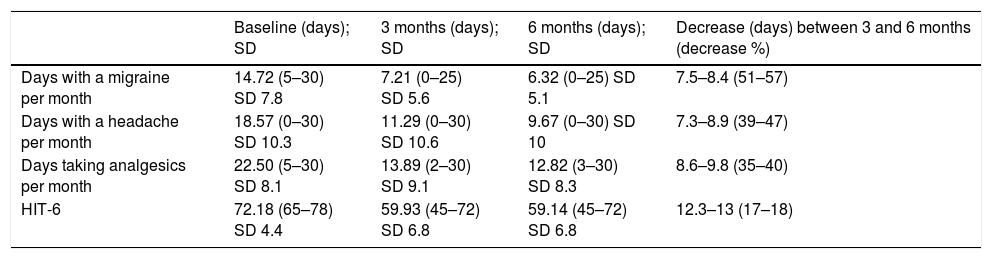
array:24 [ "pii" => "S2387020621006896" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.03.028" "estado" => "S300" "fechaPublicacion" => "2022-01-21" "aid" => "5670" "copyright" => "Elsevier España, S.L.U.. All rights reserved" "copyrightAnyo" => "2021" "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2022;158:96-7" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "Traduccion" => array:1 [ "es" => array:19 [ "pii" => "S0025775321002347" "issn" => "00257753" "doi" => "10.1016/j.medcli.2021.03.021" "estado" => "S300" "fechaPublicacion" => "2022-01-21" "aid" => "5670" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2022;158:96-7" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "es" => array:10 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Carta al Editor</span>" "titulo" => "Erenumab para el tratamiento de migraña crónica farmacorresistente" "tienePdf" => "es" "tieneTextoCompleto" => "es" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "96" "paginaFinal" => "97" ] ] "titulosAlternativos" => array:1 [ "en" => array:1 [ "titulo" => "Erenumab for the treatment of chronic resistant migraine" ] ] "contieneTextoCompleto" => array:1 [ "es" => true ] "contienePdf" => array:1 [ "es" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Almudena Layos-Romero, Alberto Andrés López, Laura Rojas Bartolomé" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Almudena" "apellidos" => "Layos-Romero" ] 1 => array:2 [ "nombre" => "Alberto" "apellidos" => "Andrés López" ] 2 => array:2 [ "nombre" => "Laura" "apellidos" => "Rojas Bartolomé" ] ] ] ] ] "idiomaDefecto" => "es" "Traduccion" => array:1 [ "en" => array:9 [ "pii" => "S2387020621006896" "doi" => "10.1016/j.medcle.2021.03.028" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "en" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621006896?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775321002347?idApp=UINPBA00004N" "url" => "/00257753/0000015800000002/v1_202201120526/S0025775321002347/v1_202201120526/es/main.assets" ] ] "itemSiguiente" => array:19 [ "pii" => "S2387020621006902" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.12.002" "estado" => "S300" "fechaPublicacion" => "2022-01-21" "aid" => "5691" "copyright" => "Elsevier España, S.L.U." "documento" => "article" "crossmark" => 1 "subdocumento" => "sco" "cita" => "Med Clin. 2022;158:98" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Image in medicine</span>" "titulo" => "Acute myeloid leukemia onset with bilateral retinal hemorrhages" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:1 [ "paginaInicial" => "98" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Debut de leucemia aguda mieloblástica con hemorragias retinianas bilaterales" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0015" "etiqueta" => "Figure 3" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr3.jpeg" "Alto" => 471 "Ancho" => 478 "Tamanyo" => 40014 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at0015" "detalle" => "Figure " "rol" => "short" ] ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Maria Huguet, Marta Balboa, Susana Vives" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Maria" "apellidos" => "Huguet" ] 1 => array:2 [ "nombre" => "Marta" "apellidos" => "Balboa" ] 2 => array:2 [ "nombre" => "Susana" "apellidos" => "Vives" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S002577532100258X" "doi" => "10.1016/j.medcli.2021.04.015" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S002577532100258X?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621006902?idApp=UINPBA00004N" "url" => "/23870206/0000015800000002/v1_202201220701/S2387020621006902/v1_202201220701/en/main.assets" ] "itemAnterior" => array:18 [ "pii" => "S2387020621006951" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.03.031" "estado" => "S300" "fechaPublicacion" => "2022-01-21" "aid" => "5671" "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2022;158:95-6" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Refractory juvenile dermatomyositis: Response to tofacitinib" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "95" "paginaFinal" => "96" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Dermatomiositis juvenil refractaria: respuesta al tofacitinib" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 1558 "Ancho" => 2500 "Tamanyo" => 203816 ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">(A) X-ray findings of calcification on the patient's two legs before being treated with tofacitinib, there were a lot of calcifications on both legs. (B) X-ray findings of calcification on the patient's two legs, after 17 months’ treatment with tofacitinib, calcifications regressed. In (A), the white dot image in the figure is calcification as shown by the red arrow. In (B), the calcification as shown by the red arrow has disappeared.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Qingfang Zhou, Ruohang Weng, Yu Xia" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Qingfang" "apellidos" => "Zhou" ] 1 => array:2 [ "nombre" => "Ruohang" "apellidos" => "Weng" ] 2 => array:2 [ "nombre" => "Yu" "apellidos" => "Xia" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "en" => array:9 [ "pii" => "S0025775321002359" "doi" => "10.1016/j.medcli.2021.03.022" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "en" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775321002359?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621006951?idApp=UINPBA00004N" "url" => "/23870206/0000015800000002/v1_202201220701/S2387020621006951/v1_202201220701/en/main.assets" ] "en" => array:14 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Erenumab for the treatment of chronic resistant migraine" "tieneTextoCompleto" => true "saludo" => "Dear Editor:" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "96" "paginaFinal" => "97" ] ] "autores" => array:1 [ 0 => array:4 [ "autoresLista" => "Almudena Layos-Romero, Alberto Andrés López, Laura Rojas Bartolomé" "autores" => array:3 [ 0 => array:4 [ "nombre" => "Almudena" "apellidos" => "Layos-Romero" "email" => array:1 [ 0 => "almudenalayos@gmail.com" ] "referencia" => array:2 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">a</span>" "identificador" => "aff0005" ] 1 => array:2 [ "etiqueta" => "*" "identificador" => "cor0005" ] ] ] 1 => array:3 [ "nombre" => "Alberto Andrés" "apellidos" => "López" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">b</span>" "identificador" => "aff0010" ] ] ] 2 => array:3 [ "nombre" => "Laura" "apellidos" => "Rojas Bartolomé" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">c</span>" "identificador" => "aff0015" ] ] ] ] "afiliaciones" => array:3 [ 0 => array:3 [ "entidad" => "Servicio de Neurología, Hospital General Universitario de Albacete, Albacete, Spain" "etiqueta" => "a" "identificador" => "aff0005" ] 1 => array:3 [ "entidad" => "Sección de Neurología, Hospital General de Almansa, Almansa, Spain" "etiqueta" => "b" "identificador" => "aff0010" ] 2 => array:3 [ "entidad" => "Sección de Neurología, Hospital de Hellín, Hellín, Spain" "etiqueta" => "c" "identificador" => "aff0015" ] ] "correspondencia" => array:1 [ 0 => array:3 [ "identificador" => "cor0005" "etiqueta" => "⁎" "correspondencia" => "Corresponding author." ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Erenumab para el tratamiento de migraña crónica farmacorresistente" ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="par0005" class="elsevierStylePara elsevierViewall">A headache is one of the most frequent reasons for neurological consultations in Primary Care settings. Migraines have a high prevalence among these cases (14% of the population)<a class="elsevierStyleCrossRef" href="#bib0005"><span class="elsevierStyleSup">1</span></a> and are associated with significant disability, causing repercussions on the patients’ personal and working lives. This situation is magnified in the case of chronic migraines; that is, suffering from a headache for more than 15 days per month (eight days meeting migraine criteria) for more than three months.</p><p id="par0010" class="elsevierStylePara elsevierViewall">Treatment of migraines is based on hygienic measures, including the promotion of routines that improve the experience of pain, the use of a simple and effective analgesic regimen, and preventive treatment in case of frequent, disabling attacks or with a poor symptomatic response. Preventive treatments are initially oral, non-specific (antidepressants, neuromodulators, and antihypertensives, among others), and have significant side effects.</p><p id="par0015" class="elsevierStylePara elsevierViewall">The calcitonin gene-related peptide (CGRP) is one of the vasoactive peptides released into the meninges through the activation of the trigeminovascular system. These molecules cause sterile inflammation and are determinants in the onset of pain.<a class="elsevierStyleCrossRef" href="#bib0010"><span class="elsevierStyleSup">2</span></a> In the last year, monclonal antibodies against the CGRP or its receptor have been approved for subjects suffering from migraines for eight or more days a month (high-frequency episodic migraines and chronic migraines) and three or more failures of previous treatments used at sufficient doses for three months, provided that one of these treatments is botulinum toxin in case of chronic migraines.</p><p id="par0020" class="elsevierStylePara elsevierViewall">In this paper we describe our experience using erenumab 70 mg, a drug targeting the CGRP receptor. We identified a total of 28 patients (22 female and six male), with a mean age of 47 years (22–68), with chronic migraines with a mean clinical evolution of 24 years (5–47), and a mean of 7.6 previous preventive treatments (1–14). The following efficacy variables were collected: days with a migraine per month (DMM), days with a headache per month (DHM), and days requiring analgesics per month (DAM). Patients suffering from a headache due to abusing analgesics were quantified. The Headache Impact Test (HIT-6) questionnaire scale was used as a measure of disability (with a HIT-6 score >60 indicating severe disability, of 55–60 indicating moderate disability, of 50–54 indicating mild disability, and <50 indicating no disability). The recommendations of the official guidelines were used as treatment response criteria,<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a> i.e., a decrease >50% in the DMM, the DHM, or the DAM, and >5 points in the HIT-6 scale. Side effects were also collected.</p><p id="par0025" class="elsevierStylePara elsevierViewall">A decrease of 7.5, 7.3, and 8.6 was observed in the DMM, DHM, and DAM, respectively, after three months of treatment (<a class="elsevierStyleCrossRef" href="#tbl0005">Table 1</a>). After six months, this decrease was of 8.4, 8.9, and 9.8 in the DMM, DHM, and DAM, respectively. The number of patients meeting analgesic abuse criteria decreased from 12 to 9. The HIT-6 score dropped from 72 to 59 points, which entailed an improvement in all disability levels: of 100% cases of severe disability, only 35% continued to present with this degree, 21% had a moderate disability, 39% had a mild disability, and 4% had no disability. Of all patients, only four (14%) reported constipation, although one of them had to discontinue the drug because of this symptom. No other side effects were detected.</p><elsevierMultimedia ident="tbl0005"></elsevierMultimedia><p id="par0030" class="elsevierStylePara elsevierViewall">These results support the effectiveness and safety of erenumab in subjects with chronic migraines, with a halving in the number of monthly migraine attacks and headaches, a 40% reduction in the number of days of analgesic consumption, and an improvement in self-perceived disability. Compared with the results reported from studies on the drug,<a class="elsevierStyleCrossRef" href="#bib0020"><span class="elsevierStyleSup">4</span></a> our case series included patients with a longer clinical evolution of the condition, a greater number of previous preventive measures, and all had received an ineffecive treatment course with botulinum toxin and were highly disabled. Despite this, the reduction in the DMM, DHM, and DAM met efficacy criteria according to international guidelines, with a low rate of side effects.</p></span>" "pdfFichero" => "main.pdf" "tienePdf" => true "NotaPie" => array:1 [ 0 => array:2 [ "etiqueta" => "☆" "nota" => "<p class="elsevierStyleNotepara" id="npar0005">Please cite this article as: Layos-Romero A, López AA, Rojas Bartolomé L. Erenumab para el tratamiento de migraña crónica farmacorresistente. Med Clin (Barc). 2022;158:96–97.</p>" ] ] "multimedia" => array:1 [ 0 => array:8 [ "identificador" => "tbl0005" "etiqueta" => "Table 1" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at0005" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:1 [ "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black"> \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Baseline (days); SD \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">3 months (days); SD \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">6 months (days); SD \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Decrease (days) between 3 and 6 months (decrease %) \t\t\t\t\t\t\n \t\t\t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Days with a migraine per month \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">14.72 (5–30) SD 7.8 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">7.21 (0–25) SD 5.6 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">6.32 (0–25) SD 5.1 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">7.5–8.4 (51–57) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Days with a headache per month \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">18.57 (0–30) SD 10.3 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">11.29 (0–30) SD 10.6 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">9.67 (0–30) SD 10 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">7.3–8.9 (39–47) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Days taking analgesics per month \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">22.50 (5–30) SD 8.1 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">13.89 (2–30) SD 9.1 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">12.82 (3–30) SD 8.3 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">8.6–9.8 (35–40) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">HIT-6 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">72.18 (65–78) SD 4.4 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">59.93 (45–72) SD 6.8 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">59.14 (45–72) SD 6.8 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">12.3–13 (17–18) \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab2809566.png" ] ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Results after three and six months of treatment.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bibs0005" "bibliografiaReferencia" => array:4 [ 0 => array:3 [ "identificador" => "bib0005" "etiqueta" => "1" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Epidemiology of headache in Europe" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:5 [ 0 => "L.J. Stovner" 1 => "J.A. Zwart" 2 => "K. Hagen" 3 => "G.M. Terwindt" 4 => "J. Pascual" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1111/j.1468-1331.2006.01184.x" "Revista" => array:6 [ "tituloSerie" => "Eur J Neurol." "fecha" => "2006" "volumen" => "13" "paginaInicial" => "333" "paginaFinal" => "345" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/16643310" "web" => "Medline" ] ] ] ] ] ] ] ] 1 => array:3 [ "identificador" => "bib0010" "etiqueta" => "2" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "CGRP en migraña: de la fisiopatología a la terapéutica" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "S. Santos-Lasaosa" 1 => "R. Belvís" 2 => "M.L. Cuadrado" 3 => "S. Díaz-Insa" 4 => "A. Gago-Veiga" 5 => "A.L. Guerrero-Peral" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.nrl.2019.03.013" "Revista" => array:2 [ "tituloSerie" => "Neurología." "fecha" => "2019" ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bib0015" "etiqueta" => "3" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "S. Sacco" 1 => "L. Bendtsen" 2 => "M. Ashina" 3 => "U. Reuter" 4 => "G. Terwindt" 5 => "D.D. Mitsikostas" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1186/s10194-018-0955-y" "Revista" => array:6 [ "tituloSerie" => "J Headache Pain." "fecha" => "2019" "volumen" => "20" "paginaInicial" => "1" "paginaFinal" => "33" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/30616570" "web" => "Medline" ] ] ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bib0020" "etiqueta" => "4" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "S. Tepper" 1 => "M. Ashina" 2 => "U. Reuter" 3 => "J.L. Brandes" 4 => "D. Dolezil" 5 => "S. Silberstein" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S1474-4422(17)30083-2" "Revista" => array:6 [ "tituloSerie" => "Lancet Neurol." "fecha" => "2017" "volumen" => "16" "paginaInicial" => "425" "paginaFinal" => "434" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/28460892" "web" => "Medline" ] ] ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/23870206/0000015800000002/v1_202201220701/S2387020621006896/v1_202201220701/en/main.assets" "Apartado" => array:4 [ "identificador" => "43309" "tipo" => "SECCION" "en" => array:2 [ "titulo" => "Letters to the Editor" "idiomaDefecto" => true ] "idiomaDefecto" => "en" ] "PDF" => "https://static.elsevier.es/multimedia/23870206/0000015800000002/v1_202201220701/S2387020621006896/v1_202201220701/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621006896?idApp=UINPBA00004N" ]
Journal Information
Vol. 158. Issue 2.
Pages 96-97 (January 2022)
Share
Download PDF
More article options
Vol. 158. Issue 2.
Pages 96-97 (January 2022)
Letter to the Editor
Erenumab for the treatment of chronic resistant migraine
Erenumab para el tratamiento de migraña crónica farmacorresistente
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail