Edited by: Dr. Juan González Moreno - Hospital Universitario, Spain. Dra. Inés Losada López - Hospital Universitario, Spain
More infoTafamidis is the only approved transthyretin stabiliser approved for the treatment of variant transthyretin amyloidosis (A-ATTRv) related polyneuropathy (PNP). The aim of this study is to analyse the effectiveness of tafamidis in a real-world setting in Spain.
MethodsThis is a national multicenter study in which patients with V30M A-ATTR related PN treated with tafamidis for at least 1 year were included. Clinical, demographic, analytical and neurophysiological variables were analysed.
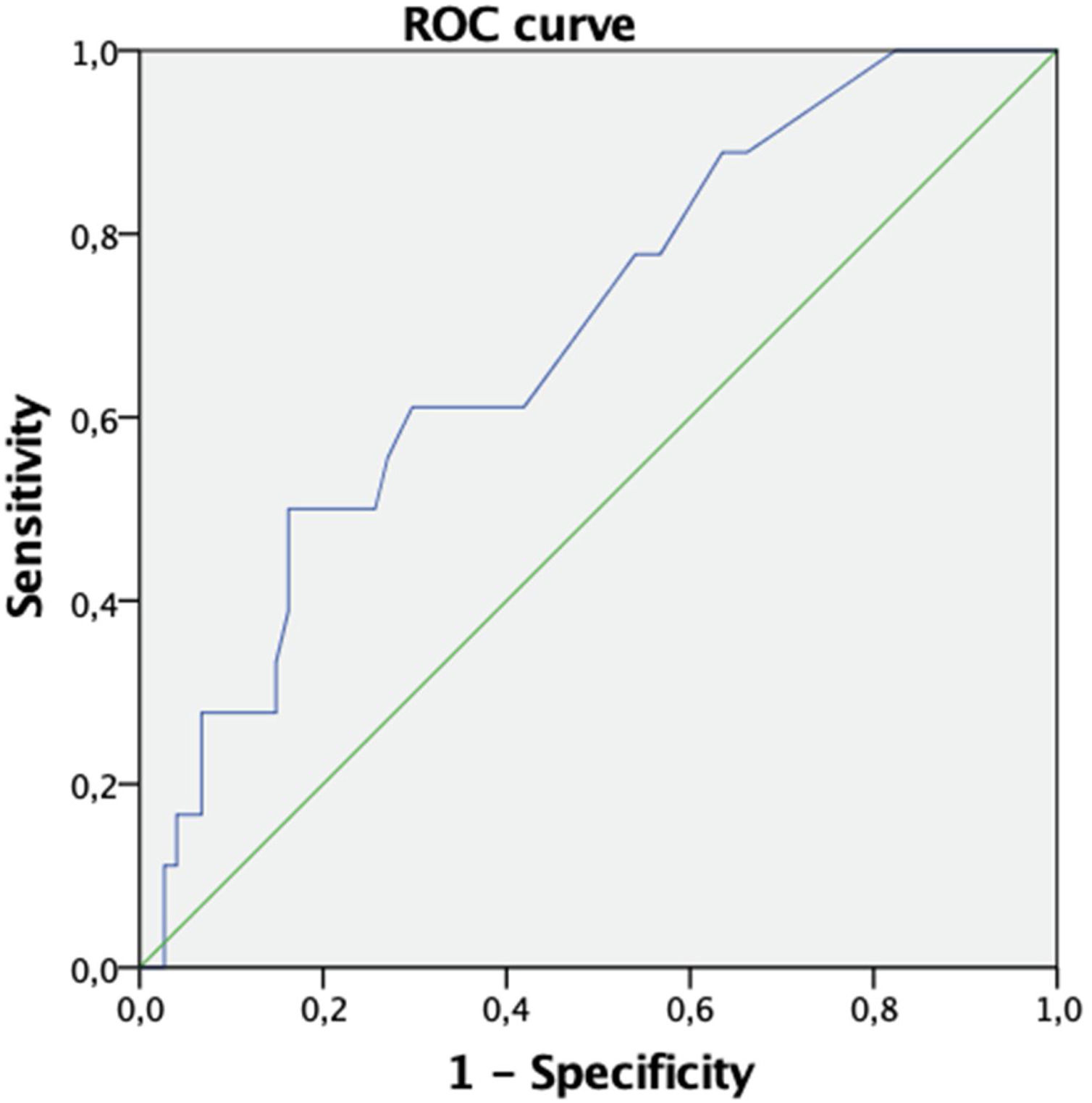
Results100 patients were recruited. Overall, 47 patients (47%) were classified as complete responders, 32 (32%) as partial responders and 21 (21%) as non-responders. The median duration of treatment with tafamidis was 35 months. Better treatment response was shown in patients with in polyneuropathy disability score (PND) I, lower neuropathy impairment score (NIS), compound muscle action potential (CMAP) and Norfolk QoL questionnaire. Higher albumin levels and lower NTproBNP levels were also associated with better treatment response. A basal NIS≥15 predicts that the patient could be a non-responder with a 60% probability.
ConclusionsOur results reinforce the tafamidis efficacy to treat A-ATTRv-PNP if started early in the disease course. Patients with the V30M variant, NIS<15 and PND I are the most appropriate subjects for this treatment.
Tafamidis es el único estabilizador de la transtirretina aprobado para el tratamiento de la polineuropatía (PN) relacionada con la amiloidosis por transtirretina variante (A-ATTRv). El objetivo de este estudio es analizar la eficacia de tafamidis en vida real en España.
MétodosSe trata de un estudio multicéntrico nacional en el que se incluyó a pacientes con PN relacionada con V30M A-ATTR tratados con tafamidis durante al menos un año. Se analizaron variables clínicas, demográficas, analíticas y neurofisiológicas.
ResultadosSe reclutó a 100 pacientes. En conjunto, 47 pacientes (47%) fueron clasificados como respondedores completos, 32 (32%) como respondedores parciales y 21 (21%) como no respondedores. La duración media del tratamiento con tafamidis fue de 35 meses. Se observó una mejor respuesta al tratamiento en los pacientes con polyneuropathy disability score (PND) 1 y con neuropathy impairment score (NIS), compound muscle action potential (CMAP) y cuestionario Norfolk QoL más bajos. Los niveles más altos de albúmina y los niveles más bajos de NTproBNP también se asociaron a una mejor respuesta al tratamiento. Un NIS basal ≥ 15 predice que el paciente podría ser no respondedor con una probabilidad del 60%.
ConclusionesNuestros resultados refuerzan la eficacia de tafamidis para tratar la A-ATTRv-PN si se inicia precozmente en el curso de la enfermedad. Los pacientes con la variante V30M, NIS<15 y PND I son los sujetos más apropiados para este tratamiento.