SARS-CoV-2 is an RNA virus belonging to the coronavirus family that causes COVID-19. Since the description in china of the first cases until our current pandemic, this disease has presented major clinical challenges for health systems in every country. From a clinical point of view, it can manifest itself in various ways: from asymptomatic, through bilateral pneumonia, and on to adult respiratory distress syndromes.1 To date, the diagnosis has been based primarily on tests that confirm the presence of SARS-CoV-2, since the symptoms that patients present can be common to other viruses, bacteria or even some atypical bacteria. We must not forget to evaluate these symptoms so as to rule them out as possible causative agents of the disease we observe. Up to now, when it is suspected that SARS-CoV-2 is the cause of the infection, the gold standard determination is real-time reverse-transcription polymerase chain reaction [RT-PCR]).2 However, despite the usual high sensitivity and specificity of the RT-PCR tests, on several occasions clinicians have encountered patients with high clinical suspicion (epidemiological, clinical, analytical, and radiological criteria) and with repeatedly negative PCR results. For this reason, first of all we wonder whether the location from where the specimen is obtained is ideal. The test is performed with nasopharyngeal exudate because that is the region where the virus experiences a higher rate of replication. However, other alternative specimens could also be used, such as oropharyngeal exudate, sputum, saliva or bronchoalveolar lavage (the latter implies a greater risk for the personnel who obtain the specimen). Secondly, we question whether the specimen is collected and transported to the laboratory in a suitable manner and without contamination and, thirdly, if the specimen has been optimally processed to obtain the maximum performance of the test.
Given that RT-PCR is a test that requires at least 4−6 hours to complete and its costs are high, recent efforts in the diagnosis of COVID-19 have also focused on enzyme-linked immunosorbent assays [ELISA]), and on rapid antigen and antibody tests (Table 1). On the one hand, the ELISA test is an immunoenzymatic test that determines the presence of IgM and IgG antibodies, or a combination of IgM + IgA. The cost of this test is low, and it takes about 3.5−4 hours. On the other hand, the rapid test is a lateral flow chromatographic immunoassay, and it enables results to be obtained easily in 20−60 min, but with low sensitivity.
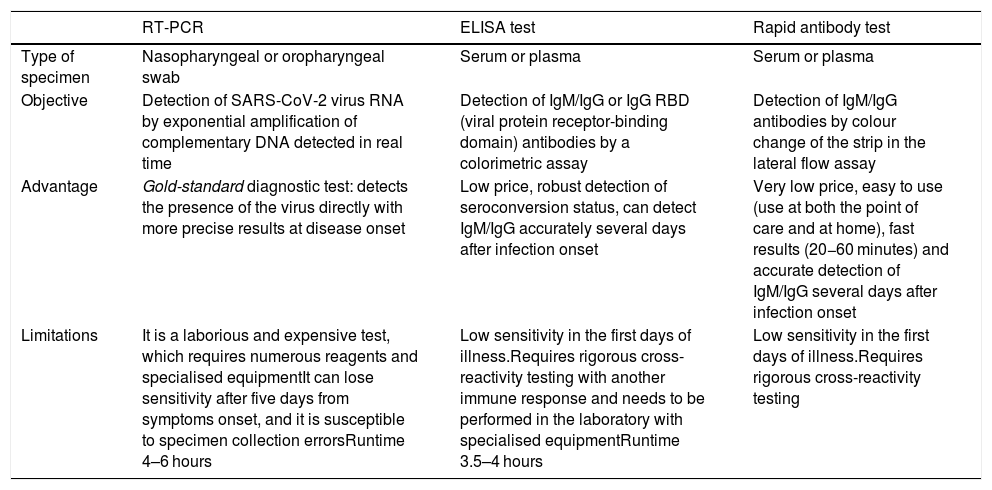
Comparison of the principal diagnostic tests against COVID-19.
| RT-PCR | ELISA test | Rapid antibody test | |
|---|---|---|---|
| Type of specimen | Nasopharyngeal or oropharyngeal swab | Serum or plasma | Serum or plasma |
| Objective | Detection of SARS-CoV-2 virus RNA by exponential amplification of complementary DNA detected in real time | Detection of IgM/IgG or IgG RBD (viral protein receptor-binding domain) antibodies by a colorimetric assay | Detection of IgM/IgG antibodies by colour change of the strip in the lateral flow assay |
| Advantage | Gold-standard diagnostic test: detects the presence of the virus directly with more precise results at disease onset | Low price, robust detection of seroconversion status, can detect IgM/IgG accurately several days after infection onset | Very low price, easy to use (use at both the point of care and at home), fast results (20−60 minutes) and accurate detection of IgM/IgG several days after infection onset |
| Limitations | It is a laborious and expensive test, which requires numerous reagents and specialised equipmentIt can lose sensitivity after five days from symptoms onset, and it is susceptible to specimen collection errorsRuntime 4–6 hours | Low sensitivity in the first days of illness.Requires rigorous cross-reactivity testing with another immune response and needs to be performed in the laboratory with specialised equipmentRuntime 3.5–4 hours | Low sensitivity in the first days of illness.Requires rigorous cross-reactivity testing |
It should be noted that the rapid antibody tests and the ELISA tests provide additional information on the patient's immune status compared to the RT-PCR test3 (Table 1), although it is still unknown if they can indicate a patient’s immunity to future reinfections. The weak points of these techniques are low sensitivity (close to 50%) in the first 7 days of the disease (increasing as days go by to 88%), and the results being affected by the patient’s immune status.4 For these reasons, the information provided by these tests is of little use in many cases and of no use at all in some cases, such as in patients with some types of immunodeficiencies. However, the advantages of the rapid tests include the speed in which they provide the results, simplicity of use and price.2 In addition, the confirmation of suspected cases of COVID-19 through serological tests could help reduce the risk of exposure to patients, as well as avoid carrying out a RT-PCR and thus reserve its use for other patients.5
Therefore, despite the fact that the current diagnostic tests for a new virus have some deficiencies, the performance is steadily improving, and possible errors in how and from where the specimens are obtained are being corrected, converting them into a foundation to help the clinician make diagnostic-therapeutic decisions.
Please cite this article as: Hernández-Pérez JM, Martín-González E, Pino-Yanes M. Virtudes y dificultades en los test diagnósticos de la infección por el SARS-CoV-2. Med Clin (Barc). 2020;155:464–465.