To determine the predictive factors of pulmonary thromboembolic (PTE) in patients with SARS-CoV-2 infection (COVID-19) assessed in the emergency department at a tertiary hospital during the first pandemic wave.
MethodsObservational single-center study conducted in a retrospective cohort of patients with confirmed SARS-CoV-2 infection (or high clinical-radiological suspicion) who underwent PTE screening by computed tomography pulmonary angiography (CTPA). Predictive factors of PTE were explored using logistic regression, creating two predictive models (without or with D-dimer values).
ResultsOut of a total of 274 CTPA performed, 70 procedures presented diagnostic findings of PTE, representing a cumulative incidence of 25.54% (95% confidence interval [CI]: 20.49–31.14). In the non-D-dimer based model, respiratory rate >22 bpm (odds ratio [OR]: 3.162; 95% CI: 1.627–6.148; p = 0.001) and the absence of findings suggestive of COVID-19 in plain chest X-ray (OR: 3.869; 95% CI: 0.869–17.225; p = 0.076) were predictors of PTE. In the D-dimer-based model, tachypnea remained as a predictive factor (OR: 4.967; 95% CI: 2.053–12.018; p < 0.001), as well as D-dimers > 3000 ng/mL (OR: 7.494; 95% CI: 3.038–18.485; p < 0.001).
ConclusionsThe presence of tachypnea (>22 bpm) and the absence of radiological findings suggestive of SARS-CoV-2 infection in the chest X-ray, in addition to D-dimer values >3000 ng/mL, were identified as predictive factors of PTE in patients with COVID-19.
Pretendemos determinar los factores predictores de enfermedad tromboembólica pulmonar (ETEP) en pacientes con infección por SARS-CoV-2 (COVID-19) atendidos en el servicio de urgencias de un hospital terciario durante la primera ola pandémica.
MétodosEstudio observacional unicéntrico realizado en una cohorte retrospectiva de pacientes con infección confirmada por SARS-CoV-2 (o alta sospecha clínico-radiológica de COVID-19) sometidos a despistaje de ETEP mediante tomografía computarizada de arterias pulmonares (TCAP). Se exploraron los factores predictores de ETEP mediante regresión logística, creándose dos modelos predictivos (sin o con los valores de dímeros-D).
ResultadosDe un total de 274 TCAP realizados, 70 procedimientos presentaron hallazgos diagnósticos de ETEP, representando una incidencia acumulada de 25,54% (intervalo de confianza [IC] 95%: 20,49–31,14). En el modelo no ajustado por el nivel de dímeros-D, la frecuencia respiratoria >22 rpm (odds ratio [OR]: 3,162; IC 95%: 1,627–6,148; p = 0,001) y la ausencia de hallazgos sugerentes de COVID-19 en la radiología simple de tórax (OR: 3,869; IC 95%: 0,869–17,225; p = 0,076) fueron predictores de ETEP. En el segundo modelo se mantuvo la presencia de taquipnea (OR: 4,967; IC 95%: 2,053–12,018; p < 0,001), identificándose además un nivel de dímeros-D > 3.000 ng/mL (OR: 7,494; IC 95%: 3,038–18,485; p < 0,001).
ConclusionesLa presencia de taquipnea (>22 rpm) y la ausencia de hallazgos radiológicos sugestivos de infección por SARS-CoV-2 en la radiografía simple de tórax, además de los valores de dímero-D > 3.000 ng/mL, fueron identificados como factores predictores de ETEP en pacientes con COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first described in December 2019 in Wuhan, Hubei province, China.1 Since then, coronavirus disease 2019 (COVID-19) has spread to become a pandemic affecting more than 106 million people in almost every country in the world. Global mortality as of February 2021 exceeds 2.3 million people, with Spain being one of the most affected countries in Western Europe, with over 63,000 deaths.2
Numerous studies support the ability of SARS-CoV-2 to invade vascular endothelial cells through angiotensin-converting enzyme 2 (ACE 2) expressed on the cell surface. This phenomenon is subsequently accompanied by endothelial inflammation, complement activation, thrombin generation, platelet and leukocyte recruitment, and the onset of innate and adaptive immune responses, causing an underlying thrombophilia with a predisposition to the formation of thrombotic phenomena in different sites.
There are certain parameters that allow this prothrombotic status to be assessed, such as the presence of elevated levels of D-dimer and other coagulation disorders,3 C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), markers of myocardial damage, and N-terminal prohormone of brain natriuretic peptide (NT-proBNP).4
Diagnosis of thromboembolic disease superimposed on COVID-19 disease is a priority from the time of patient admission in order to guide the application of targeted prophylactic and therapeutic anticoagulation strategies, as appropriate. The objective of the present study, therefore, is to identify the factors associated with the presence of pulmonary embolism (PE) in patients with a confirmed diagnosis (or high suspicion) of COVID-19, and the development of a predictive model that allows determining, from the emergency department, the probability of this complication.
Material and methodsStudy designWe conducted a single-center observational analytical study based on a retrospective cohort, following the STROBE recommendations for observational studies. The study was approved by the Drug Research Ethics Committee (CEIm) of the Hospital Universitario 12 de Octubre (code 20/229).
Patients and study settingAdult patients seen in the emergency department of the Hospital Universitario 12 de Octubre with a high epidemiological, clinical and radiological suspicion and/or microbiological confirmation (by polymerase chain reaction [RT-PCR]) of SARS-CoV-2 infection (COVID-19) who underwent pulmonary artery computed tomography (PACT) for suspected PE between 15 March and 25 April 2020 were included in the study. The criteria applied to request screening for PE were a low correlation between respiratory symptoms and normal plain X-ray, no improvement in O2 saturation despite high-flow oxygen therapy, and/or elevated D-dimer in the absence of other inflammatory parameters. Patients were excluded if it was impossible to perform a PACT despite its indication (allergy to contrasts, claustrophobia, etc.), if the PACT was performed for a different indication, or if the clinical information was not accessible.
All patients who underwent a PACT during that period were located through the computerised registry of the radiology service, excluding those cases that did not meet the eligibility criteria. A series of demographic, epidemiological, clinical, laboratory, and radiological variables were collected using a standardized form through the hospital’s electronic medical record, which was then included in an anonymized database. These variables were selected for their potential relationship with the outcome variable (presence of PE) based on their pathophysiological plausibility and the published literature in this regard.
Pulmonary artery computed tomography protocolAll the PACT procedures were performed using a 64- or 16-slice CT scanner (Phillips Brilliance, Phillips Medical System, Eindhoven, The Netherlands). The acquisition parameters were: 120 kVp, 50–350 mA with dose modulation, collimation of 64 × 0.625 mm or 16 × 0.75 mm, rotation time 0.8 s, and reconstruction section thickness of 2 mm and 1 mm of reconstruction interval in mediastinal and parenchymal windows.
A bolus tracking technique was used with a threshold of 150 Hounsfield units (HU) measured in the main pulmonary artery after intravenous injection of 50–90 mL of non-ionic iodinated contrast medium (Ioexol 350 mg/mL, GE Healthcare, Cork, Ireland). The flow rate was 4 mL/s using an injector (Medrad, Stellant, Pittsburgh, PA, USA).
All examinations were reported by a thoracic or emergency physician radiologist with at least 15 years of experience, or by a fourth-year radiology resident supervised by an attending radiologist. Possible discrepancies in the interpretation of the PACT were resolved by consensus.
Primary and secondary objectivesThe main objective was to identify the predictive factors of the presence of PE on a PACT. As secondary objectives, we conducted a descriptive study of the epidemiological, clinical, laboratory and radiological characteristics of patients diagnosed with PE and analysed the differences with patients who did not have PE on PACT.
Statistical analysisIn the descriptive study, qualitative variables were expressed as absolute and relative frequency distributions, while quantitative variables were expressed as mean or median with standard deviation (SD) or interquartile range (IQR), with 95% confidence intervals (95% CI).
To establish differences between the characteristics of the study groups, Pearson’s chi2 test or Fisher’s test were used for comparisons between qualitative variables and the Student’s t-test or the parametric Mann–Whitney U-test for quantitative variables according to whether or not they conformed to a normal distribution, respectively. A 95% CI was established.
The associations between the independent variables and the main variable were estimated using odds ratios (OR) with their corresponding 95% CI.
Treatment of missing values was performed by full data analysis (listwise), by default in the SPSS statistical software used.
A series of continuous variables were dichotomized for analysis according to different criteria: respiratory rate ≥22 cpm on patient arrival at the emergency department based on the cut-off point established on the qSOFA scale; quotient between the saturation and the inspired O2 fraction (SpO2/FiO2) ≥ 420 based on its median value; LDH ≥ 350 IU/L based on its upper limit of normal; and D-dimer ≥ 3000 ng/mL after determination of the optimal point for the prediction of PE using Youden’s J statistic (J = sensitivity + specificity − 1). The area under the receiver operating characteristics curve (AUC-ROC) was also estimated as an index of discriminative capacity. Previous studies show D-dimer as an independent predictor of PE in the context of COVID-19, with an optimal cut-off point of 5000 ng/mL.5
A multivariate analysis was carried out using a logistic regression model (backward-stepwise) to determine the impact of each of the predictive factors on the primary endpoint of the study (presence of PE), controlling for the effect of possible confounding factors, obtaining two models (without D-dimers or introducing this variable with the aforementioned cut-off point of 3000 ng/mL). The predictive capacity of both models was analysed, following the recommendations of the TRIPOD guidelines, based on the AUC-ROC obtained.
The selection criteria for the variables in the model were based on their significance in the univariate analysis (p < 0.05) and their biological plausibility. All statistical analyses were performed using SPSS version 23.0 (SPSS, IBM Corp, Armonk, NY, USA).
ResultsA total of 274 patients were included (Appendix B Fig. S1), 179 (65.32%) of whom were diagnosed with SARS-CoV-2 infection by RT-PCR. The remaining 95 patients (34.67%) were diagnosed on the basis of a clinical-radiological presentation highly suggestive of COVID-19 in a compatible epidemiological context.
A total of 70 patients had findings compatible with PE of varying degrees of severity on TCAP, representing a cumulative incidence of 25.54% (95% CI: 20.49–31.14).
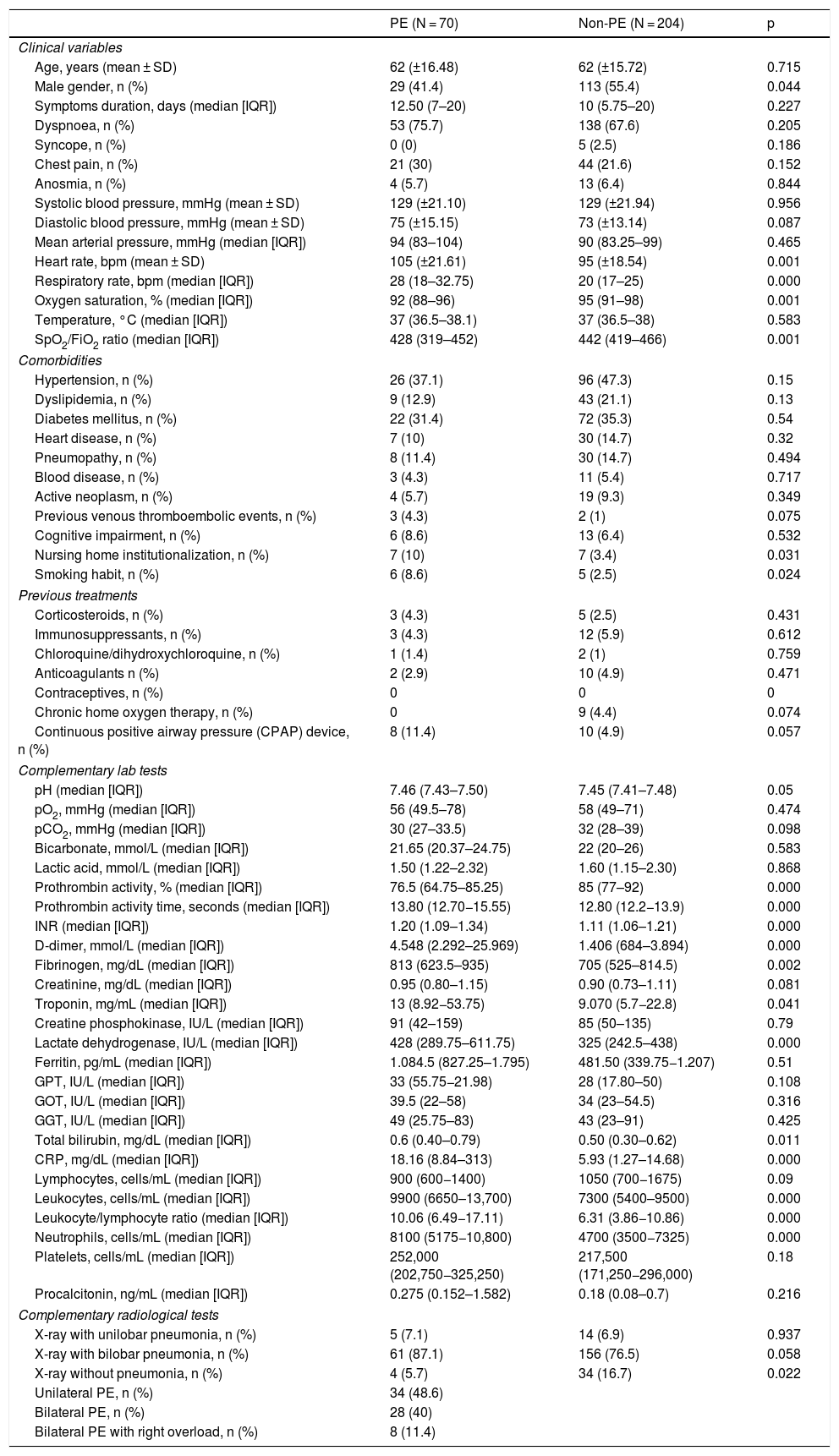
For the analysis of predictors of the presence of PE, variables for which statistically significant differences were observed between the group of patients with and without PE were chosen (Table 1). Patients with PE had the following clinical characteristics: mean age 62 years, with a prevalence of women (41 patients [58.9%]). The median duration of symptoms until assessment in the emergency department was 12.50 days (IQR: 7–20 days), and dyspnoea was reported as the main symptom during history-taking (53 patients [75.7%]). Other less common symptoms were chest pain (21 [30.0%]) and anosmia (4 [5.7%]).
Baseline characteristics of the sample analysed with and without PE.
| PE (N = 70) | Non-PE (N = 204) | p | |
|---|---|---|---|
| Clinical variables | |||
| Age, years (mean ± SD) | 62 (±16.48) | 62 (±15.72) | 0.715 |
| Male gender, n (%) | 29 (41.4) | 113 (55.4) | 0.044 |
| Symptoms duration, days (median [IQR]) | 12.50 (7–20) | 10 (5.75–20) | 0.227 |
| Dyspnoea, n (%) | 53 (75.7) | 138 (67.6) | 0.205 |
| Syncope, n (%) | 0 (0) | 5 (2.5) | 0.186 |
| Chest pain, n (%) | 21 (30) | 44 (21.6) | 0.152 |
| Anosmia, n (%) | 4 (5.7) | 13 (6.4) | 0.844 |
| Systolic blood pressure, mmHg (mean ± SD) | 129 (±21.10) | 129 (±21.94) | 0.956 |
| Diastolic blood pressure, mmHg (mean ± SD) | 75 (±15.15) | 73 (±13.14) | 0.087 |
| Mean arterial pressure, mmHg (median [IQR]) | 94 (83–104) | 90 (83.25–99) | 0.465 |
| Heart rate, bpm (mean ± SD) | 105 (±21.61) | 95 (±18.54) | 0.001 |
| Respiratory rate, bpm (median [IQR]) | 28 (18–32.75) | 20 (17–25) | 0.000 |
| Oxygen saturation, % (median [IQR]) | 92 (88–96) | 95 (91–98) | 0.001 |
| Temperature, °C (median [IQR]) | 37 (36.5–38.1) | 37 (36.5–38) | 0.583 |
| SpO2/FiO2 ratio (median [IQR]) | 428 (319–452) | 442 (419–466) | 0.001 |
| Comorbidities | |||
| Hypertension, n (%) | 26 (37.1) | 96 (47.3) | 0.15 |
| Dyslipidemia, n (%) | 9 (12.9) | 43 (21.1) | 0.13 |
| Diabetes mellitus, n (%) | 22 (31.4) | 72 (35.3) | 0.54 |
| Heart disease, n (%) | 7 (10) | 30 (14.7) | 0.32 |
| Pneumopathy, n (%) | 8 (11.4) | 30 (14.7) | 0.494 |
| Blood disease, n (%) | 3 (4.3) | 11 (5.4) | 0.717 |
| Active neoplasm, n (%) | 4 (5.7) | 19 (9.3) | 0.349 |
| Previous venous thromboembolic events, n (%) | 3 (4.3) | 2 (1) | 0.075 |
| Cognitive impairment, n (%) | 6 (8.6) | 13 (6.4) | 0.532 |
| Nursing home institutionalization, n (%) | 7 (10) | 7 (3.4) | 0.031 |
| Smoking habit, n (%) | 6 (8.6) | 5 (2.5) | 0.024 |
| Previous treatments | |||
| Corticosteroids, n (%) | 3 (4.3) | 5 (2.5) | 0.431 |
| Immunosuppressants, n (%) | 3 (4.3) | 12 (5.9) | 0.612 |
| Chloroquine/dihydroxychloroquine, n (%) | 1 (1.4) | 2 (1) | 0.759 |
| Anticoagulants n (%) | 2 (2.9) | 10 (4.9) | 0.471 |
| Contraceptives, n (%) | 0 | 0 | 0 |
| Chronic home oxygen therapy, n (%) | 0 | 9 (4.4) | 0.074 |
| Continuous positive airway pressure (CPAP) device, n (%) | 8 (11.4) | 10 (4.9) | 0.057 |
| Complementary lab tests | |||
| pH (median [IQR]) | 7.46 (7.43–7.50) | 7.45 (7.41–7.48) | 0.05 |
| pO2, mmHg (median [IQR]) | 56 (49.5–78) | 58 (49–71) | 0.474 |
| pCO2, mmHg (median [IQR]) | 30 (27–33.5) | 32 (28–39) | 0.098 |
| Bicarbonate, mmol/L (median [IQR]) | 21.65 (20.37–24.75) | 22 (20–26) | 0.583 |
| Lactic acid, mmol/L (median [IQR]) | 1.50 (1.22–2.32) | 1.60 (1.15–2.30) | 0.868 |
| Prothrombin activity, % (median [IQR]) | 76.5 (64.75–85.25) | 85 (77–92) | 0.000 |
| Prothrombin activity time, seconds (median [IQR]) | 13.80 (12.70−15.55) | 12.80 (12.2−13.9) | 0.000 |
| INR (median [IQR]) | 1.20 (1.09–1.34) | 1.11 (1.06–1.21) | 0.000 |
| D-dimer, mmol/L (median [IQR]) | 4.548 (2.292–25.969) | 1.406 (684–3.894) | 0.000 |
| Fibrinogen, mg/dL (median [IQR]) | 813 (623.5–935) | 705 (525–814.5) | 0.002 |
| Creatinine, mg/dL (median [IQR]) | 0.95 (0.80–1.15) | 0.90 (0.73–1.11) | 0.081 |
| Troponin, mg/mL (median [IQR]) | 13 (8.92−53.75) | 9.070 (5.7−22.8) | 0.041 |
| Creatine phosphokinase, IU/L (median [IQR]) | 91 (42–159) | 85 (50–135) | 0.79 |
| Lactate dehydrogenase, IU/L (median [IQR]) | 428 (289.75–611.75) | 325 (242.5–438) | 0.000 |
| Ferritin, pg/mL (median [IQR]) | 1.084.5 (827.25–1.795) | 481.50 (339.75−1.207) | 0.51 |
| GPT, IU/L (median [IQR]) | 33 (55.75−21.98) | 28 (17.80–50) | 0.108 |
| GOT, IU/L (median [IQR]) | 39.5 (22–58) | 34 (23–54.5) | 0.316 |
| GGT, IU/L (median [IQR]) | 49 (25.75–83) | 43 (23–91) | 0.425 |
| Total bilirubin, mg/dL (median [IQR]) | 0.6 (0.40–0.79) | 0.50 (0.30–0.62) | 0.011 |
| CRP, mg/dL (median [IQR]) | 18.16 (8.84–313) | 5.93 (1.27–14.68) | 0.000 |
| Lymphocytes, cells/mL (median [IQR]) | 900 (600−1400) | 1050 (700−1675) | 0.09 |
| Leukocytes, cells/mL (median [IQR]) | 9900 (6650–13,700) | 7300 (5400–9500) | 0.000 |
| Leukocyte/lymphocyte ratio (median [IQR]) | 10.06 (6.49−17.11) | 6.31 (3.86−10.86) | 0.000 |
| Neutrophils, cells/mL (median [IQR]) | 8100 (5175−10,800) | 4700 (3500−7325) | 0.000 |
| Platelets, cells/mL (median [IQR]) | 252,000 (202,750−325,250) | 217,500 (171,250−296,000) | 0.18 |
| Procalcitonin, ng/mL (median [IQR]) | 0.275 (0.152–1.582) | 0.18 (0.08–0.7) | 0.216 |
| Complementary radiological tests | |||
| X-ray with unilobar pneumonia, n (%) | 5 (7.1) | 14 (6.9) | 0.937 |
| X-ray with bilobar pneumonia, n (%) | 61 (87.1) | 156 (76.5) | 0.058 |
| X-ray without pneumonia, n (%) | 4 (5.7) | 34 (16.7) | 0.022 |
| Unilateral PE, n (%) | 34 (48.6) | ||
| Bilateral PE, n (%) | 28 (40) | ||
| Bilateral PE with right overload, n (%) | 8 (11.4) | ||
SD: standard deviation; PE: pulmonary embolism; IQR: interquartile range.
Regarding previous comorbidities, statistically significant differences were found between patients with and without PE in nursing home institutionalization (7 [10.0%] versus 7 [3.4%], respectively; p = 0.031) and smoking habit (6 [8.6%] versus 5 [2.5%]; p = 0.024). There were no differences in relation to the use of medications that could have influenced the development of PE (oral steroids, immunosuppressants, chloroquine or hydroxychloroquine, anticoagulation with prophylactic or therapeutic indication, and contraceptives in any form). None of the patients had a previous indication for home oxygen or use of overnight CPAP.
In relation to laboratory parameters, LDH levels (428 IU/L [IQR: 289.75–611.75] versus 325 IU/L [IQR: 242.5–438]; p < 0.001) and CRP (18.16 mg/dL [IQR: 8.84–313] versus 5.93 mg/dL [IQR: 1.27–14.68]; p < 0.001) were higher in patients with PE, as were the leukocyte and neutrophil counts and the leukocyte/lymphocyte ratio (Table 1).
Differences were found in all the parameters related to coagulation, highlighting the value of the D-dimer in terms of the relevance given to this parameter associated with COVID-19, both as a variable associated with the inflammatory phase and as a predictor of thromboembolic event (4548 ng/mL [IQR: 2292–25,969] versus 1406 ng/mL [IQR: 684–3894] in patients with and without PE; p < 0.001).
Six patients died in the PE group (8.6%) versus 22 patients (10.8%) in the non-PE group, with no significant differences observed.
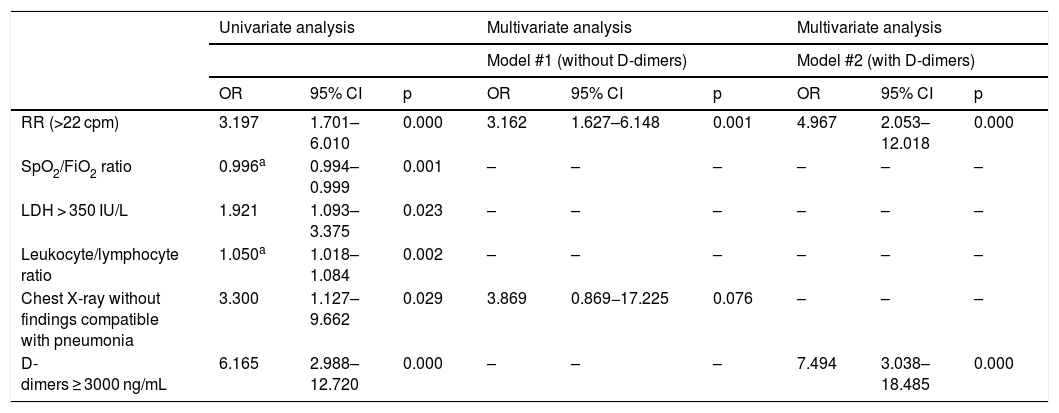
In the first multivariate analysis (not including D-dimer levels), respiratory rate > 22 cpm on patient arrival at the emergency department (OR: 3.162; 95% CI: 1.627–6.148; p = 0.001) and the absence of findings compatible with pneumonia on plain chest X-ray (OR: 3.869; 95% CI: 0.869–17.225; p = 0.076) acted as predictors of PE. When entering the presence of D-dimer levels D > 3.000 ng/mL (OR: 7.494; 95% CI: 3.038−18.485; p < 0.001) in the model, only tachypnoea > 22 rpm maintained its statistically significant association with the presence of PE (OR: 4.967; 95% CI: 2.053–12.018; p < 0.001) (Table 2). The discriminative capacity of both models, estimated using the corresponding AUC-ROC, was 0.679 (95% CI: 0.602−0.756) and 0.730 (95% CI: 0.654–0.806), respectively.
Univariate and multivariate predictive models of PE in patients with COVID-19.
| Univariate analysis | Multivariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model #1 (without D-dimers) | Model #2 (with D-dimers) | ||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| RR (>22 cpm) | 3.197 | 1.701–6.010 | 0.000 | 3.162 | 1.627–6.148 | 0.001 | 4.967 | 2.053–12.018 | 0.000 |
| SpO2/FiO2 ratio | 0.996a | 0.994–0.999 | 0.001 | – | – | – | – | – | – |
| LDH > 350 IU/L | 1.921 | 1.093–3.375 | 0.023 | – | – | – | – | – | – |
| Leukocyte/lymphocyte ratio | 1.050a | 1.018–1.084 | 0.002 | – | – | – | – | – | – |
| Chest X-ray without findings compatible with pneumonia | 3.300 | 1.127–9.662 | 0.029 | 3.869 | 0.869−17.225 | 0.076 | – | – | – |
| D-dimers ≥ 3000 ng/mL | 6.165 | 2.988–12.720 | 0.000 | – | – | – | 7.494 | 3.038–18.485 | 0.000 |
PE: pulmonary embolism; RR: respiratory rate; CI: confidence interval; LDH: lactate dehydrogenase; OR: odds ratio.
Patients with COVID-19 develop life-threatening thromboembolic events associated with the severity of the individual. We found a cumulative incidence of PE of 25.54% in our study, in line with other studies such as that of Klok et al. or the multicentre study by Helms et al. (31% and 11.7%, respectively), conducted in severely or critically ill patients.6,7 Middledorp et al. reported a cumulative incidence of PE at 7, 14 and 21 days after admission to the intensive care unit of 26%, 47% and 59%, respectively, while in general hospitalisation the figures were 5.8%, 9.2% and 9.2%,5,8 while in studies of mild to moderate patients it is less than 7%. Even so, still much higher than the data reported for the general population, which range from 1.3% to 2.5%.9
All the patients in the study were on anticoagulant therapy, at least with a prophylactic indication with LMWH since their admission, raising the reasonable doubt of presenting PE on admission to the emergency department or the possibility of having developed these thromboembolic events despite prophylaxis, as demonstrated by numerous studies, especially those conducted in critically ill patients.7 In Spain, the recommendations for thromboprophylaxis and antithrombotic treatment in patients with COVID-19 published by the Spanish Society of Thrombosis and Haemostasis (SETH) are maintained.
To date, the risk factors associated with the development of PE in these patients have not been clearly identified.10,11 Scientific evidence of its relationship with classic prothrombotic factors has not been demonstrated; probably because, from a pathophysiological point of view, a high percentage of events are secondary to the development of thrombosis in situ rather than distal thrombosis.12,13 In the multivariate analysis (Table 2) we only found, as factors independently associated with PE, the presence of tachypnoea and the absence of infiltrates in the chest X-ray, as well as elevated D-dimer levels, which per se are already high in patients with a worse prognosis. The use of clinical prediction scores for thromboembolic events does not seem to be particularly useful either.
The rest of the clinical, laboratory and radiological parameters analysed, although they show statistically significant differences with respect to the patients in whom PE was not found on PACT, are present in the most advanced and severe stages of COVID-19. Thus, we currently do not have sufficiently effective tools to help predict whether a patient with SARS-CoV-2 infection has PE on arrival at the emergency department.
Some limitations in the present study should be noted. A second model has been made including the D-dimer value in view of the number of studies that support its relationship with PE, although there were up to 30% of missing values in both groups during data collection. Although blood gas values on admission have been analysed, given the conditions experienced during this stage of the pandemic, numerous studies have been lost, in addition to not being able to ensure the conditions under which the sample was taken, as most patients were on oxygen, at least with nasal prongs at the time of care in the emergency department.
We conclude that, despite having identified certain risk factors that are associated with the presence of PE (such as tachypnoea > 22 cpm, absence of infiltrate on chest X-ray or elevated D-dimer levels), screening for this complication in patients with COVID-19 in the emergency department remains a challenge.
FundingMario Fernández-Ruiz is the grantee of a “Miguel Servet” contract (CP18/00073) issued by the Carlos III Health Institute, Ministry of Science and Innovation.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Gil Mosquera M, Fernández-Ruiz M, Sanz Rodríguez E, Mata Martínez A, Ibáñez Sanz L, Muñoz Martín D, et al. Predicción del desarrollo de tromboembolia pulmonar en pacientes con infección por SARS-CoV-2. Med Clin (Barc). 2022;158:206–210.