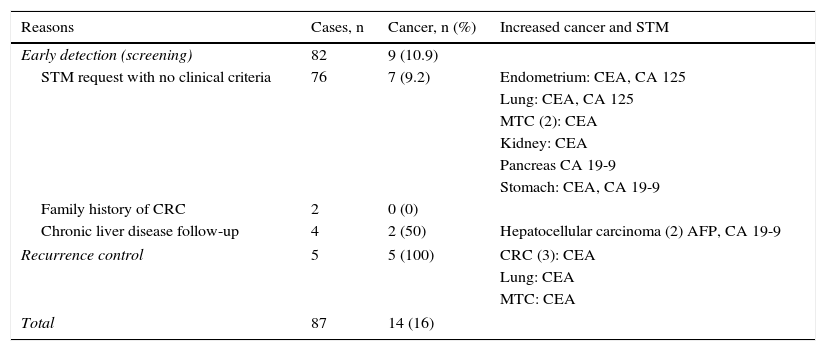
array:24 [ "pii" => "S2387020616001054" "issn" => "23870206" "doi" => "10.1016/j.medcle.2016.02.040" "estado" => "S300" "fechaPublicacion" => "2015-10-05" "aid" => "3281" "copyright" => "Elsevier España, S.L.U.. All rights reserved" "copyrightAnyo" => "2015" "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2015;145:319-20" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:2 [ "total" => 5 "HTML" => 5 ] "Traduccion" => array:1 [ "es" => array:19 [ "pii" => "S0025775315001876" "issn" => "00257753" "doi" => "10.1016/j.medcli.2015.03.008" "estado" => "S300" "fechaPublicacion" => "2015-10-05" "aid" => "3281" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2015;145:319-20" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:2 [ "total" => 66 "formatos" => array:2 [ "HTML" => 40 "PDF" => 26 ] ] "es" => array:10 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Carta científica</span>" "titulo" => "Marcadores tumorales séricos en pacientes asintomáticos" "tienePdf" => "es" "tieneTextoCompleto" => "es" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "319" "paginaFinal" => "320" ] ] "titulosAlternativos" => array:1 [ "en" => array:1 [ "titulo" => "Serum tumour markers in asymptomatic patients" ] ] "contieneTextoCompleto" => array:1 [ "es" => true ] "contienePdf" => array:1 [ "es" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Joaquim Torné Cachot, José Manuel Baucells Azcona, Jesús Blanch Falp, David Blancas Altabella" "autores" => array:4 [ 0 => array:2 [ "nombre" => "Joaquim" "apellidos" => "Torné Cachot" ] 1 => array:2 [ "nombre" => "José Manuel" "apellidos" => "Baucells Azcona" ] 2 => array:2 [ "nombre" => "Jesús" "apellidos" => "Blanch Falp" ] 3 => array:2 [ "nombre" => "David" "apellidos" => "Blancas Altabella" ] ] ] ] ] "idiomaDefecto" => "es" "Traduccion" => array:1 [ "en" => array:9 [ "pii" => "S2387020616001054" "doi" => "10.1016/j.medcle.2016.02.040" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "en" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020616001054?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775315001876?idApp=UINPBA00004N" "url" => "/00257753/0000014500000007/v1_201509120059/S0025775315001876/v1_201509120059/es/main.assets" ] ] "itemSiguiente" => array:19 [ "pii" => "S2387020616000991" "issn" => "23870206" "doi" => "10.1016/j.medcle.2016.02.034" "estado" => "S300" "fechaPublicacion" => "2015-10-05" "aid" => "3191" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2015;145:321-2" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:2 [ "total" => 5 "formatos" => array:2 [ "HTML" => 3 "PDF" => 2 ] ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Usefulness of PET/CT in IgG4-related disease" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "321" "paginaFinal" => "322" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Utilidad de la PET/TC en la enfermedad por IgG4" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 1750 "Ancho" => 2333 "Tamanyo" => 333154 ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">PET/CT study (maximum intensity projection or MIP) and imaging of pretreatment axial slices (A) showing right lung mass with increased glucose metabolism consistent with inflammatory pseudotumor FNA (arrow) and hypermetabolic, mediastinal hilar, supraclavicular, axillary and inguinal lymphadenopathy and remarkable diffuse hypermetabolism in the bilateral salivary gland and renal parenchyma (arrows). Study after treatment, at 5 months (B), showing the virtual disappearance of glucose metabolism in the lungs, lymph nodes, kidney and salivary glands.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Montserrat Cortés-Romera, Aida Sabaté-Llobera, Cristina Gámez-Cenzano, Joan Torras-Ambròs" "autores" => array:4 [ 0 => array:2 [ "nombre" => "Montserrat" "apellidos" => "Cortés-Romera" ] 1 => array:2 [ "nombre" => "Aida" "apellidos" => "Sabaté-Llobera" ] 2 => array:2 [ "nombre" => "Cristina" "apellidos" => "Gámez-Cenzano" ] 3 => array:2 [ "nombre" => "Joan" "apellidos" => "Torras-Ambròs" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S002577531400880X" "doi" => "10.1016/j.medcli.2014.11.019" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S002577531400880X?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020616000991?idApp=UINPBA00004N" "url" => "/23870206/0000014500000007/v2_201604010141/S2387020616000991/v2_201604010141/en/main.assets" ] "itemAnterior" => array:19 [ "pii" => "S2387020616000954" "issn" => "23870206" "doi" => "10.1016/j.medcle.2016.02.030" "estado" => "S300" "fechaPublicacion" => "2015-10-05" "aid" => "3177" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2015;145:318-9" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:2 [ "total" => 3 "HTML" => 3 ] "en" => array:10 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Scientific letter</span>" "titulo" => "Concurrent lymphoid and Philadelphia chromosome-negative myeloproliferative neoplasms" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "318" "paginaFinal" => "319" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Neoplasias mieloproliferativas crónicas con cromosoma Filadelfia negativo y neoplasias linfoides concurrentes" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Jose Miguel Torregrosa, Gloria Soler, Shirley Cancio, Francisca Ferrer-Marin" "autores" => array:4 [ 0 => array:2 [ "nombre" => "Jose Miguel" "apellidos" => "Torregrosa" ] 1 => array:2 [ "nombre" => "Gloria" "apellidos" => "Soler" ] 2 => array:2 [ "nombre" => "Shirley" "apellidos" => "Cancio" ] 3 => array:2 [ "nombre" => "Francisca" "apellidos" => "Ferrer-Marin" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775314008288" "doi" => "10.1016/j.medcli.2014.11.012" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775314008288?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020616000954?idApp=UINPBA00004N" "url" => "/23870206/0000014500000007/v2_201604010141/S2387020616000954/v2_201604010141/en/main.assets" ] "en" => array:14 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Scientific letter</span>" "titulo" => "Serum tumour markers in asymptomatic patients" "tieneTextoCompleto" => true "saludo" => "Dear Editor," "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "319" "paginaFinal" => "320" ] ] "autores" => array:1 [ 0 => array:4 [ "autoresLista" => "Joaquim Torné Cachot, José Manuel Baucells Azcona, Jesús Blanch Falp, David Blancas Altabella" "autores" => array:4 [ 0 => array:4 [ "nombre" => "Joaquim" "apellidos" => "Torné Cachot" "email" => array:1 [ 0 => "18112jtc@comb.cat" ] "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">*</span>" "identificador" => "cor0005" ] ] ] 1 => array:2 [ "nombre" => "José Manuel" "apellidos" => "Baucells Azcona" ] 2 => array:2 [ "nombre" => "Jesús" "apellidos" => "Blanch Falp" ] 3 => array:2 [ "nombre" => "David" "apellidos" => "Blancas Altabella" ] ] "afiliaciones" => array:1 [ 0 => array:2 [ "entidad" => "Servicio de Medicina Interna, Hospital Sant Camil, Consorci Sanitari del Garraf, Sant Pere de Ribes, Barcelona, Spain" "identificador" => "aff0005" ] ] "correspondencia" => array:1 [ 0 => array:3 [ "identificador" => "cor0005" "etiqueta" => "⁎" "correspondencia" => "Corresponding author." ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Marcadores tumorales séricos en pacientes asintomáticos" ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="par0005" class="elsevierStylePara elsevierViewall">In recent decades we are witnessing an increasing use of serum tumour markers (STMs). Its overuse in the early cancer detection is causing a new clinical situation that is usually referred to specialized care for assessment. We are talking about high levels of STMs in asymptomatic patients. This explains this study, whose main purpose is to know why STMs have been requested, assess suitability and analyze the characteristics of this group of patients.</p><p id="par0010" class="elsevierStylePara elsevierViewall">From January 2006 to March 2014 all asymptomatic patients referred to outpatient clinics have been prospectively recorded for the study of any high STM. The following variables were assessed: sex, age, origin, smoking, chronic diseases (CD), reasons for request, number of tests performed, STM type and levels and cancer diagnosis. The suitability of the request was assessed as recommended by the National Academy of Clinical Biochemistry (NACB).<a class="elsevierStyleCrossRef" href="#bib0040"><span class="elsevierStyleSup">1</span></a> Eighty-seven patients were recorded, including 36 males (41.3%) with a mean age of 59.5 years (38–86). Primary Care with 57 cases (65.5%) and outpatient visits with 27 (31%) were the main sources. An average 2.4 tests per patient (0–4) were performed. Primary STMs were: CEA in 52 cases (59.7%), 19-9 CA in 16 (24%) and 2 increased STMs in 8 (9.1%). Cancer was diagnosed in 14 patients (16%), 5 (100%) in the control of recurrence, 2 (50%) in high risk liver disease follow-up and 7 (9.2%) as early detection. 98% patients without cancer were smokers (41%) and/or had any CD (59%). In 26 patients (30%) increasing CEA was associated with smoking. The grounds for STM request and cancer diagnosis are detailed in <a class="elsevierStyleCrossRef" href="#tbl0005">Table 1</a>. STM request was appropriate in 9 cases (10.3%), 4 (4.8%) as early detection and 5 (100%) in the recurrence control. In patients without cancer and with any CD and/or smoking, CEA levels were in all cases <20<span class="elsevierStyleHsp" style=""></span>μg/ml, while the CA 19-9 was <100<span class="elsevierStyleHsp" style=""></span>U/ml in 10 cases (59%) and ranging from 100 to 250<span class="elsevierStyleHsp" style=""></span>U/ml in 4 (24%). 50% patients with two high STM levels were diagnosed with cancer.</p><elsevierMultimedia ident="tbl0005"></elsevierMultimedia><p id="par0015" class="elsevierStylePara elsevierViewall">The usefulness of STM in the management and treatment of patients with cancer and in the diagnosis of suspected cases of malignancy is provided in the NACB recommendations.<a class="elsevierStyleCrossRef" href="#bib0040"><span class="elsevierStyleSup">1</span></a> The low sensitivity and specificity in early stages discourages its use in the asymptomatic population as early diagnosis, except for alpha-fetoprotein combined with ultrasound when monitoring chronic hepatitis B, C and cirrhosis.<a class="elsevierStyleCrossRef" href="#bib0045"><span class="elsevierStyleSup">2</span></a> In this study we have the following relevant data: major source from primary care, the STM request as a strategy for early detection in 94% cases and the high prevalence of CD and/or smoking. The latter data shows that these health conditions are perceived as high risk and possibly one of the reasons for requesting STM. Most studies assessing the suitability of STM are hospital audits.<a class="elsevierStyleCrossRefs" href="#bib0050"><span class="elsevierStyleSup">3,4</span></a> The suitability described in medical literature is very variable (5–69%), depending on various factors such as the level of care, the ownership of the hospital, the areas where the study was conducted and compliance with clinical guidelines.<a class="elsevierStyleCrossRefs" href="#bib0050"><span class="elsevierStyleSup">3–5</span></a> The low positive predictive value in asymptomatic population and the many benign conditions that can cause false positives, including various prevalent CDs are 2 important factors to discourage the use of STMs as a screening test.<a class="elsevierStyleCrossRefs" href="#bib0045"><span class="elsevierStyleSup">2,6</span></a> It is obvious, as demonstrated in our cases, that the frequent request of STMs in asymptomatic patients with or without CD can help identify a number of cancers. This circumstance might be considered insignificant, given the ignorance of the actual prevalence of requests for STMs as a strategy for early detection and the various arguments described above. The results of our study, similar to those described by other authors, prove the use and understanding of the STMs is very inappropriate, which shows the frequent misunderstanding of STMS indications and failure to follow clinical guidelines.<a class="elsevierStyleCrossRefs" href="#bib0050"><span class="elsevierStyleSup">3–5,7</span></a> There is sufficient evidence to discourage from using STMs for early diagnosis in asymptomatic patients with nonspecific symptomatology and those with CD, except for cases of high risk chronic liver disease. Improper use is not beneficial and is confusing. It causes an emotional impact on the patient, an unnecessary use of resources and exposure to iatrogenesis related to additional examinations.</p></span>" "pdfFichero" => "main.pdf" "tienePdf" => true "NotaPie" => array:1 [ 0 => array:2 [ "etiqueta" => "☆" "nota" => "<p class="elsevierStyleNotepara" id="npar0005">Please cite this article as: Torné Cachot J, Baucells Azcona JM, Blanch Falp J, Blancas Altabella D. Marcadores tumorales séricos en pacientes asintomáticos. Med Clin (Barc). 2015;145:319–320.</p>" ] ] "multimedia" => array:1 [ 0 => array:8 [ "identificador" => "tbl0005" "etiqueta" => "Table 1" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at1" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:2 [ "leyenda" => "<p id="spar0010" class="elsevierStyleSimplePara elsevierViewall">CRC, colorectal cancer; MTC, medullary thyroid carcinoma.</p>" "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Reasons \t\t\t\t\t\t\n \t\t\t\t</th><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Cases, n \t\t\t\t\t\t\n \t\t\t\t</th><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Cancer, n (%) \t\t\t\t\t\t\n \t\t\t\t</th><th class="td" title="table-head " align="left" valign="top" scope="col" style="border-bottom: 2px solid black">Increased cancer and STM \t\t\t\t\t\t\n \t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleItalic">Early detection (screening)</span> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">82 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">9 (10.9) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td" title="table-entry " rowspan="6" align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>STM request with no clinical criteria</td><td class="td" title="table-entry " rowspan="6" align="char" valign="top">76</td><td class="td" title="table-entry " rowspan="6" align="char" valign="top">7 (9.2)</td><td class="td" title="table-entry " align="left" valign="top">Endometrium: CEA, CA 125 \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">Lung: CEA, CA 125 \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">MTC (2): CEA \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">Kidney: CEA \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">Pancreas CA 19-9 \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">Stomach: CEA, CA 19-9 \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Family history of CRC \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">2 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">0 (0) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleHsp" style=""></span>Chronic liver disease follow-up \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">4 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">2 (50) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="left" valign="top">Hepatocellular carcinoma (2) AFP, CA 19-9 \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td" title="table-entry " colspan="4" align="left" valign="top"><span class="elsevierStyleVsp" style="height:0.5px"></span></td></tr><tr title="table-row"><td class="td" title="table-entry " rowspan="3" align="left" valign="top"><span class="elsevierStyleItalic">Recurrence control</span></td><td class="td" title="table-entry " rowspan="3" align="char" valign="top">5</td><td class="td" title="table-entry " rowspan="3" align="char" valign="top">5 (100)</td><td class="td" title="table-entry " align="left" valign="top">CRC (3): CEA \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">Lung: CEA \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top">MTC: CEA \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td" title="table-entry " colspan="4" align="left" valign="top"><span class="elsevierStyleVsp" style="height:0.5px"></span></td></tr><tr title="table-row"><td class="td-with-role" title="table-entry ; entry_with_role_rowhead " align="left" valign="top"><span class="elsevierStyleItalic">Total</span> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">87 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="char" valign="top">14 (16) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="table-entry " align="" valign="top"> \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab1024708.png" ] ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Reasons for requesting serum tumour markers and cancer diagnosis.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bibs0005" "bibliografiaReferencia" => array:7 [ 0 => array:3 [ "identificador" => "bib0040" "etiqueta" => "1" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "C.M. Sturgeon" 1 => "M.J. Duffy" 2 => "U.H. Stenman" 3 => "H. Lilja" 4 => "N. Brünner" 5 => "D.W. Chan" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1373/clinchem.2008.105601" "Revista" => array:6 [ "tituloSerie" => "Clin Chem" "fecha" => "2008" "volumen" => "54" "paginaInicial" => "e11" "paginaFinal" => "e79" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/19042984" "web" => "Medline" ] ] ] ] ] ] ] ] 1 => array:3 [ "identificador" => "bib0045" "etiqueta" => "2" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Serum tumour markers: how to order and interpret them" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "C.M. Sturgeon" 1 => "L.C. Lai" 2 => "M.J. Duffy" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Br Med J" "fecha" => "2009" "volumen" => "339" "paginaInicial" => "852" "paginaFinal" => "858" ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bib0050" "etiqueta" => "3" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "An audit of tumour marker utilization in Greece" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "G. Ntaios" 1 => "A. Hatzitolios" 2 => "A. Chatzinikolaou" 3 => "P. Karalazou" 4 => "C. Savopoulos" 5 => "M. Karamouzis" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.ejim.2008.07.026" "Revista" => array:6 [ "tituloSerie" => "Eur J Intern Med" "fecha" => "2009" "volumen" => "20" "paginaInicial" => "e66" "paginaFinal" => "e69" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/19393482" "web" => "Medline" ] ] ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bib0055" "etiqueta" => "4" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Towards evidence-based use of serum tumour marker requests: an audit of use in a tertiary hospital" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:5 [ 0 => "S. Loi" 1 => "A.M. Haydon" 2 => "J. Shapiro" 3 => "M.A. Schwarz" 4 => "H.G. Schneider" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1111/j.1445-5994.2004.00671.x" "Revista" => array:6 [ "tituloSerie" => "Intern Med J" "fecha" => "2004" "volumen" => "34" "paginaInicial" => "545" "paginaFinal" => "550" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/15482267" "web" => "Medline" ] ] ] ] ] ] ] ] 4 => array:3 [ "identificador" => "bib0060" "etiqueta" => "5" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Tumour markers in internal medicine: a low-cost test or an unnecessary expense? A retrospective study based on appropriateness" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "D. Arioli" 1 => "M. Pipino" 2 => "E. Boldrini" 3 => "E. Amateis" 4 => "A. Cristani" 5 => "P. Ventura" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1007/s11739-007-0028-8" "Revista" => array:6 [ "tituloSerie" => "Intern Emerg Med" "fecha" => "2007" "volumen" => "2" "paginaInicial" => "88" "paginaFinal" => "94" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/17622496" "web" => "Medline" ] ] ] ] ] ] ] ] 5 => array:3 [ "identificador" => "bib0065" "etiqueta" => "6" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Tumor markers in clinical practice: a review focusing on common solid cancers" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "M.G. Duffy" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1159/000338393" "Revista" => array:6 [ "tituloSerie" => "Med Princ Pract" "fecha" => "2013" "volumen" => "22" "paginaInicial" => "4" "paginaFinal" => "11" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/22584792" "web" => "Medline" ] ] ] ] ] ] ] ] 6 => array:3 [ "identificador" => "bib0070" "etiqueta" => "7" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Tumour marker requesting in primary care and the role of the laboratory" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:2 [ 0 => "P.L. Walker" 1 => "M. Crook" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1136/jcp.2010.085357" "Revista" => array:6 [ "tituloSerie" => "J Clin Pathol" "fecha" => "2011" "volumen" => "64" "paginaInicial" => "443" "paginaFinal" => "446" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/21270062" "web" => "Medline" ] ] ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/23870206/0000014500000007/v2_201604010141/S2387020616001054/v2_201604010141/en/main.assets" "Apartado" => array:4 [ "identificador" => "43311" "tipo" => "SECCION" "en" => array:2 [ "titulo" => "Scientific letters" "idiomaDefecto" => true ] "idiomaDefecto" => "en" ] "PDF" => "https://static.elsevier.es/multimedia/23870206/0000014500000007/v2_201604010141/S2387020616001054/v2_201604010141/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020616001054?idApp=UINPBA00004N" ]
Journal Information
Vol. 145. Issue 7.
Pages 319-320 (October 2015)
Share
Download PDF
More article options
Vol. 145. Issue 7.
Pages 319-320 (October 2015)
Scientific letter
Serum tumour markers in asymptomatic patients
Marcadores tumorales séricos en pacientes asintomáticos
Visits
9
Joaquim Torné Cachot
, José Manuel Baucells Azcona, Jesús Blanch Falp, David Blancas Altabella
Corresponding author
Servicio de Medicina Interna, Hospital Sant Camil, Consorci Sanitari del Garraf, Sant Pere de Ribes, Barcelona, Spain
This item has received
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail