Atrial fibrillation (AF) has the close relation to thyroid dysfunction and these two diseases lead to poor cardiovascular outcomes. But the prognostic value of thyroid diseases in AF remains unclear. We aimed to determine whether history of thyroid diseases is associated with risk of in-hospital cardiovascular outcomes in AF.
MethodsBased on the data from the CCC-AF (Improving Care for Cardiovascular Diseases in China-Atrial Fibrillation) project, 31,486 inpatients with a definitive diagnosis of AF and record of history of thyroid diseases were included. Logistic regression analysis was performed to investigate the relationship between history of thyroid diseases and risk of in-hospital major adverse cardiovascular events (MACE) in AF.
ResultsAmong AF patients, 503 (1.6%) had a history of hypothyroidism, 642 (2.0%) had a history of hyperthyroidism and 30,341 (96.4%) had no thyroid dysfunction. During this hospitalization, 5146 (16.3%) AF patients suffered from MACE. The incidence was 13.1% in hypothyroidism, 16.3% in euthyroidism and 19.0% in hyperthyroidism, in which there was a significant difference among three groups (p=0.028). Multivariable logistic regression analysis revealed that history of hypothyroidism decreased but history of hyperthyroidism increased the risk of in-hospital MACE in AF patients (adjusted odds ratio [OR]=0.603; 95% confidence interval [CI], 0.449–0.811; p=0.001 versus adjusted OR=1.327; 95% CI, 1.060–1.661; p=0.013).
ConclusionHistory of hypothyroidism was an independent protective factor, whereas history of hyperthyroidism was an independent risk factor for in-hospital cardiovascular outcomes in AF. Our study indicated that hyperthyroidism should be treated aggressively in order to improve the prognosis of AF.
La fibrilación auricular (FA) está estrechamente relacionada con la disfunción tiroidea, y estas 2 enfermedades conducen a resultados cardiovasculares deficientes. Pero el valor pronóstico de las enfermedades tiroideas en la FA sigue sin estar claro. Nuestro objetivo era determinar si la historia de enfermedades tiroideas está asociada con el riesgo de resultados cardiovasculares intrahospitalarios en la FA.
MétodosEn base a los datos del proyecto de mejora de la atención de las enfermedades cardiovasculares en China - fibrilación auricular (CCC-FA, por sus siglas en inglés), se incluyeron 31.486 pacientes hospitalizados con un diagnóstico definitivo de FA y un registro de antecedentes de enfermedades tiroideas. Se realizó un análisis de regresión logística para investigar la relación entre la historia de las enfermedades tiroideas y el riesgo de eventos cardiovasculares adversos importantes intrahospitalarios (MACE) en FA.
ResultadosEntre los pacientes con FA, 503 (1,6%) tenían antecedentes de hipotiroidismo, 642 (2,0%) antecedentes de hipertiroidismo y 30.341 (96,4%) no tenía disfunción tiroidea. Durante esta hospitalización, 5.146 (16,3%) pacientes con FA sufrieron de MACE. La incidencia fue del 13,1% en hipotiroidismo, del 16,3% en eutiroidismo y del 19,0% en hipertiroidismo, en los que hubo una diferencia significativa entre 3 grupos (p=0,028). El análisis de regresión logística multivariable reveló que la historia de hipotiroidismo disminuyó, pero la historia de hipertiroidismo aumentó el riesgo de MACE intrahospitalario en pacientes con FA (relación de probabilidades ajustadas [OR]: 0,603; intervalo de confianza [IC] del 95%: 0,449-0,811; p=0,001 frente a OR ajustado 1,327; IC del 95%: 1,060-1,661; p=0,013).
ConclusiónLa historia del hipotiroidismo fue un factor protector independiente, mientras que la historia del hipertiroidismo fue un factor de riesgo independiente para los resultados cardiovasculares intrahospitalarios en la FA. Nuestro estudio indicó que el hipertiroidismo debe ser tratado agresivamente para mejorar el pronóstico de la FA.
Atrial fibrillation (AF) is the most common arrhythmia of clinical significance.1 It has been reported that the estimated numbers of men and women with AF worldwide were 20.9 million and 12.6 million in 2010, respectively, even with higher incidence and prevalence rates in developed countries.2 In China, AF was observed in 1.57% of the general population aged 40 years and older.3 Despite good progress in the management of such patients, AF is one of the major causes of stroke, heart failure (HF), sudden death, and cardiovascular morbidity in the world.4 Therefore, identification of risk factors is crucial for the prognosis of AF.
Hyperthyroidism, as a common endocrine disorder, is a recognized risk factor for AF.5 A shortening of the atrial refractory period and an increased supraventricular ectopic activity may explain the causal link between hyperthyroidism and AF.6,7 Not only that, it has been reported that new-onset AF seemed to be a predictor of hyperthyroidism.8 These evidences indicate a close link between AF and hyperthyroidism. AF with hyperthyroidism combined may not only cause a failure in a reversion to sinus rhythm but also worsen the cardiovascular prognosis.9–12 So additional attention should be paid to patients with AF and hyperthyroidism.
Hypothyroidism, the typical hemodynamic changes of which are opposite to those of hyperthyroidism, accompanies hypercholesterolemia, diastolic hypertension and subsequent atherosclerosis and myocardial infarction.13,14 Despite based on these observations, hypothyroidism has not been associated with increased or decreased risk of AF.15 Besides accompanied cardiovascular manifestations, hypothyroidism was associated with a higher risk of bleeding events in AF.16 However, the prognosis of AF patients with hypothyroidism needs to be further determined.
Based on the observations above, we found that there were complicated relationships among AF, hyperthyroidism and hypothyroidism and any single disease was related to cardiovascular outcomes. However, whether history of thyroid diseases have effects on the in-hospital cardiovascular outcomes in AF patients is unknown. In the present study, we aimed to investigate the relationship between history of thyroid diseases and risk of in-hospital cardiovascular outcomes in AF.
Patients and methodsStudy designThe CCC-AF (Improving Care for Cardiovascular Disease in China-AF) project is a nationwide registry and quality improvement study with an ongoing database focusing on quality of AF care. This study was launched in 2015 as a collaborative initiative of the American Heart Association and Chinese Society of Cardiology. Details of the design and methodology of the CCC-AF project have been published.17 Briefly, a certain number of inpatients with AF are monthly enrolled in 150 hospitals across 30 provinces in China. A web-based data collection platform is used to collect clinical information for patients with AF. The quality improvement initiative, including monthly benchmarked reports on hospital quality, training sessions, regular webinars and recognitions of hospital quality achievement, ensures data quality.
Study populationOn the basis of principal discharge diagnosis, 31,486 patients with AF from 150 hospitals were registered from February 2015 to June 2017. All patients with the record of history of thyroid diseases were included in this study. Institutional review board approval was granted for this research by the ethics committee of Beijing Anzhen Hospital, Capital Medical University. No informed consent was required.
Information collectionData used included patients’ demographics, medical history, results of laboratory testing at admission, pre-hospital medications and in-hospital managements. Current smokers were defined as people who smoked within 1 year. Harmful use of alcohol was defined as excessive use to the point that it caused damage to health. Family history of AF was defined as patients’ first or second-degree relative having AF. Hypertension was defined as having a history of hypertension or taking anti-hypertension medications. Coronary artery disease was defined as having a history of coronary artery disease or receiving revascularization procedure. Diabetes mellitus was defined as having a history of diabetes or receiving glucose-lowering treatment. Body mass index was calculated as dividing weight in kilograms by the square of height in meters. CHADS2 score was calculated by age, congestive heart failure, hypertension, diabetes and stroke or transient ischemic attacks.
In-hospital cardiovascular outcomesComposite end points, including in-hospital cardiac death, HF, cardiogenic shock and stroke were defined as major adverse cardiovascular events (MACE).18 The date of the first MACE was obtained from medical records.
Statistical analysisContinuous variables were presented as mean (standard deviation [SD]). Kolmogorov–Smirnov test was used to test the normality of continuous variables distribution. One-way ANOVA and Kruskal–Wallis test were used for the comparisons of continuous variables as appropriate. Categorical variables were shown as frequencies and percentages. χ2 test was used to analyze the differences between categorical variables. Logistic univariate and multivariate regression analysis were performed to determine the relationship between history of thyroid diseases and the risk of in-hospital cardiovascular outcomes in patients with AF. All computations were performed with SPSS software v20.0 (SPSS Inc., Chicago, IL, USA). A statistically significant difference was defined at p<0.05 using a two-tailed test.
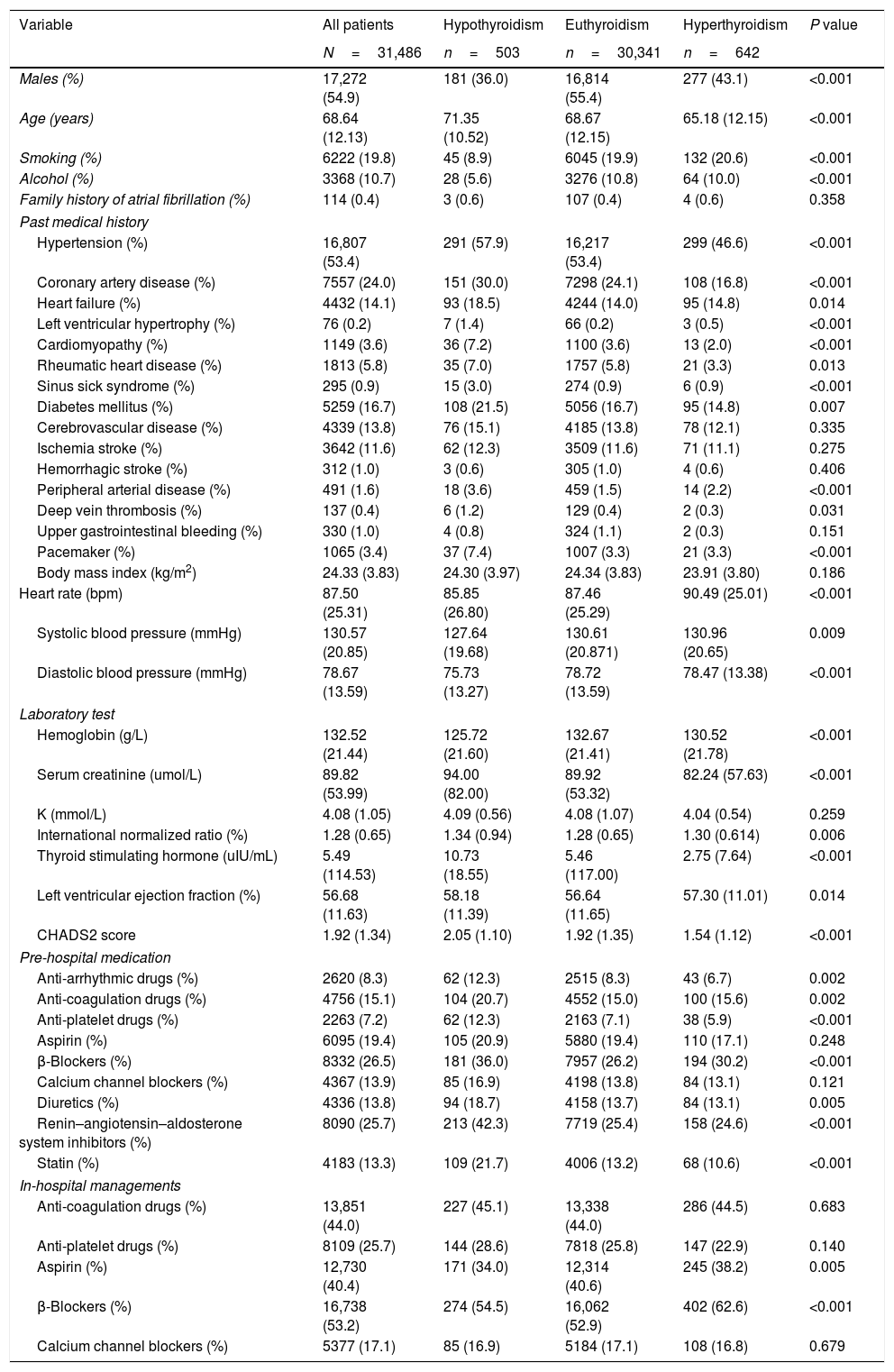
ResultsClinical characteristics of the study populationThe 31,486 patients were divided into 3 groups according to the history of thyroid diseases: euthyroidism in 30,341 (96.4%), hypothyroidism in 503 (1.6%), hyperthyroidism in 642 (2.0%). The main baseline characteristics of these 3 groups are shown in Table 1. 54.9% were males, but the majority of patients with hypothyroidism or hyperthyroidism were females. The mean age was 68.64 (12.13) years. Elder patients tended to have hypothyroidism and young patients tended to have hyperthyroidism. Among 31,486 patients with AF, 14.1% had history with HF, and patients with hypothyroidism had significantly higher prevalence (p=0.014). CHADS2 score was 1.92 (1.34) in all patients. From hypothyroidism, euthyroidism to hyperthyroidism, the score kept decreasing (p<0.001). However, intergroup comparison revealed no significant differences among the groups with respect to history of ischemia stroke, hemorrhagic stroke, upper gastrointestinal bleeding and et al. (p>0.05, Table 1).
Characteristics of patients with atrial fibrillation according to history of thyroid dysfunction.
| Variable | All patients | Hypothyroidism | Euthyroidism | Hyperthyroidism | P value |
|---|---|---|---|---|---|
| N=31,486 | n=503 | n=30,341 | n=642 | ||
| Males (%) | 17,272 (54.9) | 181 (36.0) | 16,814 (55.4) | 277 (43.1) | <0.001 |
| Age (years) | 68.64 (12.13) | 71.35 (10.52) | 68.67 (12.15) | 65.18 (12.15) | <0.001 |
| Smoking (%) | 6222 (19.8) | 45 (8.9) | 6045 (19.9) | 132 (20.6) | <0.001 |
| Alcohol (%) | 3368 (10.7) | 28 (5.6) | 3276 (10.8) | 64 (10.0) | <0.001 |
| Family history of atrial fibrillation (%) | 114 (0.4) | 3 (0.6) | 107 (0.4) | 4 (0.6) | 0.358 |
| Past medical history | |||||
| Hypertension (%) | 16,807 (53.4) | 291 (57.9) | 16,217 (53.4) | 299 (46.6) | <0.001 |
| Coronary artery disease (%) | 7557 (24.0) | 151 (30.0) | 7298 (24.1) | 108 (16.8) | <0.001 |
| Heart failure (%) | 4432 (14.1) | 93 (18.5) | 4244 (14.0) | 95 (14.8) | 0.014 |
| Left ventricular hypertrophy (%) | 76 (0.2) | 7 (1.4) | 66 (0.2) | 3 (0.5) | <0.001 |
| Cardiomyopathy (%) | 1149 (3.6) | 36 (7.2) | 1100 (3.6) | 13 (2.0) | <0.001 |
| Rheumatic heart disease (%) | 1813 (5.8) | 35 (7.0) | 1757 (5.8) | 21 (3.3) | 0.013 |
| Sinus sick syndrome (%) | 295 (0.9) | 15 (3.0) | 274 (0.9) | 6 (0.9) | <0.001 |
| Diabetes mellitus (%) | 5259 (16.7) | 108 (21.5) | 5056 (16.7) | 95 (14.8) | 0.007 |
| Cerebrovascular disease (%) | 4339 (13.8) | 76 (15.1) | 4185 (13.8) | 78 (12.1) | 0.335 |
| Ischemia stroke (%) | 3642 (11.6) | 62 (12.3) | 3509 (11.6) | 71 (11.1) | 0.275 |
| Hemorrhagic stroke (%) | 312 (1.0) | 3 (0.6) | 305 (1.0) | 4 (0.6) | 0.406 |
| Peripheral arterial disease (%) | 491 (1.6) | 18 (3.6) | 459 (1.5) | 14 (2.2) | <0.001 |
| Deep vein thrombosis (%) | 137 (0.4) | 6 (1.2) | 129 (0.4) | 2 (0.3) | 0.031 |
| Upper gastrointestinal bleeding (%) | 330 (1.0) | 4 (0.8) | 324 (1.1) | 2 (0.3) | 0.151 |
| Pacemaker (%) | 1065 (3.4) | 37 (7.4) | 1007 (3.3) | 21 (3.3) | <0.001 |
| Body mass index (kg/m2) | 24.33 (3.83) | 24.30 (3.97) | 24.34 (3.83) | 23.91 (3.80) | 0.186 |
| Heart rate (bpm) | 87.50 (25.31) | 85.85 (26.80) | 87.46 (25.29) | 90.49 (25.01) | <0.001 |
| Systolic blood pressure (mmHg) | 130.57 (20.85) | 127.64 (19.68) | 130.61 (20.871) | 130.96 (20.65) | 0.009 |
| Diastolic blood pressure (mmHg) | 78.67 (13.59) | 75.73 (13.27) | 78.72 (13.59) | 78.47 (13.38) | <0.001 |
| Laboratory test | |||||
| Hemoglobin (g/L) | 132.52 (21.44) | 125.72 (21.60) | 132.67 (21.41) | 130.52 (21.78) | <0.001 |
| Serum creatinine (umol/L) | 89.82 (53.99) | 94.00 (82.00) | 89.92 (53.32) | 82.24 (57.63) | <0.001 |
| K (mmol/L) | 4.08 (1.05) | 4.09 (0.56) | 4.08 (1.07) | 4.04 (0.54) | 0.259 |
| International normalized ratio (%) | 1.28 (0.65) | 1.34 (0.94) | 1.28 (0.65) | 1.30 (0.614) | 0.006 |
| Thyroid stimulating hormone (uIU/mL) | 5.49 (114.53) | 10.73 (18.55) | 5.46 (117.00) | 2.75 (7.64) | <0.001 |
| Left ventricular ejection fraction (%) | 56.68 (11.63) | 58.18 (11.39) | 56.64 (11.65) | 57.30 (11.01) | 0.014 |
| CHADS2 score | 1.92 (1.34) | 2.05 (1.10) | 1.92 (1.35) | 1.54 (1.12) | <0.001 |
| Pre-hospital medication | |||||
| Anti-arrhythmic drugs (%) | 2620 (8.3) | 62 (12.3) | 2515 (8.3) | 43 (6.7) | 0.002 |
| Anti-coagulation drugs (%) | 4756 (15.1) | 104 (20.7) | 4552 (15.0) | 100 (15.6) | 0.002 |
| Anti-platelet drugs (%) | 2263 (7.2) | 62 (12.3) | 2163 (7.1) | 38 (5.9) | <0.001 |
| Aspirin (%) | 6095 (19.4) | 105 (20.9) | 5880 (19.4) | 110 (17.1) | 0.248 |
| β-Blockers (%) | 8332 (26.5) | 181 (36.0) | 7957 (26.2) | 194 (30.2) | <0.001 |
| Calcium channel blockers (%) | 4367 (13.9) | 85 (16.9) | 4198 (13.8) | 84 (13.1) | 0.121 |
| Diuretics (%) | 4336 (13.8) | 94 (18.7) | 4158 (13.7) | 84 (13.1) | 0.005 |
| Renin–angiotensin–aldosterone system inhibitors (%) | 8090 (25.7) | 213 (42.3) | 7719 (25.4) | 158 (24.6) | <0.001 |
| Statin (%) | 4183 (13.3) | 109 (21.7) | 4006 (13.2) | 68 (10.6) | <0.001 |
| In-hospital managements | |||||
| Anti-coagulation drugs (%) | 13,851 (44.0) | 227 (45.1) | 13,338 (44.0) | 286 (44.5) | 0.683 |
| Anti-platelet drugs (%) | 8109 (25.7) | 144 (28.6) | 7818 (25.8) | 147 (22.9) | 0.140 |
| Aspirin (%) | 12,730 (40.4) | 171 (34.0) | 12,314 (40.6) | 245 (38.2) | 0.005 |
| β-Blockers (%) | 16,738 (53.2) | 274 (54.5) | 16,062 (52.9) | 402 (62.6) | <0.001 |
| Calcium channel blockers (%) | 5377 (17.1) | 85 (16.9) | 5184 (17.1) | 108 (16.8) | 0.679 |
Data are presented as mean (SD) or number (%).
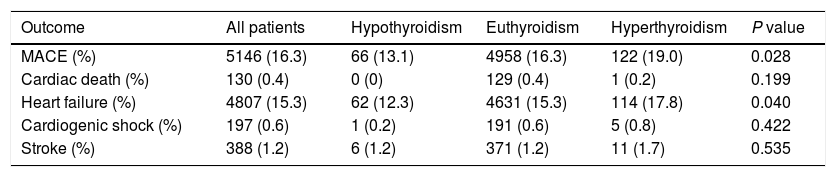
The mean hospital stay was 10 days (interquartile range: 6–12 days). At the end of the hospitalization, 5146 (16.3%) patients had MACE, including 130 (0.4%) cardiac death, 4807 (15.3%) HF, 197 (0.6%) cardiogenic shock and 388 (1.23%) stroke. Details of every endpoints in patients with history with hypothyroidism, euthyroidism and hyperthyroidism are shown in Table 2.
In-hospital cardiovascular outcomes of patients with atrial fibrillation according to history of thyroid dysfunction.
| Outcome | All patients | Hypothyroidism | Euthyroidism | Hyperthyroidism | P value |
|---|---|---|---|---|---|
| MACE (%) | 5146 (16.3) | 66 (13.1) | 4958 (16.3) | 122 (19.0) | 0.028 |
| Cardiac death (%) | 130 (0.4) | 0 (0) | 129 (0.4) | 1 (0.2) | 0.199 |
| Heart failure (%) | 4807 (15.3) | 62 (12.3) | 4631 (15.3) | 114 (17.8) | 0.040 |
| Cardiogenic shock (%) | 197 (0.6) | 1 (0.2) | 191 (0.6) | 5 (0.8) | 0.422 |
| Stroke (%) | 388 (1.2) | 6 (1.2) | 371 (1.2) | 11 (1.7) | 0.535 |
Data are presented as number (%). MACE, major adverse cardiovascular events.
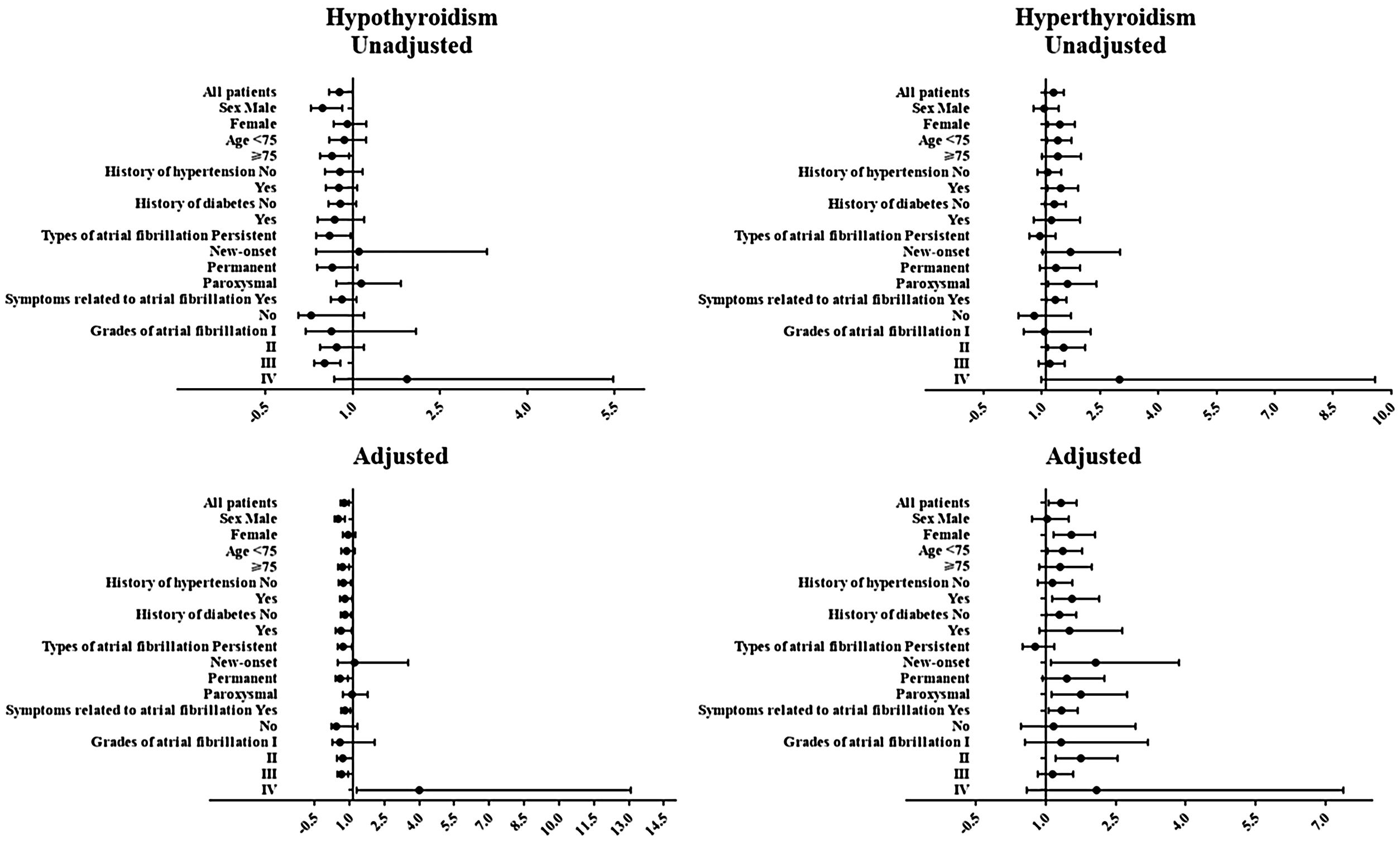
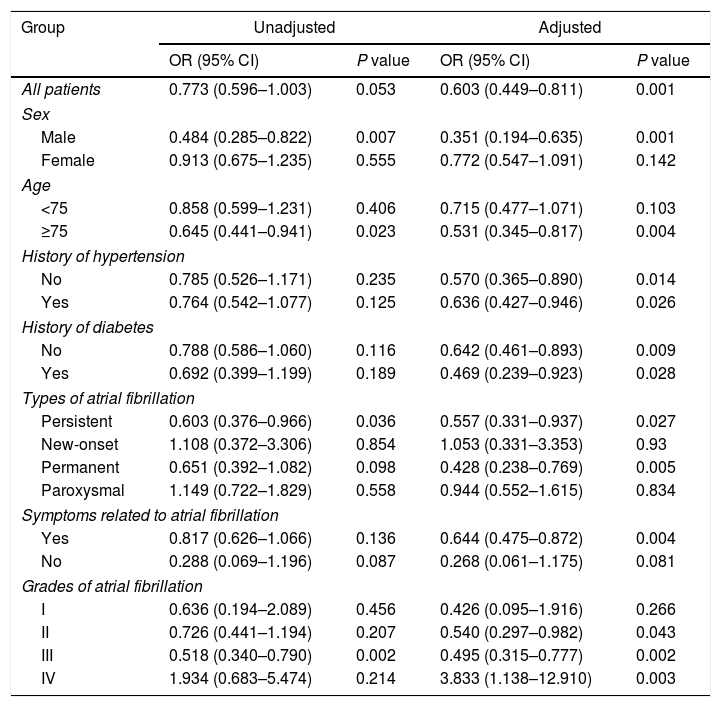
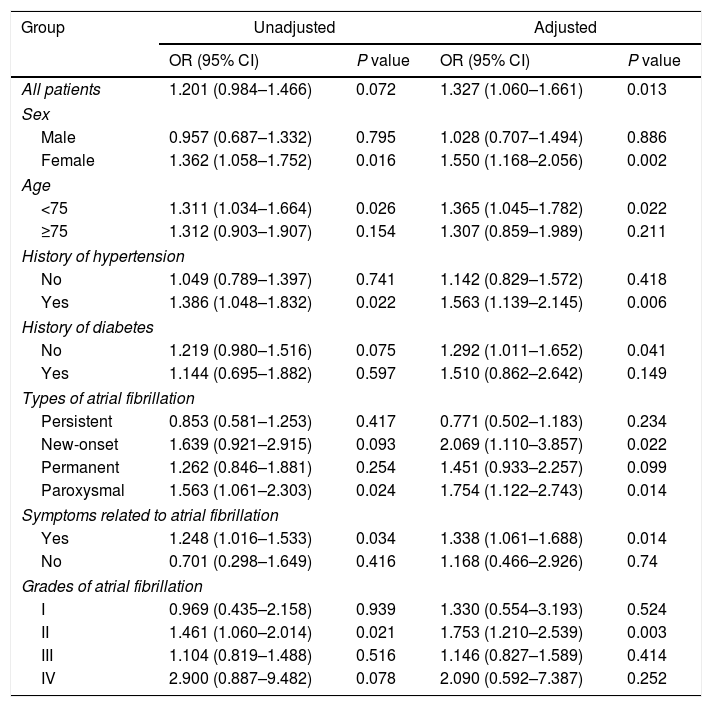
Logistic regression analysis was performed to determine the relationship between history of thyroid diseases and the risk of in-hospital cardiovascular outcomes in AF patients, results of which are presented in Fig. 1 and Tables 3 and 4. Although in univariate analysis, history of hypothyroidism or hyperthyroidism was not associated with the risk of MACE (p>0.05). After adjustment for sex, age, medical history, laboratory examination and et al. (Fig. 1 and Tables 3 and 4), history of hypothyroidism was significantly associated with a decreased risk of MACE (odds ratio [OR]=0.603; 95% confidence intervals [CI], 0.449–0.811; p=0.001). However, history of hyperthyroidism significantly increased the risk of MACE in multivariate analysis (OR=1.327; 95% CI, 1.060–1.661; p=0.013).
The relationship between history of hypothyroidism and risk of in-hospital major adverse cardiovascular events in patients with atrial fibrillation.
| Group | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| All patients | 0.773 (0.596–1.003) | 0.053 | 0.603 (0.449–0.811) | 0.001 |
| Sex | ||||
| Male | 0.484 (0.285–0.822) | 0.007 | 0.351 (0.194–0.635) | 0.001 |
| Female | 0.913 (0.675–1.235) | 0.555 | 0.772 (0.547–1.091) | 0.142 |
| Age | ||||
| <75 | 0.858 (0.599–1.231) | 0.406 | 0.715 (0.477–1.071) | 0.103 |
| ≥75 | 0.645 (0.441–0.941) | 0.023 | 0.531 (0.345–0.817) | 0.004 |
| History of hypertension | ||||
| No | 0.785 (0.526–1.171) | 0.235 | 0.570 (0.365–0.890) | 0.014 |
| Yes | 0.764 (0.542–1.077) | 0.125 | 0.636 (0.427–0.946) | 0.026 |
| History of diabetes | ||||
| No | 0.788 (0.586–1.060) | 0.116 | 0.642 (0.461–0.893) | 0.009 |
| Yes | 0.692 (0.399–1.199) | 0.189 | 0.469 (0.239–0.923) | 0.028 |
| Types of atrial fibrillation | ||||
| Persistent | 0.603 (0.376–0.966) | 0.036 | 0.557 (0.331–0.937) | 0.027 |
| New-onset | 1.108 (0.372–3.306) | 0.854 | 1.053 (0.331–3.353) | 0.93 |
| Permanent | 0.651 (0.392–1.082) | 0.098 | 0.428 (0.238–0.769) | 0.005 |
| Paroxysmal | 1.149 (0.722–1.829) | 0.558 | 0.944 (0.552–1.615) | 0.834 |
| Symptoms related to atrial fibrillation | ||||
| Yes | 0.817 (0.626–1.066) | 0.136 | 0.644 (0.475–0.872) | 0.004 |
| No | 0.288 (0.069–1.196) | 0.087 | 0.268 (0.061–1.175) | 0.081 |
| Grades of atrial fibrillation | ||||
| I | 0.636 (0.194–2.089) | 0.456 | 0.426 (0.095–1.916) | 0.266 |
| II | 0.726 (0.441–1.194) | 0.207 | 0.540 (0.297–0.982) | 0.043 |
| III | 0.518 (0.340–0.790) | 0.002 | 0.495 (0.315–0.777) | 0.002 |
| IV | 1.934 (0.683–5.474) | 0.214 | 3.833 (1.138–12.910) | 0.003 |
OR, odds ratio; CI, confidence intervals.
Adjusted for sex, age, smoking, alcohol, family history of atrial fibrillation, hypertension, coronary artery disease, heart failure, left ventricular hypertrophy, cardiomyopathy, rheumatic heart disease, sinus sick syndrome, diabetes mellitus, cerebrovascular disease, ischemia stroke, hemorrhagic stroke, peripheral arterial disease, deep vein thrombosis, upper gastrointestinal bleeding, pacemaker, heart rate, systolic blood pressure, diastolic blood pressure, hemoglobin, serum creatinine, K, pre-hospital medications and in-hospital managements.
The relationship between history of hyperthyroidism and risk of in-hospital major adverse cardiovascular events in patients with atrial fibrillation.
| Group | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| All patients | 1.201 (0.984–1.466) | 0.072 | 1.327 (1.060–1.661) | 0.013 |
| Sex | ||||
| Male | 0.957 (0.687–1.332) | 0.795 | 1.028 (0.707–1.494) | 0.886 |
| Female | 1.362 (1.058–1.752) | 0.016 | 1.550 (1.168–2.056) | 0.002 |
| Age | ||||
| <75 | 1.311 (1.034–1.664) | 0.026 | 1.365 (1.045–1.782) | 0.022 |
| ≥75 | 1.312 (0.903–1.907) | 0.154 | 1.307 (0.859–1.989) | 0.211 |
| History of hypertension | ||||
| No | 1.049 (0.789–1.397) | 0.741 | 1.142 (0.829–1.572) | 0.418 |
| Yes | 1.386 (1.048–1.832) | 0.022 | 1.563 (1.139–2.145) | 0.006 |
| History of diabetes | ||||
| No | 1.219 (0.980–1.516) | 0.075 | 1.292 (1.011–1.652) | 0.041 |
| Yes | 1.144 (0.695–1.882) | 0.597 | 1.510 (0.862–2.642) | 0.149 |
| Types of atrial fibrillation | ||||
| Persistent | 0.853 (0.581–1.253) | 0.417 | 0.771 (0.502–1.183) | 0.234 |
| New-onset | 1.639 (0.921–2.915) | 0.093 | 2.069 (1.110–3.857) | 0.022 |
| Permanent | 1.262 (0.846–1.881) | 0.254 | 1.451 (0.933–2.257) | 0.099 |
| Paroxysmal | 1.563 (1.061–2.303) | 0.024 | 1.754 (1.122–2.743) | 0.014 |
| Symptoms related to atrial fibrillation | ||||
| Yes | 1.248 (1.016–1.533) | 0.034 | 1.338 (1.061–1.688) | 0.014 |
| No | 0.701 (0.298–1.649) | 0.416 | 1.168 (0.466–2.926) | 0.74 |
| Grades of atrial fibrillation | ||||
| I | 0.969 (0.435–2.158) | 0.939 | 1.330 (0.554–3.193) | 0.524 |
| II | 1.461 (1.060–2.014) | 0.021 | 1.753 (1.210–2.539) | 0.003 |
| III | 1.104 (0.819–1.488) | 0.516 | 1.146 (0.827–1.589) | 0.414 |
| IV | 2.900 (0.887–9.482) | 0.078 | 2.090 (0.592–7.387) | 0.252 |
OR, odds ratio; CI, confidence intervals.
Adjusted for sex, age, smoking, alcohol, family history of atrial fibrillation, hypertension, coronary artery disease, heart failure, left ventricular hypertrophy, cardiomyopathy, rheumatic heart disease, sinus sick syndrome, diabetes mellitus, cerebrovascular disease, ischemia stroke, hemorrhagic stroke, peripheral arterial disease, deep vein thrombosis, upper gastrointestinal bleeding, pacemaker, heart rate, systolic blood pressure, diastolic blood pressure, hemoglobin, serum creatinine, K, pre-hospital medications and in-hospital managements.
Next, a series of subgroup analysis were carried out to investigate whether the relationship was reliable. Despite history of thyroid diseases was not associated with the risk of MACE in individual subgroups, history of hypothyroidism decreased while hyperthyroidism increased the risk of MACE in general.
DiscussionGiven that AF, hypothyroidism and hyperthyroidism have close connections to one another and causal associations with cardiovascular outcomes, the relationship between history of thyroid diseases and risk of in-hospital cardiovascular outcomes in patients with AF remains unclear. Our present study was a nationwide, multicenter study in China and found that the incidence of in-hospital MACE was higher in AF patients with history of hyperthyroidism than AF alone while it was lower in AF patients with hypothyroidism. History of hyperthyroidism was an independent risk factor of in-hospital MACE in AF patients and hypothyroidism was a protective factor. To the best of our knowledge, our study is the first analysis of an ongoing registry on the association between history of thyroid diseases and the risk of in-hospital cardiovascular outcomes in patients with AF.
Hyperthyroidism is a common disorder and affects multiple systems in the body, the most striking of which is cardiovascular effects.12 In addition to cardiovascular clinical presentations, hyperthyroidism is a known potent inducer of many cardiovascular complications such as AF.5,19 It has been reported that the association between hyperthyroidism and sustained AF still existed ever after return to the euthyroid state.20 But more importantly, increasing evidence has suggested that AF with hyperthyroidism combined worsened the cardiovascular prognosis.9–12 However, Bruere et al. revealed that history of hyperthyroidism was not an independent risk factor for stroke/systemic embolism in AF.16 Lack of direct evidence and inconsistent research results drove us to conduct the current study. In the present study, history of hyperthyroidism was an independent risk factor for in-hospital MACE in AF. There are two possible reasons for this. First, AF is closely associated with cardiovascular morbidity and mortality.4 One of the most important mechanisms is arterial embolism from the left atrium as a result of blood pooling.10,21 And antithrombotic therapy can prevent the majority of ischemic event and prolong life.4 In hyperthyroidism, a hypercoagulable and hypofibrinolytic state had been described.22 AF with hyperthyroidism combined has higher risk of embolic events than AF alone.10 Another reason is hemodynamic changes in the two kinds of disorders. AF can cause reductions in cardiac output because of shorter diastolic filling time, loss of atrial contractile function and elevated filling pressures and tachycardia-induced myocardial dysfunction.23 And long-term exposure to thyroid hormone excess also exert unfavorable effects on cardiac morphology and function because it may increase left ventricular mass, arterial stiffness and left atrial size and induce subsequent diastolic dysfunction.24 These mechanisms are related to the occurrence of HF and poorer prognosis. In addition, several reasons, including relatively small sample size and single end point may explain the negative result in the previous study. To the best of our knowledge, our analysis is one of the largest in patients with AF and history of hyperthyroidism and with a composite end point to investigate the association between history of hyperthyroidism and in-hospital MACE in AF. Our results indicated that hyperthyroidism should be aggressively treated in order to improve the prognosis in patients with AF.
Hypothyroidism refers to another common pathological condition of thyroid hormone deficiency, which is opposite of hyperthyroidism. Hypothyroidism also has clinical implication related to nearly all major organs, but the cardiovascular system is the most robustly studied.25 Although the association between hypothyroidism and the incidence of AF is still controversial, hypothyroidism may result in accelerated atherosclerosis and coronary artery disease, presumably because of the associated hypercholesterolemia and hypertension.9,13,15 But such an effect lacks direct evidence and was indicated from a study of subclinical hypothyroidism.14 In addition, few studies have investigated the prognostic value of hypothyroidism in AF. In the current study, interestingly, history of hypothyroidism was an independent protective factor for in-hospital MACE in AF. We present several assumptions which may explain this result. Firstly, instead of hyperthyroidism, hyperfibrinolysis is observed in hypothyroidism.22 So, we speculate that hypothyroidism may decrease the level of hypercoagulable state in AF to some extent. Bruere et al. found that history of hypothyroidism was independently associated with an increased risk of bleeding events in AF, which was consistent with our hypothesis.16 Another possible reason results from signs and symptoms of thyroid hormone deficiency in hypothyroidism. In our present study, AF patients with hypothyroidism had lower heart rate than patients with AF alone, which might decrease rapid ventricular rate induced by AF. The first step in the management of AF is to control the ventricular response and hypothyroidism can exert this function.5 Besides, more proportion of females, less smoking history and lower blood pressure might explain why history of hypothyroidism was a protective factor for in-hospital MACE in AF patients.
The current study has several limitations. First, history of thyroid diseases was obtained from patients’ self-reports. Recall bias could not be ruled out. Second, the actual state of thyroid diseases was not available at admission. Although serum thyroid stimulating hormone concentrations at admission were consistent with history of thyroid diseases, the present state of thyroid diseases could explain the relationship with prognosis better. Third, we only investigated the association between history of thyroid diseases and the risk of in-hospital outcomes. Longer follow-up is needed to determine the prognostic value of history of thyroid disorders.
ConclusionIn conclusion, history of hypothyroidism was associated with decreased risk, while history of hyperthyroidism was related with increased risk of in-hospital cardiovascular outcomes in patients with AF. Our results indicated that hyperthyroidism might be treated aggressively in AF patients in order to improve the prognosis of AF.
Data availability statementThere are no linked research data sets for this submission, because the authors do not have permission to share data.
FundingThe CCC project is a program of the American Heart Association and the Chinese Society of Cardiology. The American Heart Association has been funded by Pfizer for quality improvement initiative through an independent grant for leaning and change.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors thanked all hospitals participating in the CCC project. And the authors also thanked Meng-ge Zhou, Yue-yan Xing, Yu-wei Liu for their help.