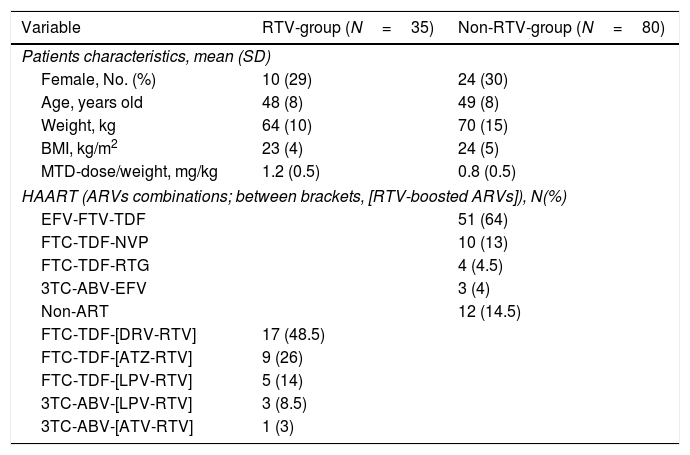
array:23 [ "pii" => "S2387020619300014" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.03.040" "estado" => "S300" "fechaPublicacion" => "2019-02-15" "aid" => "4468" "copyright" => "Elsevier España, S.L.U.. All rights reserved" "copyrightAnyo" => "2018" "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2019;152:161-2" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "itemSiguiente" => array:19 [ "pii" => "S2387020618305849" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.05.052" "estado" => "S300" "fechaPublicacion" => "2019-02-15" "aid" => "4553" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2019;152:163-4" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:10 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Myasthenia gravis and large granular lymphocytic leukemia" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "163" "paginaFinal" => "164" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Miastenia gravis y leucemia de linfocitos T grandes granulares" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Cándido Muñoz Muñoz, Christian Homedes Pedret, Josefa López Vivancos" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Cándido" "apellidos" => "Muñoz Muñoz" ] 1 => array:2 [ "nombre" => "Christian" "apellidos" => "Homedes Pedret" ] 2 => array:2 [ "nombre" => "Josefa" "apellidos" => "López Vivancos" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775318303646" "doi" => "10.1016/j.medcli.2018.05.026" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775318303646?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020618305849?idApp=UINPBA00004N" "url" => "/23870206/0000015200000004/v1_201902100719/S2387020618305849/v1_201902100719/en/main.assets" ] "itemAnterior" => array:19 [ "pii" => "S2387020618305904" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.12.011" "estado" => "S300" "fechaPublicacion" => "2019-02-15" "aid" => "4487" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2019;152:160-1" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Scientific letter</span>" "titulo" => "Consumption of the tobacco-betel-calcium hydroxide mixture, among Spanish students" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "160" "paginaFinal" => "161" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Consumo de la mezcla tabaco-betel-hidróxido cálcico entre estudiantes españoles" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 1101 "Ancho" => 1407 "Tamanyo" => 81315 ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Percentage distribution of tobacco use in different groups (F, female; M, male).</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Nansi López-Valverde, Antonio López-Valverde, BegoñaGarcía García Cenador" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Nansi" "apellidos" => "López-Valverde" ] 1 => array:2 [ "nombre" => "Antonio" "apellidos" => "López-Valverde" ] 2 => array:2 [ "nombre" => "BegoñaGarcía" "apellidos" => "García Cenador" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775318302227" "doi" => "10.1016/j.medcli.2018.03.022" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775318302227?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020618305904?idApp=UINPBA00004N" "url" => "/23870206/0000015200000004/v1_201902100719/S2387020618305904/v1_201902100719/en/main.assets" ] "en" => array:13 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Scientific letter</span>" "titulo" => "Methadone dosing in patients on ritonavir-boosted-based highly active antiretroviral therapy" "tieneTextoCompleto" => true "saludo" => "Dear Editor:" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "161" "paginaFinal" => "162" ] ] "autores" => array:1 [ 0 => array:4 [ "autoresLista" => "Roberto Lozano, Nieves Domeque, Alberto Jose Frutos" "autores" => array:3 [ 0 => array:4 [ "nombre" => "Roberto" "apellidos" => "Lozano" "email" => array:1 [ 0 => "rlozano@salud.aragon.es" ] "referencia" => array:2 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">a</span>" "identificador" => "aff0005" ] 1 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">*</span>" "identificador" => "cor0005" ] ] ] 1 => array:3 [ "nombre" => "Nieves" "apellidos" => "Domeque" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">b</span>" "identificador" => "aff0010" ] ] ] 2 => array:3 [ "nombre" => "Alberto Jose" "apellidos" => "Frutos" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">c</span>" "identificador" => "aff0015" ] ] ] ] "afiliaciones" => array:3 [ 0 => array:3 [ "entidad" => "Servicio de Farmacia, Hospital Real de Nuestra Señora de Gracia, Zaragoza, Spain" "etiqueta" => "a" "identificador" => "aff0005" ] 1 => array:3 [ "entidad" => "Servicio Psiquiatría, Hospital Real de Nuestra Señora de Gracia, Zaragoza, Spain" "etiqueta" => "b" "identificador" => "aff0010" ] 2 => array:3 [ "entidad" => "Servicio Farmacia, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain" "etiqueta" => "c" "identificador" => "aff0015" ] ] "correspondencia" => array:1 [ 0 => array:3 [ "identificador" => "cor0005" "etiqueta" => "⁎" "correspondencia" => "Corresponding author." ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Dosificación de metadona en pacientes en tratamiento con terapia antirretroviral de gran actividad incluyendo ritonavir" ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="par0005" class="elsevierStylePara elsevierViewall">Patients receiving methadone (MTD), for heroin addiction, in so-called methadone maintenance treatment (MMT) programs, are characterized by chronic diseases, abuse and/or dependence on other drugs, and a higher prevalence of mental pathologies and infectious diseases (<span class="elsevierStyleItalic">e.g.</span>, AIDS, hepatitis).<a class="elsevierStyleCrossRef" href="#bib0030"><span class="elsevierStyleSup">1</span></a></p><p id="par0010" class="elsevierStylePara elsevierViewall">In addition, due to this high prevalence of infection with the human immunodeficiency virus (HIV(+)), and the concurrent use of MTD and highly active antiretroviral therapy (HAART), it is very important to consider metabolism-based drug-drug interactions (DDIs), when adjusting the MTD-dose, and specifically when combinations of antiretroviral drugs (ARVs) include ritonavir (RTV).</p><p id="par0015" class="elsevierStylePara elsevierViewall">In fact, we know that RTV is a highly potent HIV protease inhibitor, widely used as an enhancer of other ARVs (in so-called ritonavir-boosted antiretroviral therapy; <span class="elsevierStyleItalic">e.g.</span> combinations of RTV with atazanavir, lopinavir or darunavir) and extensively metabolized by cytochrome P450 (CYP) 3A4 for which is a potent inhibitor.<a class="elsevierStyleCrossRef" href="#bib0035"><span class="elsevierStyleSup">2</span></a> Also, we know that MTD is mainly metabolized by CYP 3A4 and CYP 2B6 to which it potently induces.<a class="elsevierStyleCrossRef" href="#bib0040"><span class="elsevierStyleSup">3</span></a> Therefore, the sharing of the CYP 3A4 metabolic pathway, results in a metabolism-based DDI, between MTD and RTV.</p><p id="par0020" class="elsevierStylePara elsevierViewall">Despite being a well-known DDI, the mechanism by which RTV alters the clearance of MTD remains controversial, so is its influence on plasma MTD levels. Which finally manifests in form of great variability in the dosage of MTD, when used in conjunction with RTV: from not modifying the dose of MTD until increases up to 40%.<a class="elsevierStyleCrossRef" href="#bib0045"><span class="elsevierStyleSup">4</span></a></p><p id="par0025" class="elsevierStylePara elsevierViewall">Thus, we aimed to analyze the influence of ritonavir-based HAART on MTD-dose of patients included in MMT programs, in order to avoid the variability of its dosage.</p><p id="par0030" class="elsevierStylePara elsevierViewall">For this, a retrospective observational clinical study was conducted. The initial sample was composed of HIV(+)-patients treated with HAART and included in our MMT program, during a 10-years follow-up period (<span class="elsevierStyleItalic">n</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>130). Since it was not an experimental work no ethic committee review was required. However, it did need to meet the ethical standards of the Declaration of Helsinki.</p><p id="par0035" class="elsevierStylePara elsevierViewall">Firstly, we collected data of clinically relevant metabolism-based drug-drug interactions (DDI) between ARVs and MTD. The results showed that 26% of patients display ARVs–MTD interactions, of which the MTD–RTV interaction (23%) was the most prevalent. Secondly, after excluding patients (<span class="elsevierStyleItalic">n</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>15) who had MTD interactions with drugs other than RTV we obtained the study final sample (<span class="elsevierStyleItalic">n</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>115).</p><p id="par0040" class="elsevierStylePara elsevierViewall">From this final sample, we compared the mean value of the MTD-dose, adjusted by body weight, of HIV(+)-patients on RTV-based HAART (RTV-group; RTV dose of 100–200<span class="elsevierStyleHsp" style=""></span>mg/day; <span class="elsevierStyleItalic">n</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>35) <span class="elsevierStyleItalic">vs</span>. of HIV(+)-patients on HAART that excluded RTV (non-RTV-group; <span class="elsevierStyleItalic">n</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>80). As result, we found that average MTD-dose in the RTV-group was 50% higher than non-RTV-group (1.2<span class="elsevierStyleHsp" style=""></span>±<span class="elsevierStyleHsp" style=""></span>0.5<span class="elsevierStyleHsp" style=""></span>mg/kg <span class="elsevierStyleItalic">vs</span> 0.8<span class="elsevierStyleHsp" style=""></span>±<span class="elsevierStyleHsp" style=""></span>0.5<span class="elsevierStyleHsp" style=""></span>mg/kg, 95% CI<span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>0.199 to 0.601, <span class="elsevierStyleItalic">p</span><span class="elsevierStyleHsp" style=""></span><<span class="elsevierStyleHsp" style=""></span>.0001), <a class="elsevierStyleCrossRef" href="#tbl0005">Table 1</a>.</p><elsevierMultimedia ident="tbl0005"></elsevierMultimedia><p id="par0045" class="elsevierStylePara elsevierViewall">These data coincide with those of the authors who reported a significant decrease in plasma MTD values (up to 40%),<a class="elsevierStyleCrossRef" href="#bib0045"><span class="elsevierStyleSup">4</span></a> when used in conjunction with RTV; also, support the observations of Kharasch et al.,<a class="elsevierStyleCrossRef" href="#bib0050"><span class="elsevierStyleSup">5</span></a> that CYP3A does not mediate the interaction between MTD and RTV. In addition, the results suggest that RTV would first inhibit CYP3A4, increasing plasma MTD levels and, this increase, in turn, would secondarily cause an increase in the self-induction of CYP2B6. Thus, the MTD-inhibition of CYP3A4 together with the self-induction of CYP2B6, would result in an overall decrease of plasma MTD levels; which, ultimately, could lead to opiate withdrawal symptoms (for example, fatigue, anxiety, restlessness), and cause patients to require higher doses of MTD.</p><p id="par0050" class="elsevierStylePara elsevierViewall">In conclusion, up to date, MTD-RTV continues to be the most prevalent DDI among HIV(+)-patients receiving HAART and included in MMT programs. These patients, due to the decrease of the values of MTD in plasma when administered in conjunction with RTV-boosted-based HAART (100–200<span class="elsevierStyleHsp" style=""></span>mg/day), need to increase their dose of MTD (mg/kg-body weight) by 50%.</p><p id="par0055" class="elsevierStylePara elsevierViewall">Finally, it should be noted, as the most important limitation of this DDI analysis and due to the size of the sample and the absence of data, that it could not evaluate the influence of other factors, such as sex, age, the different combination of ARVs, and concomitant treatments for other infectious and psychiatric co-morbidities.</p></span>" "pdfFichero" => "main.pdf" "tienePdf" => true "multimedia" => array:1 [ 0 => array:8 [ "identificador" => "tbl0005" "etiqueta" => "Table 1" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at1" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:2 [ "leyenda" => "<p id="spar0010" class="elsevierStyleSimplePara elsevierViewall">ABV: abacavir; ARV: antiretroviral; ATV: atazanavir; DRV: darunavir; EFV: efavirenz; FTC: emtricitabine; HAART: highly active antiretroviral therapy; HIV: human immunodeficiency virus; LPV: lopinavir; MTD: methadone; NVP: nevirapine; RTV: ritonavir; RTG: raltegravir; 3TC: lamivudine; TDF: tenofovir; BMI: Body Mass Index.</p>" "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Variable \t\t\t\t\t\t\n \t\t\t\t</th><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">RTV-group (<span class="elsevierStyleItalic">N</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>35) \t\t\t\t\t\t\n \t\t\t\t</th><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Non-RTV-group (<span class="elsevierStyleItalic">N</span><span class="elsevierStyleHsp" style=""></span>=<span class="elsevierStyleHsp" style=""></span>80) \t\t\t\t\t\t\n \t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td" title="table-entry " colspan="3" align="left" valign="top"><span class="elsevierStyleItalic">Patients characteristics, mean (SD)</span></td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Female, No. (%) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">10 (29) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">24 (30) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Age, years old \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">48 (8) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">49 (8) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Weight, kg \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">64 (10) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">70 (15) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>BMI, kg/m<span class="elsevierStyleSup">2</span> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">23 (4) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">24 (5) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>MTD-dose/weight, mg/kg \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">1.2 (0.5) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">0.8 (0.5) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td" title="table-entry " colspan="3" align="left" valign="top"><span class="elsevierStyleVsp" style="height:0.5px"></span></td></tr><tr title="table-row"><td class="td" title="table-entry " colspan="3" align="left" valign="top"><span class="elsevierStyleItalic">HAART (ARVs combinations; between brackets, [RTV-boosted ARVs]), N(%)</span></td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>EFV-FTV-TDF \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">51 (64) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>FTC-TDF-NVP \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">10 (13) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>FTC-TDF-RTG \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">4 (4.5) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>3TC-ABV-EFV \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">3 (4) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Non-ART \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">12 (14.5) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>FTC-TDF-[DRV-RTV] \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">17 (48.5) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>FTC-TDF-[ATZ-RTV] \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">9 (26) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>FTC-TDF-[LPV-RTV] \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">5 (14) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>3TC-ABV-[LPV-RTV] \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">3 (8.5) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>3TC-ABV-[ATV-RTV] \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">1 (3) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab1959485.png" ] ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Patients characteristics and antirretroviral drug regimens.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bibs0015" "bibliografiaReferencia" => array:5 [ 0 => array:3 [ "identificador" => "bib0030" "etiqueta" => "1" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Pharmacological maintenance treatments of opiate addiction" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "J. Bell" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1111/bcp.12051" "Revista" => array:6 [ "tituloSerie" => "Br J Clin Pharmacol" "fecha" => "2014" "volumen" => "77" "paginaInicial" => "253" "paginaFinal" => "263" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/23210630" "web" => "Medline" ] ] ] ] ] ] ] ] 1 => array:3 [ "identificador" => "bib0035" "etiqueta" => "2" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Multidose pharmacokinetics of ritonavir and zidovudine in human immunodeficiency virus-infected patients" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:6 [ 0 => "A. Cato" 1 => "J. Qian" 2 => "A. Hsu" 3 => "B. Levy" 4 => "J. Leonard" 5 => "R. Granneman" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Antimicrob Agents Chemother" "fecha" => "1998" "volumen" => "2" "paginaInicial" => "1788" "paginaFinal" => "1793" ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bib0040" "etiqueta" => "3" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Mechanism of autoinduction of methadone N-demethylation in human hepatocytes" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:4 [ 0 => "S.D. Campbell" 1 => "A. Crafford" 2 => "B.L. Williamson" 3 => "E.D. Kharasch" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1213/ANE.0b013e3182918252" "Revista" => array:6 [ "tituloSerie" => "Anesth Analg" "fecha" => "2013" "volumen" => "117" "paginaInicial" => "52" "paginaFinal" => "60" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/23733841" "web" => "Medline" ] ] ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bib0045" "etiqueta" => "4" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Decreased methadone effect after ritonavir initiation" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:2 [ 0 => "S.M. Geletko" 1 => "A.D. Erickson" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:6 [ "tituloSerie" => "Pharmacotherapy" "fecha" => "2000" "volumen" => "20" "paginaInicial" => "93" "paginaFinal" => "94" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/10641980" "web" => "Medline" ] ] ] ] ] ] ] ] 4 => array:3 [ "identificador" => "bib0050" "etiqueta" => "5" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Cytochrome P4503A does not mediate the interaction between methadone and ritonavir–lopinavir" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:2 [ 0 => "E.D. Kharasch" 1 => "K. Stubbert" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1124/dmd.113.053991" "Revista" => array:6 [ "tituloSerie" => "Drug Metab Dispos" "fecha" => "2013" "volumen" => "41" "paginaInicial" => "2166" "paginaFinal" => "2174" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/24067429" "web" => "Medline" ] ] ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/23870206/0000015200000004/v1_201902100719/S2387020619300014/v1_201902100719/en/main.assets" "Apartado" => array:4 [ "identificador" => "43311" "tipo" => "SECCION" "en" => array:2 [ "titulo" => "Scientific letters" "idiomaDefecto" => true ] "idiomaDefecto" => "en" ] "PDF" => "https://static.elsevier.es/multimedia/23870206/0000015200000004/v1_201902100719/S2387020619300014/v1_201902100719/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020619300014?idApp=UINPBA00004N" ]
Journal Information
Vol. 152. Issue 4.
Pages 161-162 (February 2019)
Vol. 152. Issue 4.
Pages 161-162 (February 2019)
Scientific letter
Methadone dosing in patients on ritonavir-boosted-based highly active antiretroviral therapy
Dosificación de metadona en pacientes en tratamiento con terapia antirretroviral de gran actividad incluyendo ritonavir
Visits
4
This item has received
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail