortality was 3.3; 95% CI, 1.3–8.5 (age-adjusted hazard ratio for mortality 2.1; 95% CI, 0.8–5.8) if tocilizumab was administered after the onset of ARDS.
ConclusionEarly administration of tocilizumab in patients needing oxygen supplementation may be critical to patient recovery. Our preliminary data could inform bedside decisions until more data regarding the precise timing in of initiation of the treatment with tocilizumab.
Los tratamientos inmunomoduladores para la prevención del daño pulmonar están siendo ampliamente estudiados contra la COVID-19. El objetivo primario es evaluar la mortalidad a los 7 días después de la administración de tocilizumab. El objetivo secundario es el ingreso en UCI, el desarrollo de distrés respiratorio agudo e insuficiencia respiratoria aguda entre otros.
MétodosInformamos sobre los resultados preliminares de la cohorte del Hospital Universitario Vall d’Hebron en Barcelona (España), que incluye todos los pacientes consecutivos con infección confirmada por SARS-CoV-2 y que recibieron tratamiento con tocilizumab hasta el 25 de marzo 2020.
ResultadosOchenta y dos pacientes con COVID-19 recibieron al menos una dosis de tocilizumab. La edad media (±DE) fue de 59,1 (±19,8) años, el 63% eran hombres, 22% correspondía a paciente nacidos fuera de España, y la mediana (RIC) del índice de Charlson ajustado por edad en el momento basal fue de 3 (1-4) puntos. Sesenta y dos pacientes (75,6%) y 45 pacientes (54,9%) desarrollaron insuficiencia respiratoria y distrés respiratorio agudo respectivamente. La mediana de tiempo desde el inicio de los síntomas hasta el desarrollo de ditrés fue de 8 días (5-11). La mortalidad a los 7 días fue del 26,8% La hazard ratio de mortalidad fue del 3,3; IC 95% 1,3-8,5 (la hazard ratio de mortalidad ajustada por edad fue de 2,1; IC 95% 0,8-5,8) si el tocilizumab se administraba después del inicio del distrés respiratorio.
ConclusiónLa administración precoz de tocilizumab en pacientes con suplementos de oxígeno podría ser crítica para la recuperación de los pacientes. Nuestros datos podrían ayudar a tomar decisiones clínicas hasta que se disponga de más información sobre el momento adecuado para iniciar el tratamiento con tocilizumab.