To describe macular vessel density and perfusion in COVID-19 patients using coherence tomography angiography (OCTA) and to investigate whether there is a correlation between retinal vascular abnormalities and clinical and laboratory parameters.
MethodsCross-sectional analysis conducted at the Hospital Clinico San Carlos in Madrid, Spain. Patients with laboratory-confirmed COVID-19 that were attended in the Emergency Department (ED) from March 23 to March 29, 2020 were included. Fundus examination and OCTA were performed 4 weeks after being attended in ED. Macular OCTA parameters were analyzed and correlated with clinical (severity and hypoxemia- oxygen saturation<92%) and laboratory parameters during hospital stay (D-Dimer-DD, lactate dehydrogenase-LDH and C-reactive protein-CRP).
Results80 patients were included, mean age 55(SD9) years old; 46.3% male. We reported macular vessel density and perfusion measurements in COVID-19 patients. Those patients with D-Dimer≥500ng/ml during SARS-CoV-2 infection had a decrease of central vessel density (mean difference 2.2; 95%CI 0.4–3.9) and perfusion density (mean difference 4.9; 95%CI 0.9–8.9) after the acute phase of COVID-19. These variations of vessel density and perfusion density were not documented in patients with LDH≥500U/L, CRP≥10mg/L and hypoxemia.
ConclusionsCOVID-19 patients showed short-term retinal vasculature abnormalities which may be related to a prothrombotic state associated with SARS-CoV-2 infection. Since the retinal microvasculature shares many morphological and physiological properties with the vasculature of other vital organs, further research is needed to establish whether patients with increased D-Dimer levels require more careful assessment and follow-up after COVID-19.
Evaluar la densidad vascular (DV) y la perfusión vascular (PV) retiniana en pacientes con COVID-19 mediante una angiografía por tomografía de coherencia óptica (OCTA), e investigar si existe una correlación entre las anomalías vasculares de la retina y los parámetros clínicos y de laboratorio.
MétodosAnálisis transversal realizado en el Hospital Clínico San Carlos, Madrid. Se incluyeron pacientes con diagnóstico confirmado de COVID-19 atendidos en el Servicio de Urgencias (SU) del 23 al 29 de marzo del 2020. Se realizó una exploración oftalmológica y OCTA cuatro semanas después de acudir al SU. Se analizaron los parámetros maculares de OCTA y se correlacionaron con parámetros clínicos (gravedad e hipoxemia-saturación de oxígeno < 92%) y de laboratorio durante la estancia hospitalaria (dímero D [DD], lactato deshidrogenasa [LDH] y proteína C reactiva [CRP].
ResultadosSe incluyeron 80 pacientes, edad media 55 (DE nueve) años; 46,3% hombres. Las personas con DD > 500 ng/mL durante la infección por SARS-CoV-2 tuvieron una disminución de la DV central (diferencia de medias 2,2; IC 95% 0,4 a 3,9) y PV central (diferencia de medias 4,9; IC 95% 0,9 a 8,9) después de la fase aguda de COVID-19. Estas variaciones no se documentaron en los pacientes con LDH > = 500 U/L, CRP > = 10 mg/L y con hipoxemia.
ConclusionesLos pacientes con COVID-19 mostraron anomalías de la vasculatura retiniana a corto plazo que pueden estar relacionadas con un estado protrombótico asociado con la infección por SARS-CoV-2. Dado que la microvasculatura de la retina comparte muchas propiedades morfológicas y fisiológicas con la vasculatura de otros órganos vitales, es necesario seguir investigando para determinar si los pacientes con niveles elevados de DD requieren una evaluación y un seguimiento más cuidadoso.
An outbreak of a novel coronavirus named severe acute respiratory syndrome coronavirus – 2 (SARS-CoV-2) was reported in Wuhan, Hubei Province (China) in December 2019. This virus causes the coronavirus disease 2019 (COVID-19), that is making an extraordinary impact worldwide.1
COVID-19 has shown several clinical manifestations at respiratory, gastrointestinal and neurological levels, among others. SARS-CoV-2 infection has also been related to inflammatory and coagulation abnormalities.2,3 The pathophysiology of these complications is not fully understood, as well, as is the extent of these after the acute phase of the infection.
To the best of our knowledge, there are no previous reports in the medical literature that depict and quantify the short-term small vessel alterations in COVID-19 patients. Given the current situation of SARS-CoV-2 pandemic, identifying retinal vascular changes that are associated with clinical and laboratory parameters that carry an increased risk of systemic complications secondary to infection has relevant implications in the follow-up of these patients and identification of possible short- and long-term sequels of COVID-19.
To this end, the retina is a relatively accessible organ to evaluate and quantify these microvascular changes via direct examination of blood vessels. Optical coherence tomography angiography (OCTA) is a novel, rapid, and non-invasive technique that generates a three-dimensional angiogram of the retina, without the need for a contrast agent.4 Several studies have evaluated by OCTA the clinical and subclinical retinal microvascular changes associated with systemic diseases, such as diabetes mellitus, systemic arterial hypertension and infectious diseases.5–8
The main purpose of this study is to describe retinal vessel density and perfusion in patients with COVID-19 using OCTA and to investigate whether there is a correlation between retinal vascular abnormalities and clinical and laboratory parameters.
MethodsStudy designThis is a case series study with cross-sectional analysis that was carried out in Hospital Clinico San Carlos (HCSC), a tertiary, multispecialty metropolitan teaching hospital located in Madrid, Spain. The study was approved by the Clinical Research Ethics Committee and was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all patients.
Patient selectionWe selected COVID-19 patients attended in Emergency Department (ED) from March 23 to March 29, 2020. The inclusion criteria were: age 18–65 years old, positive reverse transcriptase–polymerase chain reaction (RT-PCR) test from nasopharyngeal swab for SARS-CoV-2, sample blood for a laboratory test required during the clinical evaluation in acute phase of infection and written informed consent for the participation in the study. Those patients with previous history of stroke or thromboembolic events, concomitant ophthalmic diseases, antithrombotic therapy, the requirement for longer quarantine (patients still admitted to COVID-19 unit or intensive care unit, persistence of respiratory symptoms, persistently positive PCR test, close contact of laboratory-confirmed or probable COVID-19 patients after discharge), unable to attend the hospital, or did not consent to participate in the study were excluded. The concomitant ophthalmic diseases excluded were high myopia (>6 diopters), macular disorders, optic nerve head disease, retinal vascular disorders, previous ocular surgery other than uncomplicated cataract extraction and intraocular lens implantation performed more than 6 months before enrollment and media opacity that affected examination.
Ophthalmologic examinationEvery patient underwent an ophthalmologic examination that included slit-lamp exam, fundus examination and optical coherence tomography angiography (OCTA) at least 4 weeks after being attended in the ED in order to fully comply mandatory isolation. The isolation criteria according to the World Health Organization (WHO) is 10 days after symptom onset, plus at least 3 additional days without symptoms.9 All patients underwent the examination at least 28 days after COVID-19 diagnosis, thus ensuring sufficient time. Moreover, those patients who required a longer quarantine period according to different criteria established by the public health department were excluded. All procedures followed infection control and prevention measures according to the hospital's protocol.
OCTA images were obtained using spectral-domain OCTA Zeiss Cirrus 5000 with AngioPlex (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Macular angiography imaging with 6×6mm scans was performed in both eyes for each subject. Inclusion criteria for the acceptable signal strength (SS) was 6 or higher. The optical microangiography-complex (OMAGc) algorithm analyzed the changes in complex signals. Scans were analyzed using the Cirrus OCTA software (AngioPlex™, version 11.0).
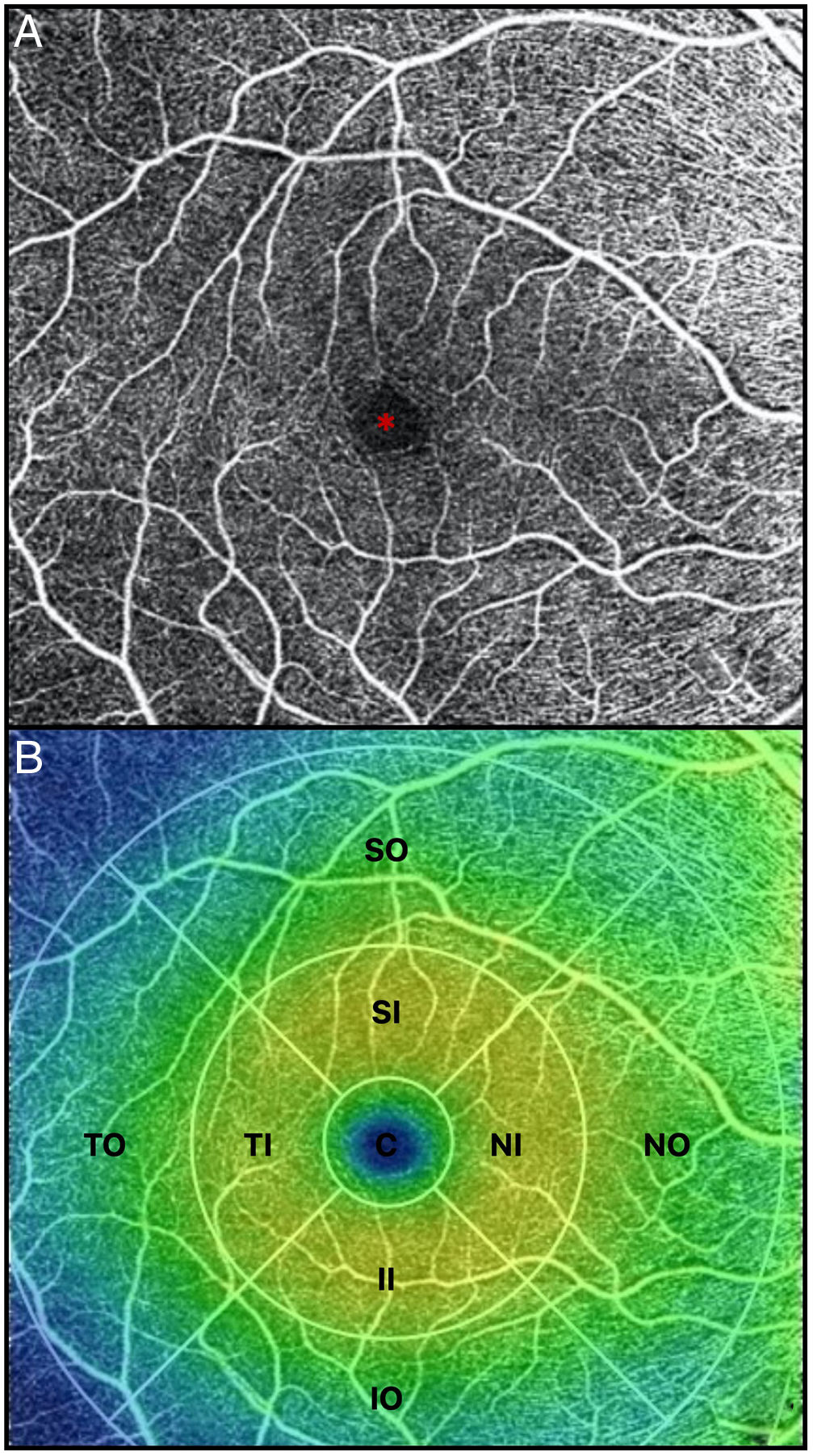
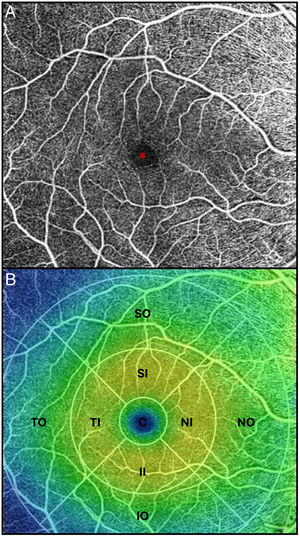
All OCTA scans were performed by the same trained examiner, and were reviewed individually by two ophthalmologists (NG and BB) for quality evaluation, excluding substandard scans. Quantification of the vessel density (VD), perfusion density (PD), and foveal avascular zone (FAZ) area, perimeter, and morphology in the superficial capillary plexus (SCP) were noted. The macular region was segmented according to the nine Early Treatment of Diabetic Retinopathy Study (ETDRS) sectors. The fovea was represented by the central circle of 1mm in diameter. The inner ring had an inner diameter of 1mm and an outer diameter of 3mm, and the outer ring had an inner diameter of 3mm and outer diameter of 6mm. Both inner and outer rings were further subdivided into superior, nasal, inferior and temporal region (Figs. 1A and 2B). The right eye was included, unless it did not meet the inclusion and exclusion criteria, in which case the left eye was included.
(A) OCTA of the macular region, showing an angiogram of the retinal circulation in the superficial capillary plexus. The image depicts the retinal arteries and veins within the macular region in a binarized OCTA image, which represents the blood vessels in white color. The central area devoid of vessels represents the foveal avascular zone (*). (B) Color image with ETDRS sectors, depicting the 9 sectors of the ETDRS grid in the macular region. The fovea is represented by the central circle (C). Both inner (I) and outer (O) rings are further subdivided into superior (S), nasal (N), inferior (I) and temporal (T) region.
Socio-demographic data (age, gender and birthplace), medical history (arterial hypertension, diabetes mellitus, dyslipidemia, obesity, chronic respiratory and heart disease, smoking), clinical findings at ED presentation (symptoms onset, baseline oxygen saturation, consolidation on chest X-ray, and clinical severity according to WHO ordinal scale10) and laboratory tests (lymphocytes count, D-Dimer – DD-, C-reactive protein – CRP- and lactate dehydrogenase – LDH-levels) during the hospital stay were collected. Regarding laboratory test, we considered the highest levels documented during the hospital stay.
Statistical analysisContinuous variables are presented as mean (standard deviation [SD]) or median (interquartile range [IQR]), as appropriate; categorical variables as numbers and percentages. A normality test was carried out for all variables based on their randomness, variance and Kolmogorov–Smirnov test. In the case of not having the possibility of parametric analysis, these were carried out with non-parametric tests to relate the variables under study. Macular OCTA parameters were analyzed according to hypoxemia at ED presentation and the highest values of DD, LDH and CRP. Laboratory data were dichotomized according to cut-off values established in reference COVID-19 studies. Thus, the cutoffs values were 500ng/ml for DD levels, 10mg/L for CRP levels and 500U/L for LDH.11 Hypoxemia was defined as oxygen saturation<92%.12 Comparisons among groups were made using t-Student for continuous variables. P value for linear trend was estimated. Subgroup analysis for clinical and laboratory variables was performed. Statistically significant differences were considered two-side p-value less than 0.05. The sample size was not calculated as this was an exploratory study. Data analysis was performed using SPSS software, version 24.00 (IBM, New Castle, NY, USA) and STATA version 15.1 (Stata Corp., College Station, TX, USA).
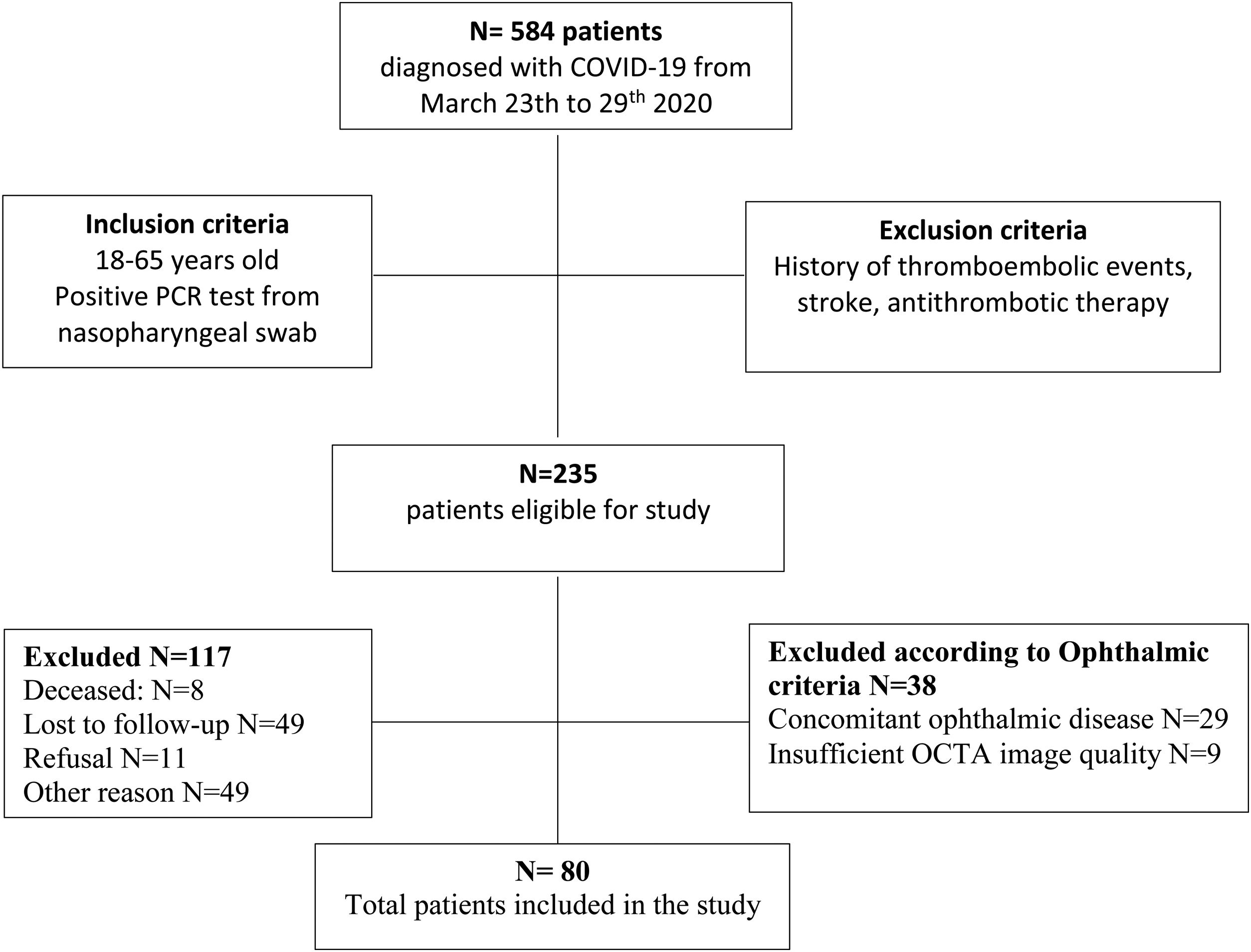
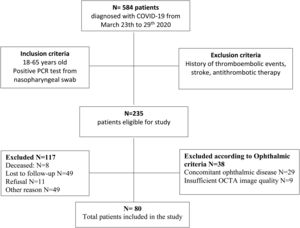
ResultsThe overall study population included 80 patients. Of the 584 patients diagnosed with COVID-19 in the Emergency Department from March 23rd to 29th 2020, 235 patients met the inclusion criteria. Of those, 8 patients died, 49 patients were lost to follow-up after emergency department discharge, 22 patients were still admitted to the COVID-19 unit, 15 patients were unable to return to the hospital due to their clinical situation, 12 patients still presented respiratory symptoms after discharge and 11 patients did not give consent. 29 patients had concomitant ocular disorders, which included: high myopia (5), age related macular degeneration (3), glaucoma (3), previous history of central serous choroidopathy (1), history of bilateral pars plana vitrectomy (1), history of retinal vascular disorders (4), moderate-severe media opacities (12). 9 patients were excluded because the OCTA image did not meet the quality criteria (signal strength, loss of fixation, segmentation error, and motion artifacts). Fig. 2 depicts the flowchart of patient selection.
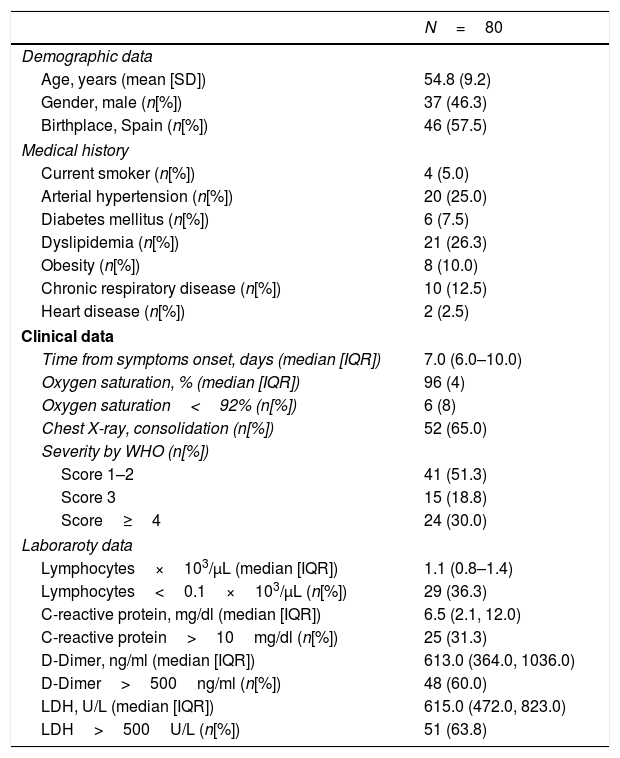
Of the 80 patients included, 37 (46.3%) were male, mean age was 54.8±9.2 years. Table 1 depicts the demographical and clinical characteristics of the patients and the laboratory results. Patients were evaluated in the emergency department 7 days (6–10) after the onset of COVID-19 symptoms. Ophthalmic examination was performed 30 days (28–32) after COVID-19 diagnosis. Slit-lamp examination of the anterior segment and the fundus revealed the above-described ophthalmic disorders in 29 patients, who were excluded from the study. Funduscopic examination of the 80 patients included was otherwise unremarkable. None of the patients reported decrease in vision or other remarkable ocular symptoms during the illness, nor until the time of the evaluation.
Clinical characteristics of COVID-19 patients included in the study.
| N=80 | |
|---|---|
| Demographic data | |
| Age, years (mean [SD]) | 54.8 (9.2) |
| Gender, male (n[%]) | 37 (46.3) |
| Birthplace, Spain (n[%]) | 46 (57.5) |
| Medical history | |
| Current smoker (n[%]) | 4 (5.0) |
| Arterial hypertension (n[%]) | 20 (25.0) |
| Diabetes mellitus (n[%]) | 6 (7.5) |
| Dyslipidemia (n[%]) | 21 (26.3) |
| Obesity (n[%]) | 8 (10.0) |
| Chronic respiratory disease (n[%]) | 10 (12.5) |
| Heart disease (n[%]) | 2 (2.5) |
| Clinical data | |
| Time from symptoms onset, days (median [IQR]) | 7.0 (6.0–10.0) |
| Oxygen saturation, % (median [IQR]) | 96 (4) |
| Oxygen saturation<92% (n[%]) | 6 (8) |
| Chest X-ray, consolidation (n[%]) | 52 (65.0) |
| Severity by WHO (n[%]) | |
| Score 1–2 | 41 (51.3) |
| Score 3 | 15 (18.8) |
| Score≥4 | 24 (30.0) |
| Laboraroty data | |
| Lymphocytes×103/μL (median [IQR]) | 1.1 (0.8–1.4) |
| Lymphocytes<0.1×103/μL (n[%]) | 29 (36.3) |
| C-reactive protein, mg/dl (median [IQR]) | 6.5 (2.1, 12.0) |
| C-reactive protein>10mg/dl (n[%]) | 25 (31.3) |
| D-Dimer, ng/ml (median [IQR]) | 613.0 (364.0, 1036.0) |
| D-Dimer>500ng/ml (n[%]) | 48 (60.0) |
| LDH, U/L (median [IQR]) | 615.0 (472.0, 823.0) |
| LDH>500U/L (n[%]) | 51 (63.8) |
*SD: standard deviation; IQR: interquartil range; WHO: World Health Organization; LDH: lactate dehydrogenase.
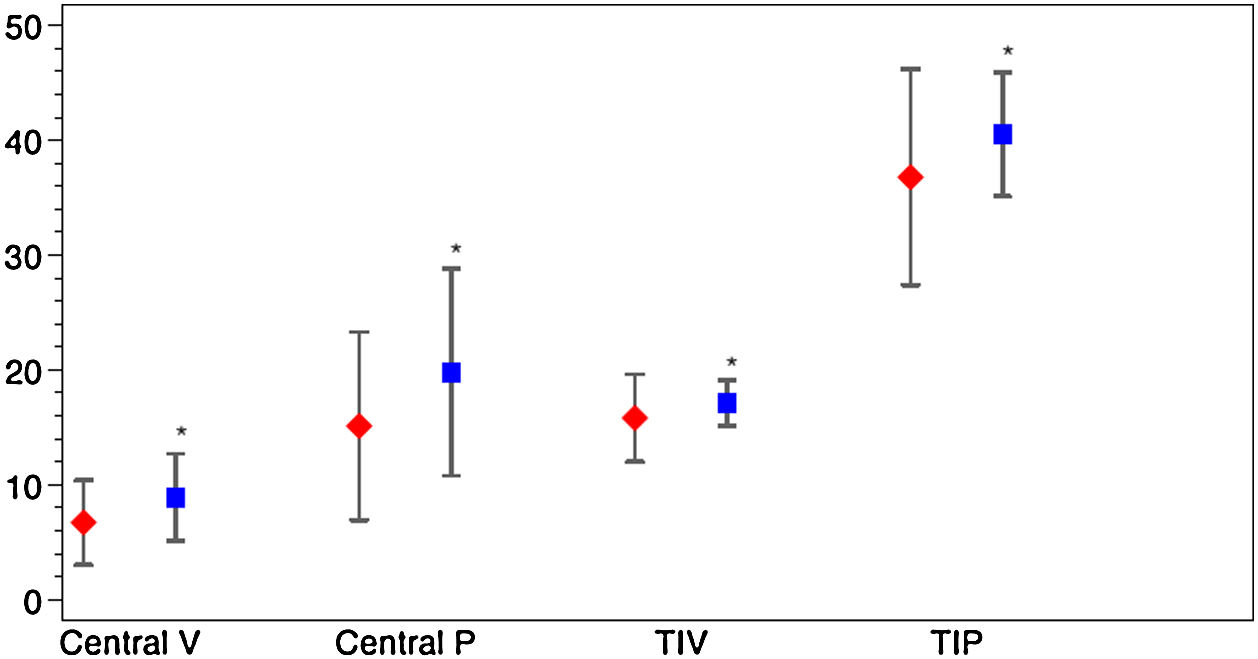
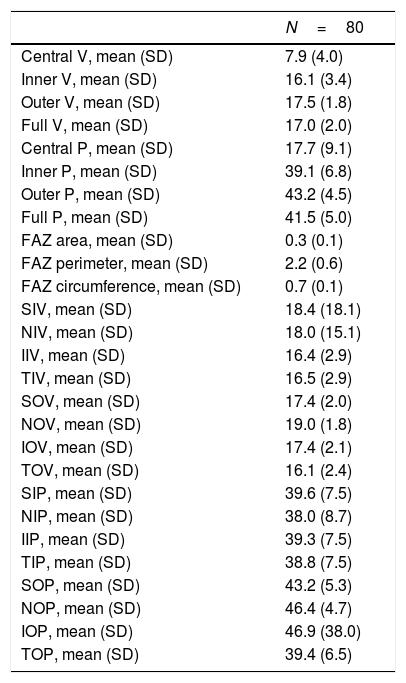
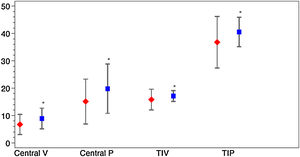
Vessel density and perfusion density measurements at the SCP of the macular region were analyzed. Table 2 depicts macular OCTA results in COVID-19 patients after the acute phase of the infection. A marked difference was observed for the variable D-dimer. Patients with levels of DD≥500ng/ml during SARS-CoV-2 infection had a decreased of central vessel density (8.8 (SD 4.0) vs 6.6 (SD 3.6), p=0.013; mean difference 2.2; 95%CI 0.4–3.9) and perfusion 19.6 (SD 9.3) vs. 14.7 (SD 8.0), p=0.018; mean difference 4.9; 95%CI 0.9–8.9) after the acute phase of COVID-19. They also showed a significant decrease of perfusion in the temporal inner sector of the macula (40.3 (SD 5.5) vs. 36.7 (SD 9.5), p=0.033; mean difference 3.6; 95%CI 0.3–6.9) and a tendency in the vessel density in that sector (17.0 (SD 2.0) vs. 15.7 (SD 3.8), p=0.053; mean difference 1.3; 95%CI 0.0–2.6). Fig. 3 represents OCTA parameters according to DD levels. Other OCTA parameters, including FAZ, showed no differences. Blood oxygen saturation levels did not reveal significant differences. The subgroups analysis according to the rest of laboratory parameters (CRP and LDH) did not show a significant difference in OCTA values. Furthermore, no differences were found in patients regarding clinical severity, presence of consolidation on chest x-ray nor comorbidities.
Macular optical coherence tomography angiography results in COVID-19 PATIENTS.
| N=80 | |
|---|---|
| Central V, mean (SD) | 7.9 (4.0) |
| Inner V, mean (SD) | 16.1 (3.4) |
| Outer V, mean (SD) | 17.5 (1.8) |
| Full V, mean (SD) | 17.0 (2.0) |
| Central P, mean (SD) | 17.7 (9.1) |
| Inner P, mean (SD) | 39.1 (6.8) |
| Outer P, mean (SD) | 43.2 (4.5) |
| Full P, mean (SD) | 41.5 (5.0) |
| FAZ area, mean (SD) | 0.3 (0.1) |
| FAZ perimeter, mean (SD) | 2.2 (0.6) |
| FAZ circumference, mean (SD) | 0.7 (0.1) |
| SIV, mean (SD) | 18.4 (18.1) |
| NIV, mean (SD) | 18.0 (15.1) |
| IIV, mean (SD) | 16.4 (2.9) |
| TIV, mean (SD) | 16.5 (2.9) |
| SOV, mean (SD) | 17.4 (2.0) |
| NOV, mean (SD) | 19.0 (1.8) |
| IOV, mean (SD) | 17.4 (2.1) |
| TOV, mean (SD) | 16.1 (2.4) |
| SIP, mean (SD) | 39.6 (7.5) |
| NIP, mean (SD) | 38.0 (8.7) |
| IIP, mean (SD) | 39.3 (7.5) |
| TIP, mean (SD) | 38.8 (7.5) |
| SOP, mean (SD) | 43.2 (5.3) |
| NOP, mean (SD) | 46.4 (4.7) |
| IOP, mean (SD) | 46.9 (38.0) |
| TOP, mean (SD) | 39.4 (6.5) |
V: vascular density; P: perfusion; FAZ: Foveal avascular zone; SIV: vessel density of superior inner sector; NIV vessel density of nasal inner sector; IIV vessel density of inferior inner sector; TIV: vessel density of inner temporal sector; SOV: vessel density of superior outer sector; NOV: vessel density of nasal outer sector; IOV: vessel density of inferior outer sector; TOV: vessel density of temporal outer sector; SIP: perfusion of superior inner sector; NIP: perfusion of nasal inner sector; IIP: perfusion of inferior inner sector; TIP: perfusion of temporal inner sector; SOP: perfusion of superior outer sector; NOP: perfusion of nasal outer sector; IOP: perfusion of inferior outer sector; TOP: temporal outer perfusion; SD: standard deviation.
Macular OCTA parameters according to D-dimer levels. The figure depicts the mean and standard deviation of the OCTA parameters that showed significant differences between COVID-19 patients with Dimer D levels≥500ng/ml (red) and those with levels<500ng/ml (blue). The OCTA parameters represented are central vascular density (Central V), central perfusion (Central P), vascular density in the temporal inner sector (TIV) and vascular perfusion in the temporal inner sector (TIP). *p value<0.05.
Although the main clinical manifestations of COVID-19 involve respiratory and gastrointestinal symptoms, there is also evidence of thromboembolic complications and blood coagulation disorders.3 D-Dimer elevation, a marker of thrombus formation, has been reported to be one of the most frequent laboratory findings noted in hospitalized COVID-19 patients, representing an independent predictor of hospital mortality.13,14 Our results revealed that patients with an increased DD, and therefore, a prothrombotic state during the acute phase of the infection, showed lower macular vessel density and perfusion in certain OCTA parameters after the infection has resolved.
Retinal vasculature may be affected by SARS-CoV-2 infection through different mechanisms. The fact that the retina is one of the highest energy-consuming tissues in the body, turns it into a particularly vulnerable tissue to ischemia.15 Retinal vascular disorders has been demonstrated to be associated with systemic coagulopathies and prothrombotic states.16,17 In addition, the retinal vasculature endothelium may be also susceptible to the direct damage by SARS-CoV-2.18 Since there is evidence of the presence of angiotensin-converting enzyme 2 (ACE2), a functional receptor of SARS-CoV-2, in arterial and venous endothelial cells, vascular damage at this level seems feasible.19 Therefore, the retinal vasculature might be altered by a conjunction of events that include thromboembolisms, a hypercoagulable state, hypoxia and endothelial cell dysfunction.
Since the eye offers a readily accessible window to evaluate subclinical and clinical retinal vasculature changes, it seems meaningful to evaluate and quantify through OCTA the retinal involvement related to COVID-19. OCTA has demonstrated to be very useful in the study of other systemic diseases that associate retinal vascular disorders, such as diabetes mellitus and arterial hypertension.20,21 Donati et al. found that the retinal vascular density showed pathological modifications between healthy subjects and hypertensive patients.6 These findings suggested that OCTA may identify pathological markers of an early vascular damage, providing a rationale for retinal vasculature evaluation by OCTA in patients with recently resolved COVID-19.
Our results show a decrease in macular vessel density in patients with higher D-dimer values. This suggests that SARS-CoV-2 may produce subclinical changes at the level of the retinal vasculature, probably related to the potential prothrombotic action and the hypercoagulable state induced by SARS-CoV-2 infection. Since the retinal microvasculature shares many morphological and physiological properties with the vasculature of other organs such as the brain, the kidney and the coronary arteries, we could hypothesize that these results could be reflected in other vital organs.22,23
COVID-19 patients have shown laboratory abnormalities in other parameters despite D-Dimer, such as a decrease in lymphocytes and platelet count, increased CRP and elevated fibrinogen and ferritin concentrations.24 However, we have not found a correlation between OCTA parameters and these abnormalities. The fact that inflammatory laboratory parameters such as CRP had not shown differences in retinal vessel density and perfusion, and one coagulation parameter, D-dimer, had, raises the hypothesis that the underlying physiopathology of these retinal findings may have a thrombotic origin. Moreover, our study found no relationship between the clinical severity of the disease and the retinal vascular changes. Therefore, the microvascular involvement related to COVID-19 might depend on the procoagulant state of the patient rather than on the severity of the illness. This would support the fact that subclinical thromboembolic events may occur in patients with increased D-dimer despite having a mild COVID-19.
The present study has several limitations. Critically ill patients are not fully represented in our sample, because some have died and others remained admitted in the hospital. Since severe COVID-19 is more prone to thromboembolic events and coagulopathies, evaluating these patients could yield more significant data on changes at the retinal vasculature level. Furthermore, patients over 65 years old were excluded from this study. Firstly, because these patients present a higher incidence of ophthalmic disease that could condition the results, and secondly, because they represent population at risk that should avoid going to hospitals unless they require emergency care during this critical situation. Thus, the differences found in a sample with younger patients may be less evident and therefore require a larger group to find differences. On the other hand, our study only evaluated OCTA parameters in the superficial capillary plexus due to the fact that the available device only provides quantitative data of the superficial plexus. Consequently, quantitative analyses of the intermediate and deep plexus could yield additional information in this regard. Finally, the assessment was performed 4 weeks after being attended in the ED, in order to follow mandatory isolation. It would have been interesting to have evaluated the patients in an earlier stage of the disease. However, the extraordinary pandemic situation, the limited resources and the restrictive access of infected patients to common areas did not allow it.
In conclusion, a growing body of evidence exhibits that retinal vascular changes may represent a novel biomarker, reflecting the sequels of an underlying microvascular disease. Our results show that OCTA can detect microvascular changes not otherwise noted on dilated clinical examination in COVID-19 patients. The fact that elevated D-Dimer values during SARS-CoV-2 infection are associated with a decrease of retinal vascular density and perfusion raises the possibility of further vascular involvement in other organs besides the eye. More robust studies are warranted to fully elucidate the significance of these findings.
Authors’ contributionAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole.
Ethics approvalThe study was approved by the Clinical Research Ethics Committee of HCSC and was conducted in accordance with the Helsinki Declaration.
Consent to participateWritten informed consent was obtained from all patients.
Consent for publicationAll authors have given their approval for this version to be published.
Availability of data and code availabilityData sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
FundingNo funding or sponsorship was received for this study or publication of this article.
Conflict of interestAll authors declare that they have no conflict of interest.
We wish to thank investigators of COVID-19_URG-HCSC Register: Juan González del Castillo, Adrián Valls Carbó, Enrique del Toro, Eduardo Cardassay, Gabriel Cozar López, María del Mar Suárez-Cadenas, Pablo Jerez Fernández, Beatriz Angós, Cristina Díaz del Arco, Esther Rodríguez Adrada, María Teresa Montalvo Moraleda, Carolina Espejo Paeres, Amanda López Picado, Carmen Martínez Valero, Juande D. Miranda, David Chaparro, Miguel Ángel García Briñón, José Luis Fernández Rueda, José Mª Leal Pozuelo, José Luis Fernández Rueda, Víctor Hernández Martín-Romo.