To determine the seroprevalence of SARS-CoV-2 in patients with immune-mediated inflammatory diseases (IMID) treated with biologic (bDMARDs) or synthetic targeted disease-modifying antirheumatic drugs (tsDMARDs).
MethodsAn observational, descriptive, prospective and cross-sectional study of analytical prevalence analysis was conducted in patients with IMID with bDMARDs or tsDMARDs. Seroprevalence was compared by measuring immunoglobulin G (IgG) against SARS-CoV-2 between October/2020 and May/2021.
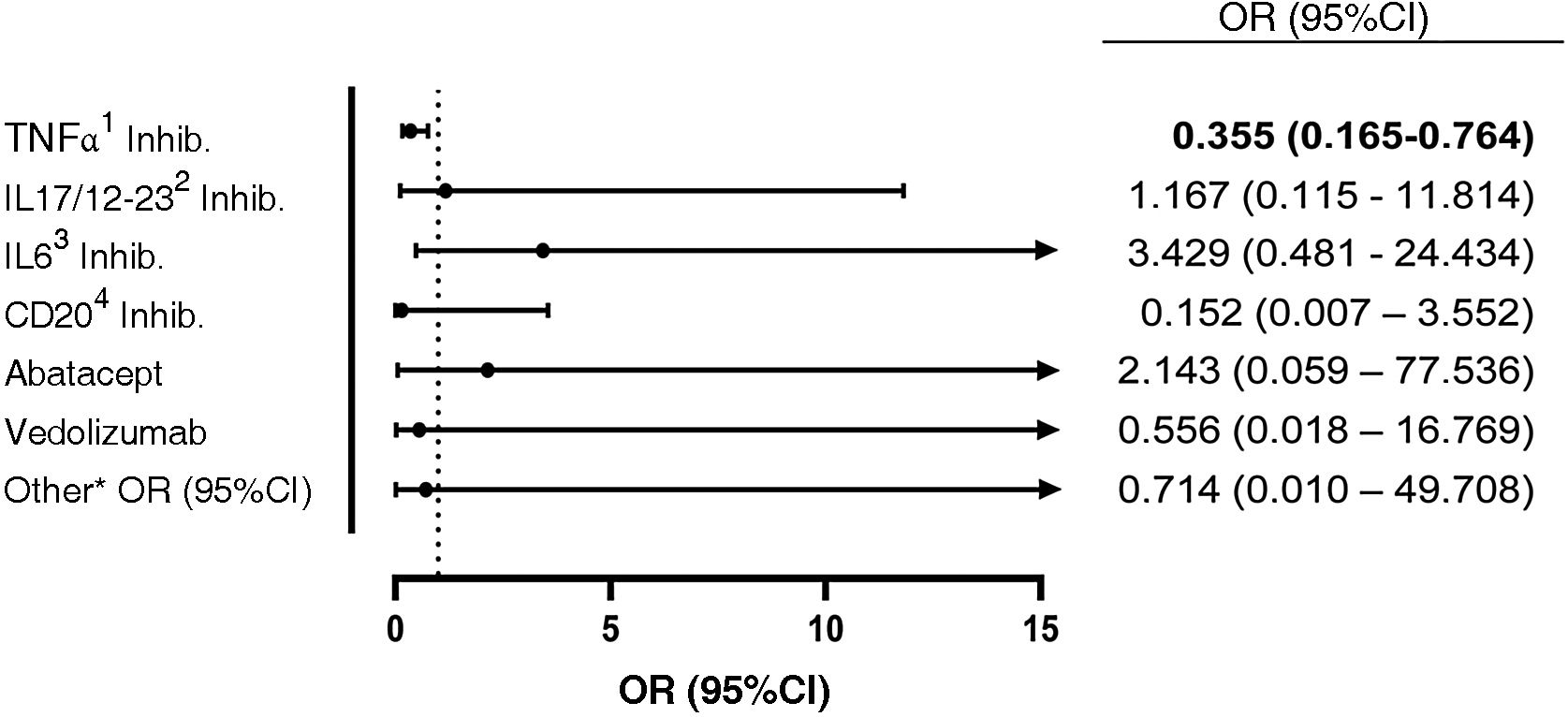
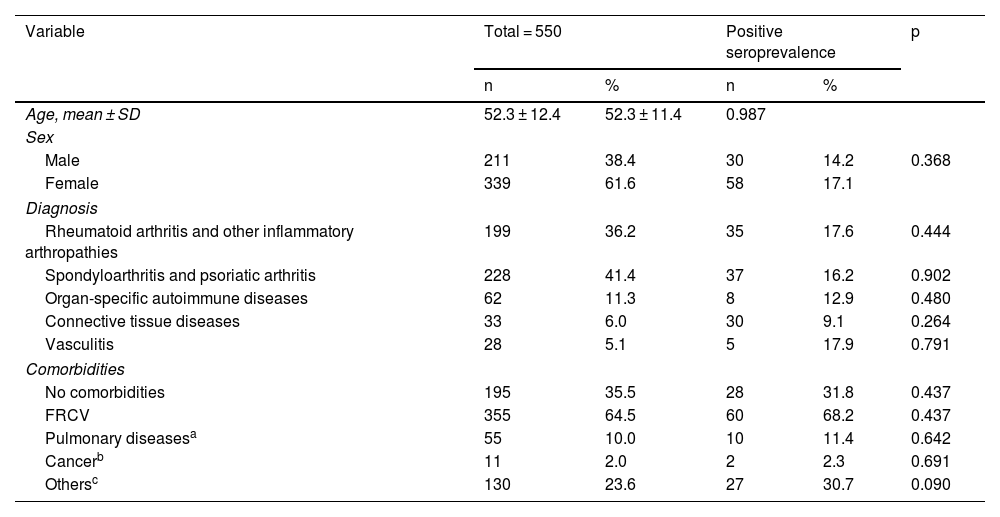
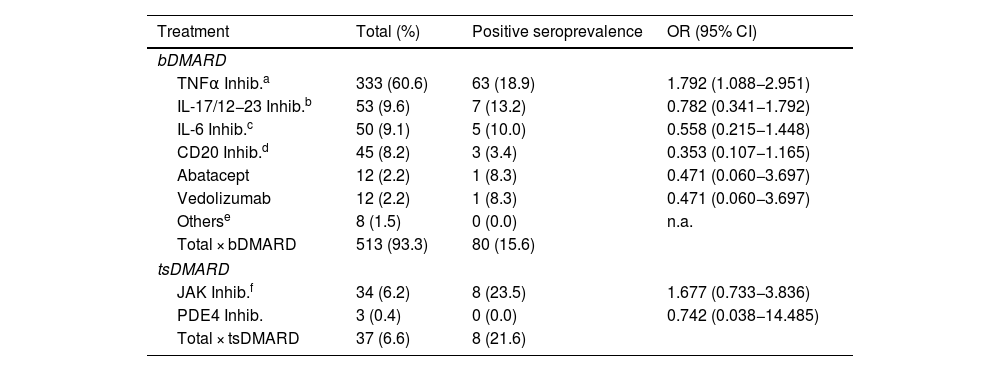
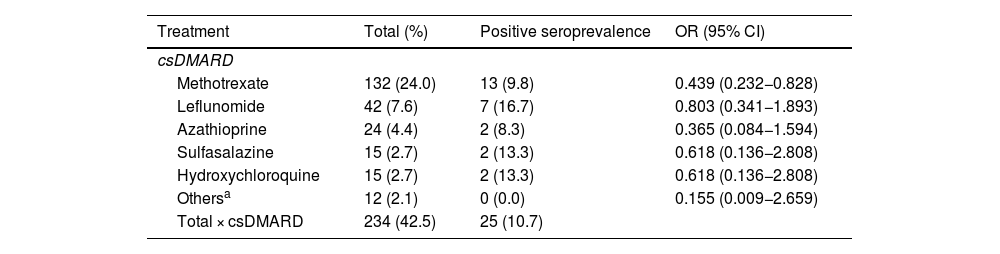
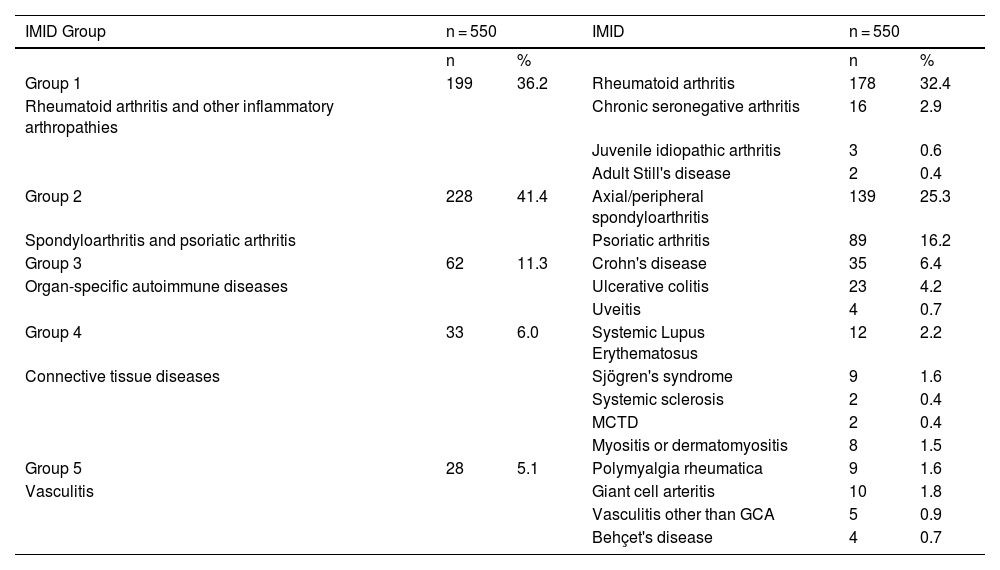
ResultsA total of 550 IMID`s patients were studied, all of them on treatment with bDMARDs or tsDMARDs. The seroprevalence of the total patient group was 16% (88/550). Patients receiving therapy with tumor necrosis factor alpha inhibitors (TNFi) had a higher seroprevalence compared to other biologic and synthetic targeted therapies (OR 1.792, [95% CI 1.088−2.951]; p = 0.021). The influence on seroprevalence of concomitant use with b/tsDMARDs of conventional synthetic DMARDs (csDMARDs) was also analyzed. A lower seroprevalence was demonstrated in the group of patients treated with TNFi and methotrexate together, compared with those on TNFi monotherapy, 10.1 vs. 24.1%, (OR 0.355, [95% CI 0.165−0.764]; p = 0.006). No significant differences were found with the other DMARDs. Regarding IMIDs, no differences in seroprevalence were identified between the different disease groups.
ConclusionPatients on treatment with TNFα inhibitors have better humoral response compared to the other b/tsDMARDs, however, when associated with methotrexate the seroprevalence decreases significantly.
Determinar la seroprevalencia del SARS-CoV-2 en pacientes con enfermedades inflamatorias inmunomediadas (IMID) tratados con fármacos antirreumáticos modificadores de la enfermedad biológica (FAMEb) o sintéticos dirigidos (FAMEsd).
MétodosSe realizó un estudio observacional, descriptivo, prospectivo y transversal de análisis de prevalencia analítica en pacientes con IMID tratados con FAMEb o FAMEsd. Se comparó la seroprevalencia midiendo la inmunoglobulina G (IgG) frente al SARS-CoV-2 entre octubre/2020 y mayo/2021.
ResultadosSe estudiaron un total de 550 pacientes con IMID, todos ellos en tratamientos con FAMEb o FAMEsd. La seroprevalencia del grupo total de pacientes fue del 16% (88/550). Los pacientes que recibieron terapia con inhibidores del factor de necrosis tumoral alfa (inhib. de TNFα) presentaron mayor seroprevalencia frente al resto de terapias biológicas y sintéticas dirigidas (OR 1,792, [IC 95% 1,088−2,951]; p = 0,021). También se analizó la influencia en la seroprevalencia del uso concomitante con los FAMEb/sd de los FAME convencionales sintéticos (FAMEcs). Se demostró una menor seroprevalencia en el grupo de pacientes tratados con inhib. de TNFα y metotrexato juntos, comparando con los que se encontraron con inhib. de TNFα en monoterapia, 10,1 vs. 24,1%, (OR 0,355, [IC 95% 0,165−0,764]; p = 0,006). Con el resto de FAMEcs no se encontraron diferencias significativas. En cuanto a las IMID, no se identifican diferencias en la seroprevalencia entre los diferentes grupos de enfermedades.
ConclusiónLos pacientes en tratamiento con inhibidores de TNFα tienen mejor respuesta humoral en comparación con los demás FAMEb/sd, sin embargo, cuando se los asocia con metotrexato la seroprevalencia disminuye de forma significativa.