Presently, there is controversy and misconception in the diagnosis and management of most congenital vascular malformations. The aim of this manuscript is to identify the current knowledge of these poorly understood and relatively uncommon pathologies. We will also review the updated terminology, classification, pathogenesis, clinical presentation, diagnosis approach and management.
Introduction
Until recently, congenital vascular malformations (CVM) suffered from a bad reputation among health professionals. Most doctors did not want to deal with CVM cases due to bad experiences in the management of these pathologies, mostly due to the lack of information and knowledge, which conditioned bad management and even worse results.1 CVM’s misconception has drastically changed due to the better understanding of pathophysiology and natural history, aspects which facilitated the creation of new CVM classifications,2 alongside the development of new diagnosis and treatment technology and the switch to a multidisciplinary approach.3,4 Great results have been accomplished with minimal morbi-mortality, few recurrences and a better acceptance of these complex nosological entities by health professionals.
The purpose of this review is to disclose current knowledge about CVM’s etiology, pathophysiology, genetic aspects, diagnosis and therapeutic approach.
Terms, definitions and classifications
Vascular anomaly is a term used to describe two pathological entities; CVM and vascular tumors represented by neonatal or infantile hemangiomas.5 However, these two conditions are very different in their anatomy, histology, pathophysiology and clinical presentation. CVMs are a result of a failure in angiogenesis, appear from birth and grow accordingly to the patient’s growth. Hemangiomas are vascular tumors originating in the endothelium; generally appearing during the neonatal period, they grow rapidly, but later devolve at 5-10 years of age in the majority of cases. Angioma is another term used indistinctly to name any vascular anomaly which causes mistakes and improper management, thus it is preferable to avoid its use. On the other hand, there is the denomination hemangioma, capillary or cavernous, originally described by Virchow in 1863 to define vascular anomalies present since birth; however, Virchow only took into account its location and did not considere other important characteristics.6 Angiodysplasia is another term coined to described CVMs, but due to its limited practical usefulness in describing a CVM and limited help in differential diagnosis its use is not very recommendable.
At the beginning of the 20th century, doctors like Klippel and Trenaunay, Parkes and Weberm, Servelle and Martorell, to name a few, reported cases of CVM, where in order to describe them they used clinical characteristics which included alterations in skin, soft tissue, bones and blood vessels and grouped them, creating syndromes which they named in accordance with the doctors who first described them.7 These names, based in eponyms, have been used for decades and even nowadays are still in use, but do not provide information about the etiology, pathophysiology and anatomy of these complex CVMs. Nevertheless, each syndrome has a historical significance and although the Hamburg classification is the one that is currently used, these classifications are still useful to some extent to describe certain mixed forms of CVMs.
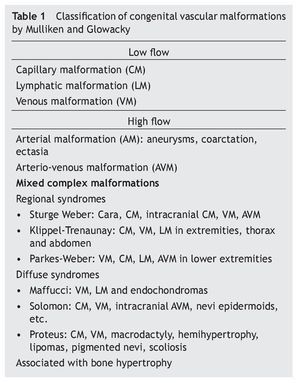
A significant step towards clearing the confusion on the subject of CVMs was the publication by Mulliken et al8 in 1982, where a differentiation between CVMs and hemangiomas was definitely made. Moreover, they created a classification based on the characteristics of the predominant endothelium, the hemodynamic state of the vascular malformation (high flow: AV, AVM or low flow: CM, VM, or LM), findings during physical examination and natural evolution (table 1). Subsequently, this classification was redefined by Mulliken et al5 in 1988.
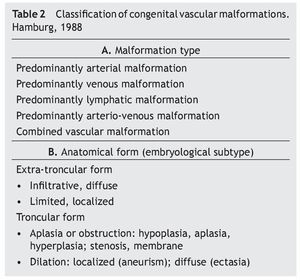
Another major development in the classification of the enigma that CVMs represent, was the creation of the Hamburg Classification (HC) (table 2). The Hamburg classification is the result of a consensus reached by experts on the subject who gathered at the 7th International Workshop on Vascular Malformations held in Hamburg, Germany in 1988.9
This classification forbids the term angioma, and properly classifies CVMs according to the type of predominantly affected vessel or its combinations, and to the embryological state where the malformation occurs using anatomic, histologic, and physiopathologic criteria as well as a hemodynamic state to define them. It has been accepted by most of the medical community because of its proven superiority in the application during clinical practice to perform a clinical-anatomical diagnosis, defining treatment and facilitating communication between different specialists.10
Within HC (table 2A) CVMs are divided into five classes, depending on the predominant vascular component: arterial malformation (AM), venous malformation (VM), lymphatic malformation (LM), arterio-venous malformations (AVM) and mixed or combined vascular malformations (MVM). At first, capillary malformations were not included in this classification because they were believed to have no clinical significance and to be equivalent to other CVMs, but in the 1992 HC revision in Denver11 they were included and later confirmed in 2002 in Seoul.12
Each CVM is divided into two types, according to the embryological stage in which it was developed (its clinical presentation, natural evolution, and the results of the treatment depend on this): troncular and extra-troncular (table 2B).
The extra-troncular type develops in a very early stage in embryological development, in which the vascular system is in reticular phase. This type of lesion is composed of remnants of embryonic mesenchymal tissue that preserve angioblast characteristics. These cells retain the potential of growing and proliferating with internal stimuli (menarche, pregnancy, etc.) or external stimuli (trauma and surgery) and present a high recurrence incidence. Extra-troncular lesions can be presented as infiltrative and diffuse lesions or localized lesions which cause mechanical compression in nearby tissues and organs.
Troncular lesions are formed in the late embryonic stage where vascular trunks are being formed,13 and at the same time are subdivided into obstructive and/or dilated lesions (figure 1). Cells forming this type of lesion have lost the ability to proliferate and are presented as a remnant of fetal vasculature, being displayed as aplastic, hypoplastic, or hyperplastic vessels (aneurysms, vascular networks, etc.). Due to the type of these cellular lesions, risk of recurrence is low, but the associated hemodynamic consequences can be severe.
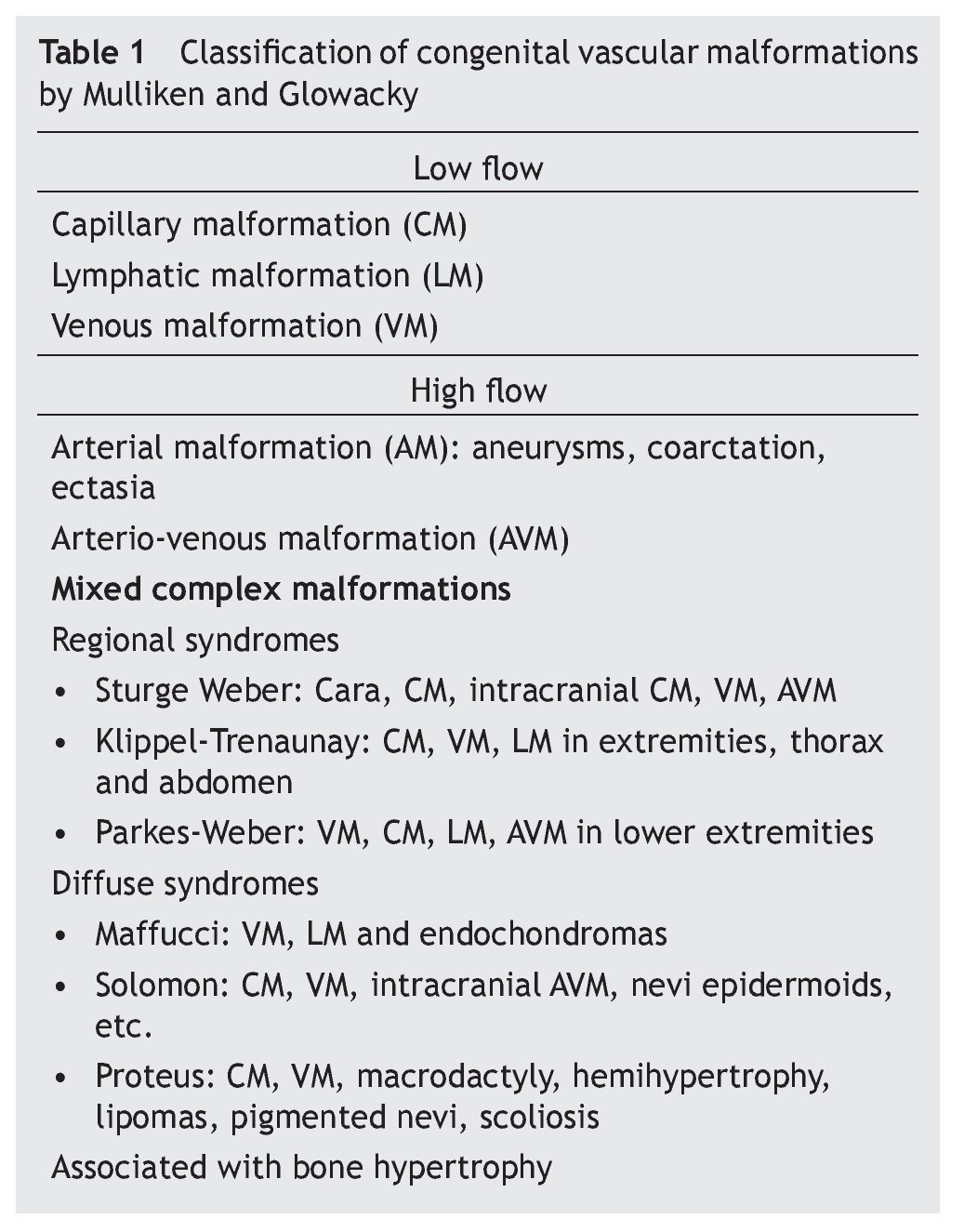
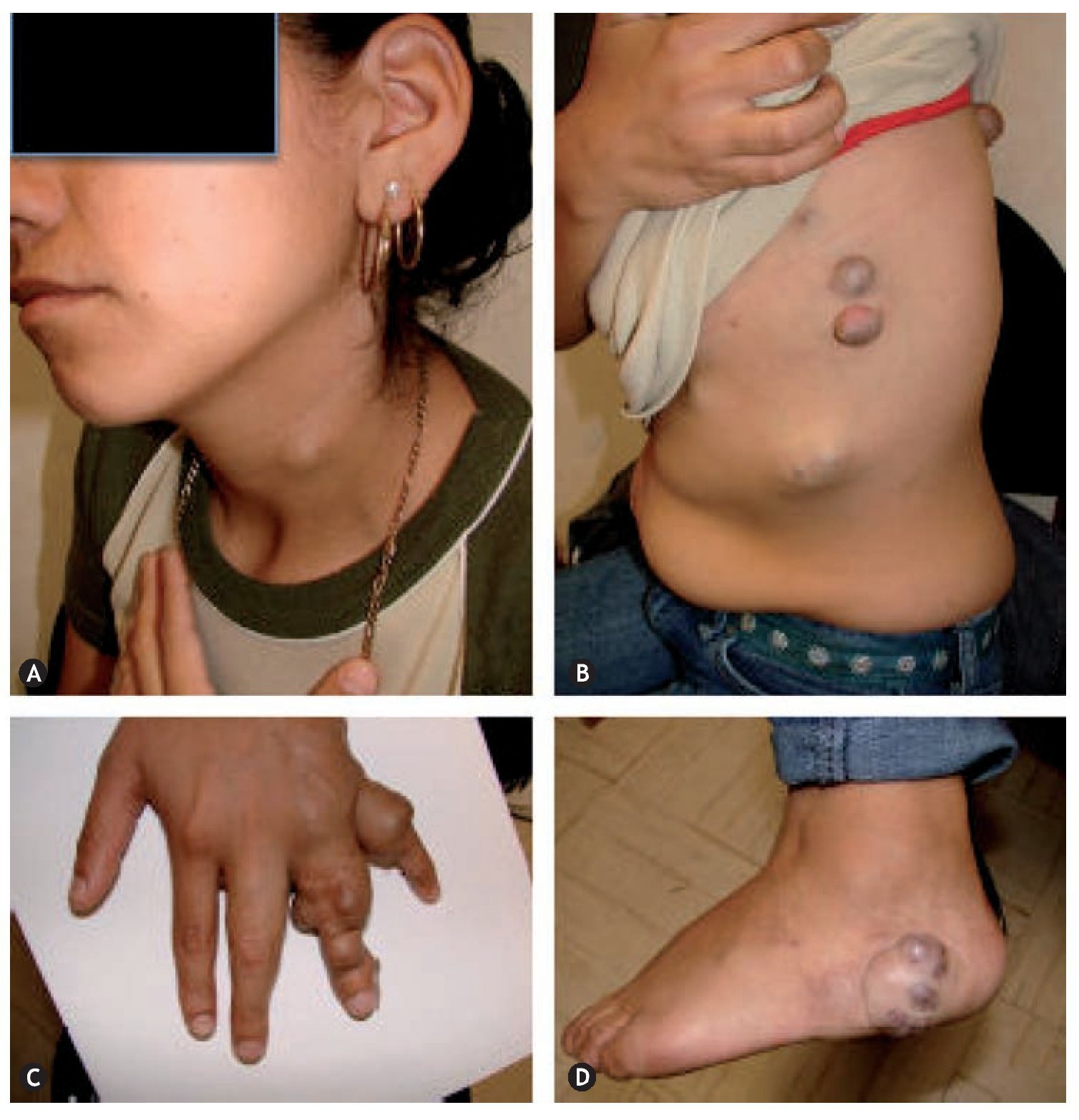
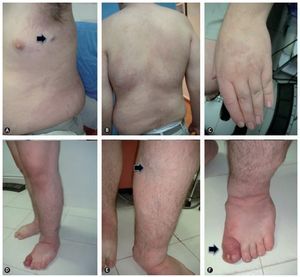
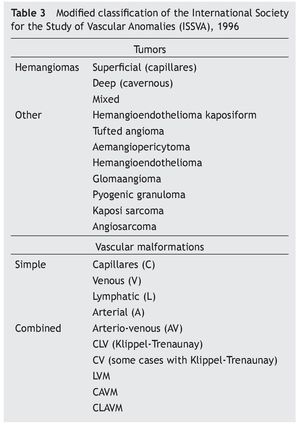
Figure 1 Klippel-Trenaunay syndrome. A: capillary malformation (port-wine stain: diffuse, irregular borders and dim color) on the lateral face of the thorax and abdomen, lymphatic cyst on the axillary region. B: scoliosis and capillary malformation on the back of the chest and abdomen. C: increase in the volume of the fingers secondary to lymphedema. D: hypertrophy and lymphedema of the lower extremities. E: venous malformation in the medial aspect of the upper third of the left leg. F: acute complication of lymphedema: onicocriptosis and cellulitis of the first toe.
After the creation of the Hamburg and Mulliken-Glowacky classifications, both with advantages and disadvantages in its use, the International Society for the Study of Vascular Anomalies (ISSVA) simplified CVMs classifications in 1996, furthermore, adding a vascular tumors section. This classification is revised every 2 years (table 3).14,15
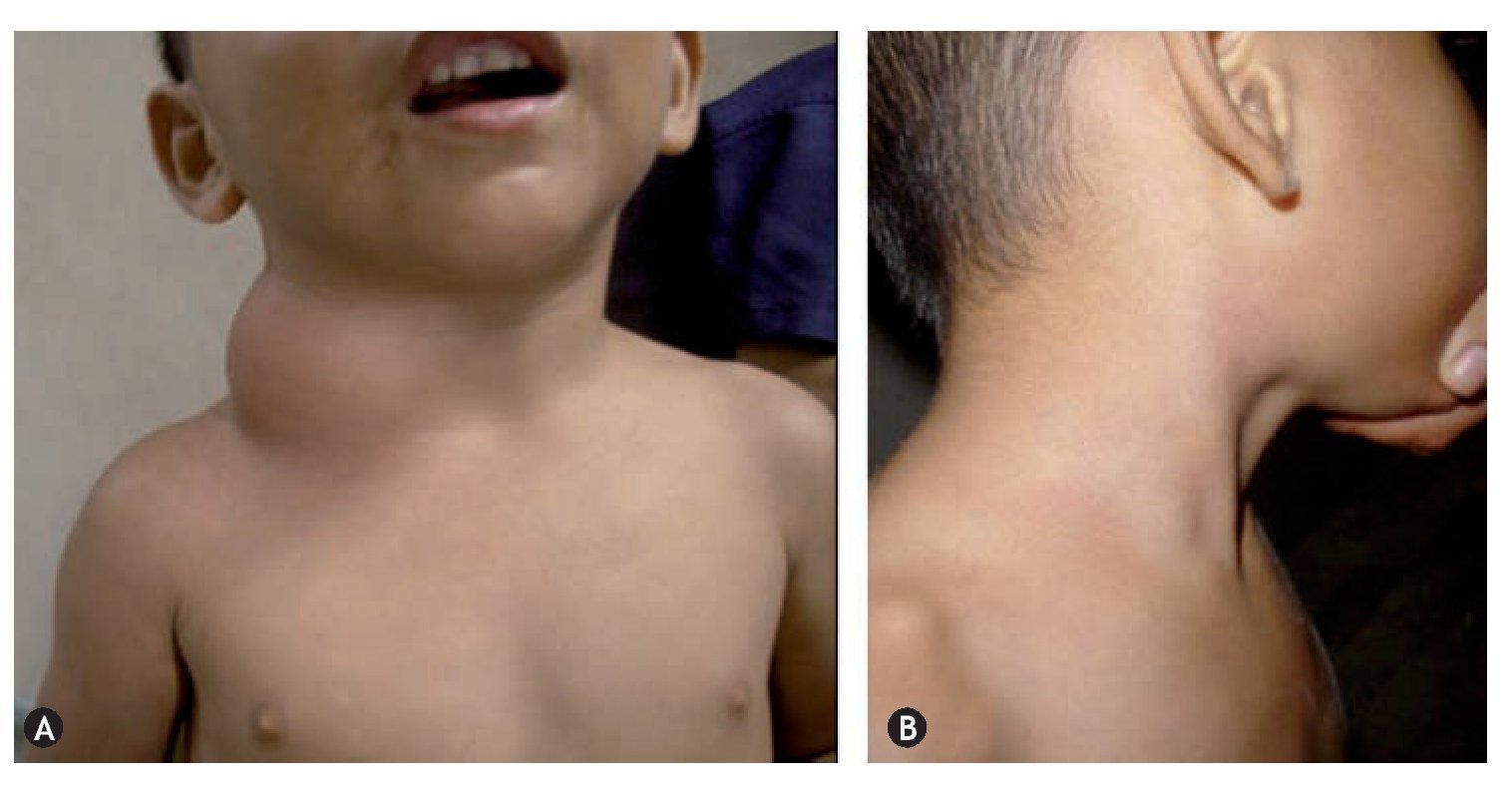
In the new classification, the components of the Klippel-Trenaunay syndromes are defined as CVM constituted by CM, VM, and LM, however, in conjunction they are referred to as hemolymphatic malformation (figure 1), those from Maffucci syndrome like VM, LM and enchondromas (figure 2) and Parkes-Weber syndrome like MVM constituted by CM, VM, LM, and VAM.
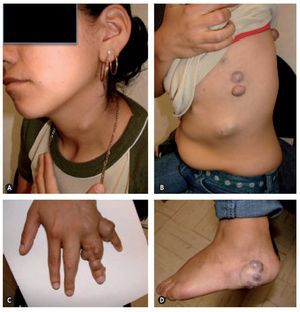
Figure 2 Maffucci symdrome. A: venous malformation (VM) on the lateral side of the neck. B: VM cysts, clustered in the anterior, lateral and posterior of the thorax. C: VM depressible, bluish, in the form of grape bunches which empty upon raising the hand and enchondroma in the phalanges. D: VM on the external side of the left heel.
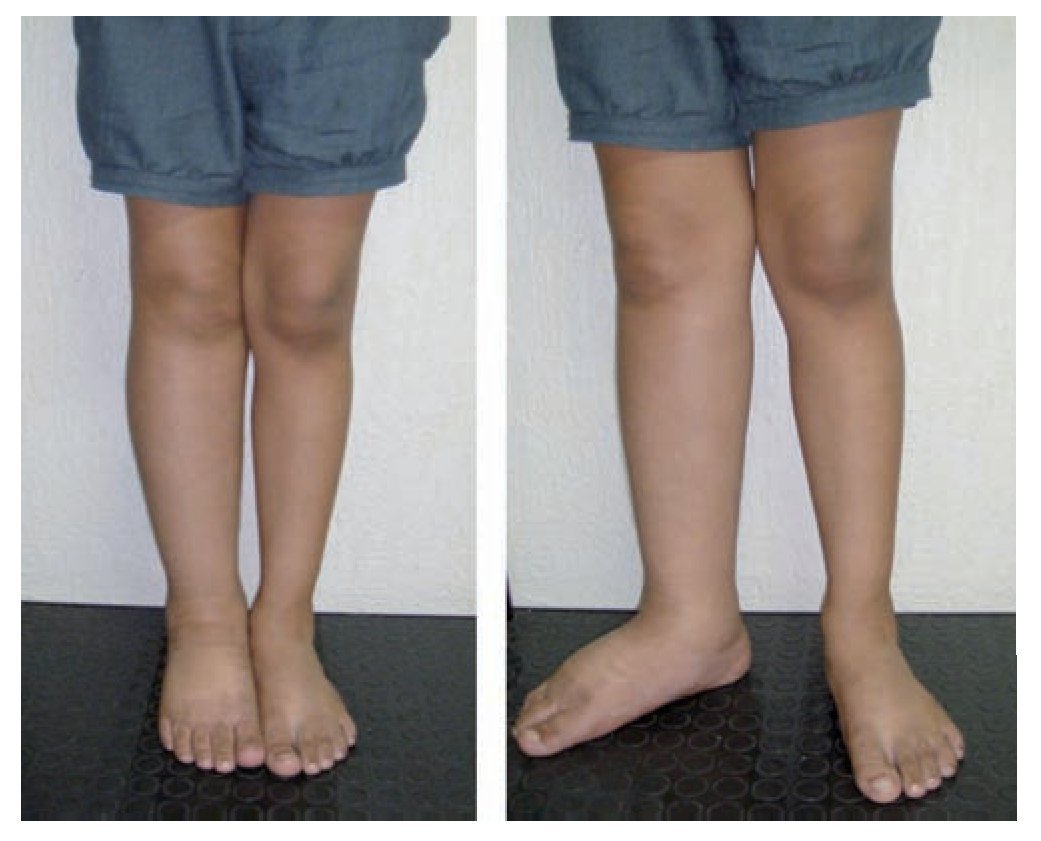
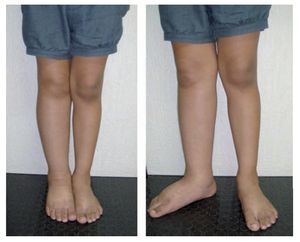
Figure 3 Early-onset primary lymphedema of the right lower extremity in a 10-year-old female patient.
On the other hand, Puig et al16 classified venous vascular malformations in function of their anatomic location, where they drain and their hemodynamic characteristics, a result that is especially useful to assess the efficacy of sclerosis therapy. In order to define the lesion’s hemodynamic characteristics, a phlebography must be performed, and with the results from this procedure venous malformations are divided into four types: I. isolated malformations without peripheral drainage; II. malformation that drains into normal veins; III. malformations that drain into dysplastic veins, and IV. malformations that drain into dilated veins.
Types I and II are the easiest to treat and have a better response to sclerotherapy.
Epidemiology
CVM incidence is 0.3-1.5%; it is high compared to congenital heart diseases (0.88%) or spina bifida (0.2%), but lower than hemangiomas, which have an incidence of 2-3% in newborns and increases to 10% at the end of the first year of life.5,17,18
Distributions between the different CVMs are as follows: venous malformations (VM) 48.5%, arterial malformations (AM) and arterio-venous malformations (AVM) 35.8%, lymphatic malformations (LM) 10%, and mixed combined malformations (MCM) 5.7%. Regarding CVM subtypes, the most frequent are extra-troncular, corresponding to 57%. In the distribution by gender, male-female ratio for VM is 1:1.2, VAM is 1:4 and LM is 1:1.
Capillary malformations (CM) appear from birth, MVs are present from birth, but become visible and symptomatic during childhood or later, MLs can manifest from birth up to adulthood (figure 4) and VAMs appear fundamentally in childhood and adolescence (figure 5).
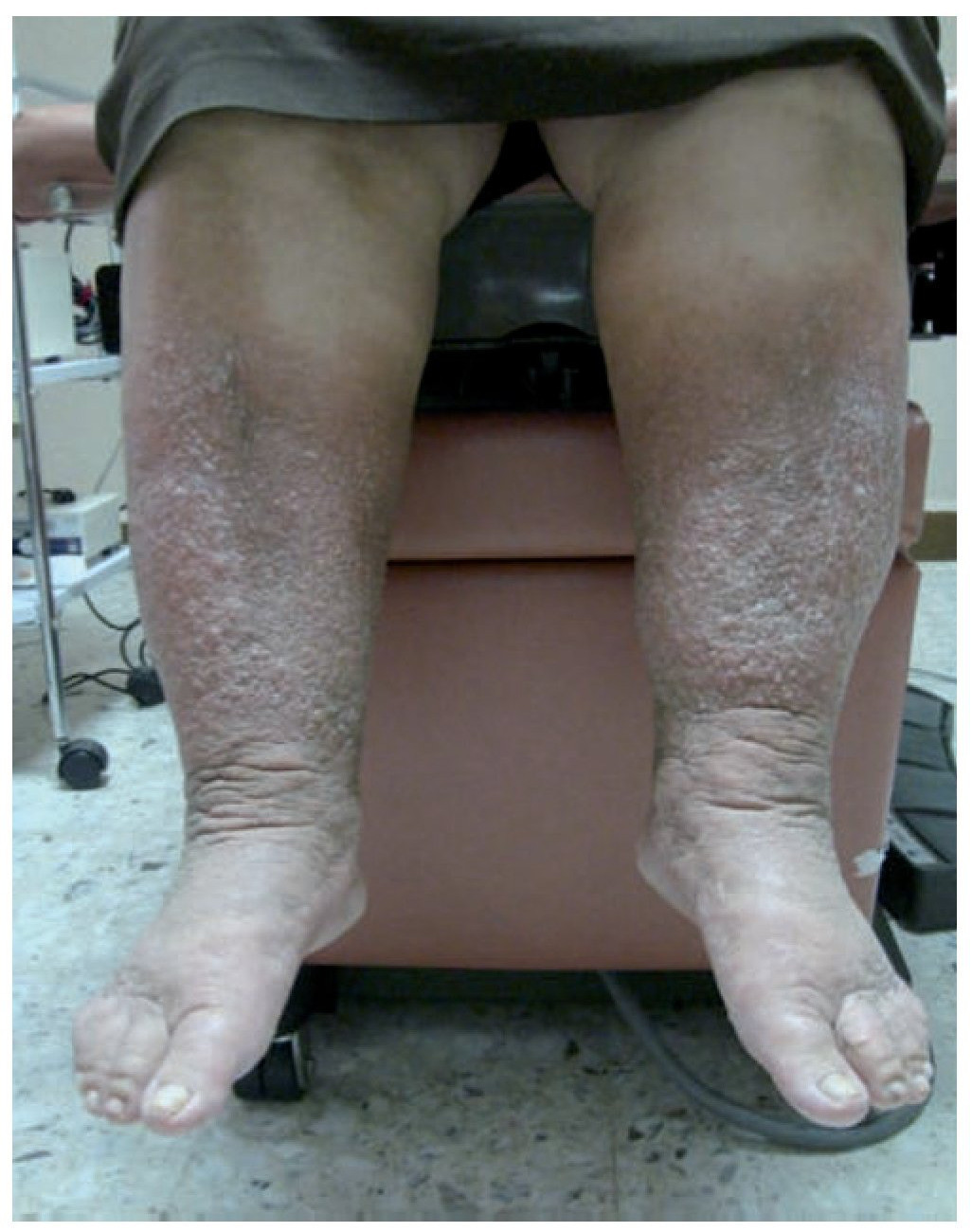
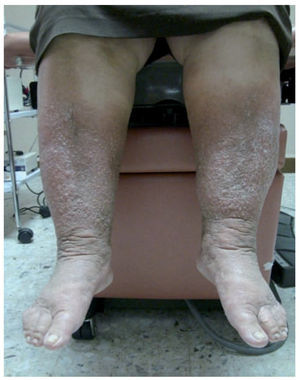
Figure 4 Late-onset primary lymphedema of both lower extremities with the presence of chronic skin changes in a patient of 62 years.
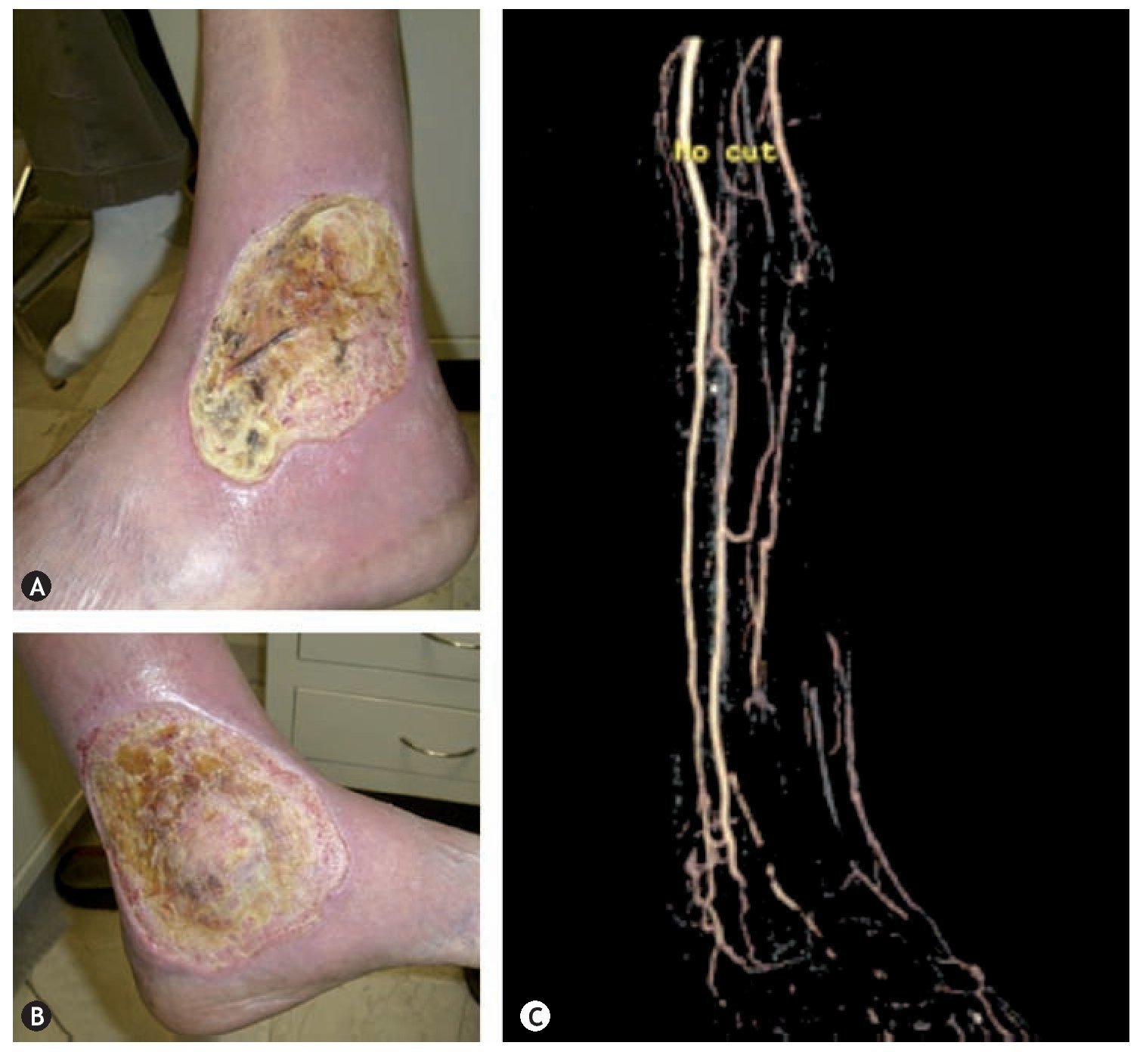
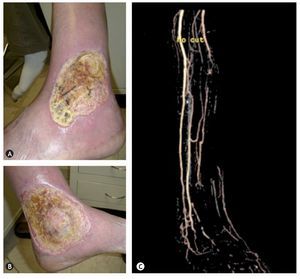
Figure 5 Arterio-venous malformation in a patient of 57 years which caused ulcers in the lower left extremity in the external malleolar (A) and medial (B) region which was accompanied by refractory pain to medical treatment and the diagnosis of AVM was confirmed by ultrasound and angio-MR (C).
Regarding their location, troncular malformations are more frequently observed in the limbs and extra-troncular malformations in the head and neck.19
Genetic aspects and pathophysiology
Vascular malformations are benign, non-tumor lesions, always present since birth, although sometimes not visible until weeks, months or years later.20,21 They are diffuse or localized mistakes in the embryological development, usually attributable to sporadic mutations. However, recent evidence points to a possible family hereditary factor. Kikkula et al22,23 found a mutation characterized by an increment in the activity of the tyrosine kinase Tie2 receptor (tunica intima endothelium kinase 2) in two families with venous malformations. Tie-2 is essential for early vessel development and an increase of its activity may produce an abnormal growth of the primary vascular plexus.24 The same workgroup has also described glomulin implication in patients with venous malformations (glomuvenous).25 Furthermore, some genetic mutations are known in cerebral cavernous mutations26 and in combined vascular malformations of the Klippel-Trenaunay-syndrome and Proteus syndrome type.28 Along the same lines, alterations in vascular neurological modulation have been described in at least two types of vascular malformations.29,30 Thus venous malformations are probably caused by a relative or absolute deficiency in the autonomic enervation of post-capillary venous plexus, while arterio-venous malformations may be caused by the same alteration, but at precapillary sphincter levels.31
In order to explain the etiology of a CM —which appears like a port-wine stain on the skin—, the term “sick dermatome” has been coined, for which a lesion is caused by an absolute or relative deficiency of the autonomic and sensory vascular innervation which originates the growth of the affected vessels, being able to reach the typical cobblestone aspect. If the defect is total, the changes will be faster as time goes by. The histological study of these hypertrophies has proven diffuse hamartomatous changes, also affecting blood vessels, epithelial connective tissue and neural skin elements, which suggest the existence of somatic mutation in the implicated area.32 On the other hand, precapillary sphincters in charge of regulating blood flow may have an autonomic innervation defect or some deficiency of the neuroreceptors in this location and be the cause of the origin of arterio-venous malformations. Depending on whether the defect is absolute or relative, the age of presentation and progression of evolutions will vary. Contrary to hemangiomas, vascular malformations do not have a growth cycle or subsequent spontaneous regression, but they persist forever and slowly grow throughout life, sometimes in relation with trauma, changes in blood or lymph pressure, infectious processes, hormone changes, etc. In these lesions it is characteristic for a vascular ectasia to be produced progressively, increasing the diameter but not the number of the vessels and expanding through hypertrophy, but not by hyperplasia, like hemangiomas do. In either case, this classical concept can be open to modification, because it presents several exceptions. For example, arterio-venous malformations frequently grow by hyperplasia and they may behave like authentic tumors. Despite the fact of an apparent endothelial quiescence, some vascular malformations may expand rapidly during pregnancy, after a surgical treatment or in response to a trauma. The pathogenesis of vascular malformations is not yet clear but its formation and progression are extremely related with angiogenesis.
Angiogenesis constitutes a complex process regulated by many factors which lead to the formation of a new functional vascularity, and includes endothelial cell differentiation, murals (pericytes), cellular proliferation and migration and the specification of blood vessels, venous or lymphatic.33
Even though to this day we do not know of a study that proves the existence of an angiogenic ceric profile in an adult patient with vascular malformations, it can be inferred, at least in theory. Marler et al34 observed levels of metalloproteinase and bFGF (basic fibroblast growth factor) in the urine in children with hemangiomas and vascular malformation in comparison to a control group. Another essay shows increased bFGF serum levels in a patient with glomangiomas.35 Moreover, some clinical observations and molecular studies proved that cerebral arterio-venous malformations present angiogenesis and vascular remodeling.36 All those findings suggest a significant role of angio-genesis in the development and maintenance of vascular malformations, perhaps not as abrupt and limited in time as it occurs with the proliferation phase of hemangiomas,37,38 but not negligible at all when it comes to explaining the pathophysiology of these lesions.
Clinical presentation
A CVM’s clinical presentation depends on the type of affected vessel, the location of the anatomical malformation and whether it is localized or diffuse. As previously mentioned, CVMs can affect arteries, veins, lymphs or capillaries independently, or appear affecting two or more types of vessels, these are called mixed lesions and are particularly named accordingly to the affected vessels (i.e. hemolymphatic malformation). The variety of clinical presentations is diverse and countless. In many cases clinical history and physical examination are enough to establish a diagnosis, nevertheless, on other occasions and especially among the pediatric population, it is very hard to stablish a correct diagnosis.
Capillary malformations
CMs are typically presented as port-wine stains on skin; they can be located in any part of the body, their way of presentation varies from small and localized lesions to extensive lesions covering several areas of the body and may infiltrate deep tissues.
CMs may be accompanied by other vascular malformations and complex syndromes, like Sturge-Weber, which is the association of a CM of the face accompanied by ocularvascular malformations and ipsilateral leptomeninges. CMs should be differentiated from lesions like nevus flamemeus neonatorum, also known as “salmon patches”; these are pink lesions located on the nape of the neck, forehead, eyelids or lips (“angel kisses”).39 In order to explain the presentation of CMs, the term “sick dermatome” was coined, thus a CM is caused by an absolute or relative deficiency of the autonomic and sensory vascular innervation at this level which originates hypertrophy of the affected vessels, possibly reaching the typical cobblestone aspect. Port-wine stains affect one or more facial dermatomes determined by the trigeminal nerve branches. V2 dermatome is the most affected (57%), followed by mandibular V3 and ophthalmic dermatome. They can be affected in an isolated or grouped manner.40
Venous malformations
VMs are the most frequent CVM, are low flow, slow growth, localized or diffuse and in general of sporadic presentation. VMs are presented as soft, ballooned, compressible, bluish lesions located superficially in the skin layers which lose their volume when the affected limb is raised, and increase upon dependence or with Valsalva maneuvers. At touch (palpation) there are no increases in temperature, thrill cannot be felt, and unless there is a thrombosis they are not painful.41 VMs can affect the skin, subcutaneous cellular tissue, muscles, limbs and any organ. Depending on their size, location and the patient’s hormonal state VMs can cause moderate to severe pain. When they affect lower limbs they can cause weakness, muscular hypertrophy or hypotrophy, bone growth disorders, pathologic fractures and mild to moderate morning pain or rigidness, which improves with compressive therapy.42 When knee articulation is affected, this becomes susceptible to lesions caused by minor trauma, manifesting itself with repetitive hemartrosis, resulting in ankyloses or degenerative arthritis.43 Migraines are a common characteristic of VMs located on the face or temporal muscle. When they affect abdominal muscles, airways, or the central nervous system they may become complicated, with symptoms provoked by bleeding, obstruction or compression. VMs located in the head and neck are usually presented in a unilateral way, provoking a mass effect causing distortion and asymmetry of facial structures.44 When they affect the tongue, palate and oropharynx they result in a significant deformity, causing dental malocclusion and if it invades airways it can cause life-threatening lesions.45 Any part of the digestive tract may be implicated, it can be presented as nodular, sessile or polypoid lesions which provoke chronic bleeding. In troncular venous lesions which manifest as venous dilation or real aneurysms, there is a thrombophilic risk, therefore the presence of venous thrombosis and pulmonary embolism is common.46,47
Lymphatic malformations
Since the establishment of the HC, the LM denomination has replaced the older names of cystic capillary hygroma and microcystic and cavernous lymphangioma, which correspond to extratroncular lesions and manifest as a benign mass, consisting of thin vessels and cystic structures of different sizes, well-defined, of a soft-spongy consistency, which are usually asymptomatic as long as there are no complications (i.e. intralesional bleeding, infections, lymphorrea),48 and are of great clinical interest because their growth may compromise the organs where they are located. They are mainly located in the head and neck and armpit, but also can be found in the mediastinum, limbs and retroperitoneum. They constitute 5% of benign masses in childhood, up to 60% of the cases can be observed from birth, 90% before 2 years of age and the rest can appear prenatally or suddenly in ulterior stages of life but usually before turning 16 years old.49,50
When they affect the oral mucous, tongue, upper or lower maxilla or neck they can also cause esthetic sequels, dyspnea, dysphonia, dysphagia and bleeding, in addition to endangering the patient’s life.51,52 If it affects the ocular region, proptosis, strabismus, or loss of vision may occur.53
Extratroncular LM can also directly or indirectly affect bone metabolism (Gorham syndrome), which causes bone over-growth and pathological fractures. Troncular LMs manifest as primary lymphoedema, of premature, early or late onset54 (figures 3 and 4).
Arterio-venous malformations
AVMs correspond to a vascular system anomaly which results in an arterio-venous shunt, which may go unnoticed or cause cardiac failure.55 The majority of AVMs are detected in patients between 20 and 40 years of age. The vessels of which AVMs are constituted consist of a thin wall, very fragile and fed by dilated arteries, which have in their walls a deficiency of elastin, connective tissue and smooth muscle.56
Most AVMs occur in independent lesions but these can also be linked to other CVMs making its diagnosis and treatment more complex.57 AVM incidence is much less than VM or LM but are hemodynamically more complex and more commonly endanger the patient’s life.4 They differentiate from other CVMs because of their varied clinical presentations, unpredictable natural history, high recurrence rate and elevated morbi-mortality.
AVMs in soft tissue manifest as a located volume increment, accompanied by a rise in local temperature, thrill presence, murmur and dilated veins (characteristic triad of AVMs). Ischemic changes and skin ulcers may occur distal to an AV short circuit, usually accompanied by refractory pain to medical treatment and intermittent bleedings (figure 5). AVMs may occur in any part of the body, however, over 50% are located in the brain or spine. Those located in the central nervous system can appear in the clinical setting as a stroke with clinical manifestations that depend on where the lesion is located.58
Diagnosis
In order to make a proper diagnosis and be able to offer the ideal treatment, we can use the following questions as a guide: Is it an hemangioma (vascular tumor) or a CVM?, what type of CVM is it?, what is the anatomic location?, and which hemodynamic alteration does it cause?
To be able to properly answer these questions, it is necessary to first have the knowledge of the different CVMs and vascular tumors so that through clinical history and physical examination we will be able to have a diagnostic orientation and subsequently choose among the different complementary tests to confirm suspicions.
The objective of a correct diagnosis is to define the type, location, extension and hemodynamic alterations of a CVM, in addition to choosing the best therapeutic option and follow up on its evolution. Diagnostic tests can be divided into: a) functional tests, useful to evaluate the physiology and hemodynamic of lesions, Doppler ultrasound and plethysmography are among these studies; b) anatomo-functional tests represented by the echo-duplex which is the first option for a CVM assessment, and can provide information of the characteristics of the lesion and its hemodynamic behavior, and c) imaging studies, which include simple x-rays, MRI, CAT scans, angiography, phlebography, lymphangiography and nuclear medicine studies like lymphangio-scyntillography and full body gammagraphy, among others.
Ultrasound
The first test to be considered in the evaluation of a patient with suspected CVM is the duplex ultrasound.59 It has the advantage of being easy to access and provides information about the anatomy of the lesion as well as its physiology. In addition, it is used for echo-guided endovascular treatments. This test allows for an immediate differentiation between low-flow (VM or LM) or high-flow lesions (AVM). VMs are shown as hypoechoic (82%), hyperechoic (8%) and isoechoic (10%).60,61 Occasionally phleboliths (as acoustic shadow) appear, which are pathognomic of the VMs. VMs flow in most of the cases appears as monophasic or biphasic (84%), and it increments after the completion of compression maneuvers; up to 16% do not present flow. It is important to determine whether or not there is a venous flow in the system (superficial or deep), as well as determine the anatomical characteristics of both systems and not to forget to assess the lateral side of the thigh where the marginal vein is usually found.62 We are able to see phleboliths which are round, non-compressible masses and with acoustic shade.63 In LMs echography is the perfect technique for the assessment of superficial lesions. In addition to being low flow, they can appear as macrocystic or microcystic lesions, appearing as hypoechoic or anechoic, and multiloculated with septa of variable thickness. Microcystic lesions with or without flow can be confused with VMs.64
AVMs appear as lesions with high systolic flow and are hyperdense because of the hypertrophy of the affected vessels. An ultrasound is also effective to assess the damage in soft tissues and muscles.65
Magnetic resonance
If the clinical history and the ultrasound indicate a low-flow CVM, magnetic resonance imaging (MRI) is the next study that should be performed, and if the diagnosis indicates high-flow, the angio-MRI and CAT scan are the indicated studies.66,67
MRI provides an excellent tissue differentiation that, along with the ability to obtain images in multiple spatial planes, makes it the best radiologic examination and a first choice study to demonstrate the anatomical relations and study surrounding tissue in contact with vascular malformations. Besides providing anatomical information, it is also capable of providing hemodynamic data, due to the phenomenon in which the MRI technique is based, the presence of fast or turbulent flow will condition a decrease in the signal’s intensity, while in those cases where there is a low flow or thrombosis the intensity will increase.68,69
VMs commonly appear as lobular lesions (single or multiple), displaying high and homogenous intensity, of a serpiginous structure or like infiltrate masses; phleboliths appear lacking a signal (Void sign).70
In LMs, it is not possible to observe a contrast, except for in the microcystic components or in the case of complex malformations of the lymphatic-venous type.71 AVMs, different from the rest, lack a solid component. The MRI studies reveal characteristically the presence of high-flow vessels shown as serpiginous morphology zones with a lack of signal in T1 as well as T2 sequences.17
The use of angiographic sequences allows for the detection of vascular structures and even the creation of a three-dimensional angiographic map when they are combined with the MIP (maximum intensity projection) reconstructions.72 MRIs are of a great value when it comes to the analysis of an AVMs extension, as well as the degree of invasion of the anatomically affected structures.73
Computerized axial tomography
One of the advantages of the computerized axial tomography (CAT) scan is the excellent demonstration of bone structures and calcifications it provides. Nevertheless, soft tissue definition is clearly lower than that offered by MRI studies. Among the disadvantages are that it is a technique based on the use of ionizing radiations which almost always needs to use contrast medium, that do not turn out to be innocuous due to their nephrotoxicity and possible adverse reactions, which on occasions can be severe.74 Also, in the case of pediatric patients, the use of sedation becomes necessary because the quality of the studies seems clearly diminished by movement. Even though the hemodynamic information provided by CAT scans is very limited, nowadays with the use of the new multislice equipment, which allows very fast image acquisition, it is possible to perform multiplane reconstructions of great quality as well as angio CAT studies. In the case of venous malformations, a CAT scan can show the presence of calcifications in relation to phleboliths and alterations in surrounding bone structures.
In spite of the administration of intravenous contrast medium, using this imaging technique may result in a difficulty delimiting the lesion in relation to the adjacent muscular planes. The lymphatic malformations appear as cystic lesions full of a homogenous liquid with an attenuation value similar to that of water, although in the lesions with liquid content rich in lymph, the attenuation is closer to that of fat. Cases that have been complicated by an infection may have a more heterogeneous density. Frequently, the walls of the cysts show a certain degree of enhancement after administration of an intravenous contrast.75 The CAT scan is also capable of showing anomalies in the bones related to arterio-venous malformations, although it does not give us specific information on vascular structures in relation to adjacent tissues.
Simple radiography
Simple radiography (SR) is useful for detecting the secondary effects of CVMs in bones and soft tissue (e.g. osteo-vascular syndrome, Gorham syndrome). An SR can detect calcified phleboliths, typical of venous vascular malformations. It can also evaluate bone changes (cortical thickening, demineralization, or osteoporosis) which can appear in up to 20% of extensive vascular malformations of the limbs.76
It develops in patients with extensive CVMs in their extremities, permitting measurement of their length and knowing exactly if dysmetry exists between either of them. Clinically, it is only possible to detect differences in length between 0.5 and 1 cm, which means that to evaluate lesser distances, we need to employ these imaging methods. On the other hand, it permits us to determine the rate of growth of each extremity, know if the dysmetries are progressive or stable and evaluate the best moment to perform the necessary therapeutic treatments to equalize the length of the extremities, aspects which are not possible to evaluate clinically. These radiographic procedures are rarely necessary before 2-3 years of age, nor after skeletal maturation, because the differences in the length of the extremities does not progress past the closure of the epiphysial cartilage.77
Because of this, we recommend performing a clinical and radiological measurement beginning at 2 years of age and be repeated annually.
Venography
In a venography result it is useful to know the anatomy of the superficial and deep veins, the state of the valves, the presence of embryonic veins, the connections between the deep and superficial systems, and the degree of venous incompetence. Disadvantages are the possibility of allergic reactions and of venous thrombosis owing to the use of iodinated contrast medium.
Arteriography
An arteriography may be necessary when the presence of arteriovenous fistulas is suspected, especially those with low flow, which are difficult to evaluate with other techniques. Apart from its diagnostic value, an arteriography is the way to perform a therapeutic embolization on an AVM.
Lymphangiogram
Historically, the lymphangiogram represents the ideal study for the evaluation of the lymphatic system. It contributes information on lymphatic drainage, the size of the vessels, alternative paths, and morphological alterations. It is a technique that is very difficult to perform and the contrast medium which is used can cause complications. The directions limit the evaluation of the lymphatic vessels in case of persistent lymphoedema, or in extensive combined malformations, when the difference in the diameter of both extremities exceeds 4 cm. Although this deals with a technique in disuse because of its complexity and slow performance, it can contribute very significant information which can be difficult to obtain by other means in some vascular malformations with associated lymphatic malformations of the thoracic cavity.78
Nuclear medicine
In the evaluation of CVMs we can count on three studies of nuclear medicine: a) the total body scintigraphy (TBS) with labeled erythrocytes, which has much value in the initial evaluation, giving information on the extent, physiology, type and subtype of the CVM; it is very useful when MRI results are ambiguous;79b) linfangio-scintigraphy,80,81 which has taken the place of lymphangiography and currently is the study of choice to confirm a diagnosis in patients with suspected lymphoedema; and c) the transarterial lung perfusion scintigraphy,82 which is used to evaluate AVMs and quantify and verify arterio-venous shunts.
Treatment
Szilagyi83 said, in 1965: “no surgical treatment should be directed in the management of any CVM and it should be considered as a palliative measure only in cases where the benefits far outweigh the risks, owing to the fact that CVMs cannot be cured through any surgical procedure.” This recommendation was followed by the medical community for decades but, with a better understanding of CVMs, this concept of incurability has changed. Every patient and any type of CVM should be evaluated by a multidisciplinary team, whose consensus will determine, after an exhaustive evaluation, the treatment of choice.84
Dr. B.B. Lee85 established, in a simple and understandable manner, the instructions for the treatment of CVMs. He divided them into absolutes and relatives. Among the absolutes were found: a) bleeding; b) cardiac failure secondary to a high flow AVM; c) complications secondary to venous hypertension; d) critical ischemia of an extremity due to the arterial steal phenomenon, and e) lesions which produce compression on important organs (respiratory tract, eyes, ears, etc.). Relatives include: a) severe, progressive and incapacitating pain; b) intermittent claudication; c) functional damage which occasionally incapacitates and loss of quality of life; d) deformities with aesthetic repercussions, which cause personality disorders; e) asymmetry in the length of the extremities, and f) recurring infections.
Conservative treatment is indicated in symptomatic patients when: a) they do not conform to the above-mentioned criteria; b) the lesion is not manageable by invasive treatments due to the anatomic location, the calculated risk being higher than the expected benefits, or the patient not signing the informed consent form, and c) the patient is receiving follow-up subsequent to undergoing surgery or minimum-invasion procedures. Within the conservative management, a measure of great importance is the use of means of gradual compression (helps to slow the progression of VMs or LMs, controls pain and decreases the incidence of complications).86 Due to the thrombophilic risk which patients with a large volume VM present, the use of a prophylaxis of low-molecular weight heparin is recommended during high-risk episodes like surgery, pregnancy, or long periods of bed rest. The use of aspirin and the suspension of oral contraceptives is also recommended, which diminish the paroxysms of pain.
The main objective in the treatment of all CVMs is the correction of the hemodynamic repercussions which produced the malformation and later treatment of the soft tissue and musculoskeletal alterations which accompanied the primary pathology. The optimal combination of surgical procedures with endovascular treatments, like liquid or foam sclerotherapy or embolization and ablation by radiofrequency or laser, should be performed in accordance with embryology, on which depends the potential of recurrence of the lesion (extra-troncular lesions are infiltrative and difficult to eradicate, with a high incidence of recurrences and complications) and the hemodynamic nature of the primary lesion. The treatment of secondary lesions frequently merits the performance of orthopedic and/or reconstructive surgery.
Venous malformations
As mentioned above, VMs are subdivided into two types: extra-troncular, which are infiltrative, with a little or great superficial component, generally cause a lot of pain and have a complex treatment; and the troncular type, which manifest as more superficial lesions that could be mistaken for venous hypertension symptoms secondary to reflux, present discomfort such as a feeling of heaviness in the legs, cramps and mild to moderate pain. Its treatment is simpler. We must acknowledge that both VM types can be present in the same patient. Conservative treatment is recommended for most VMs without bone involvement, in pediatric patients up to the age of 2 to 3 years old, where the patient is in a condition to tolerate the necessary diagnostic and therapeutic procedures, keeping in mind that in the case of a lesion which endangers the patient’s life or causes asymmetry of the extremities or bone deformities, the therapeutic approach would be more aggressive from an early age.
VMs can be treated through endovascular therapy or surgery. Both have their advantages and disadvantages. The complex nature of VMs makes the treatment more difficult. Surgery has a limited usefulness in the case of very infiltrative VMs or VMs located in places that are difficult to reach. Additionally, because VMs can respond with rapid growth to surgical trauma,87 making a latent lesion into a more aggressive one, in most of the cases endovascular therapy is preferred.
In endovascular therapy embolization, sclerotherapy, embolization and techniques of endovascular ablation with laser or radiofrequency are used. Embolization is defined as the occlusion of a blood vessel through the introduction of a foreign body; this technique is not the most recommendable for VMs, because these are formed by very dilated vessels, which generally cannot be 100% obstructed by the different utilized agents (i.e. coils, cyanoacrylate). Also, these lesions are irrigated by many vessels, making it extremely complex to occlude each one of them,88,89 in these cases sclerotherapy is preferred, which consists of injecting a substance (sclerosant) in liquid form or foam directly into the lesion, this causes endothelial damage, inflammation, thrombosis and fibrosis resulting in obliteration (sclerosis) of the treated vessels.90,91 Sclerosant agents used in VM treatments include: ethanol,92,93 sotradecol,95 Ethibloc96 and ethanolamine oleate.97 Ethanol, which has been used for over one hundred years, is considered the most effective of the sclerosant agents but also the most aggressive and with a higher incidence of local (pain, skin necrosis, compartmental syndrome, cellulitis, nervous lesion) as well as systemic (pulmonary hypertension, arrhythmias, nervous depression, pulmonary embolism, etc.) complications (7-27%),98 which may put the patient’s life at risk. For these reasons, in North America the use of sotradecol is preferred; in Europe polydocanol is the agent of choice and in Mexico, the latter is the only one available. Sclerosant agents can be administered using an injection or with the placement of a catheter, guided with the help of an ultrasound, MRI or CAT scan.99,100 Success rates for these techniques varies from 60 to 90%.101-103
Many VMs located in the pelvis or lower limbs cause venous reflux to the superficial venous system, causing complications by venous hypertension. Treatment with laser or radiofrequency is indicated improving the patient’s symptomatology and avoiding recurrences.104,105
Surgical treatment for VMs can be performed following different therapeutic guidelines:106,107
I. Surgery to reduce the hemodynamic effects of VMs (venous hypertension). This approach is not radical and it is indicated when it is not possible to perform reconstruction or completely eradicate a VM; with this technique a considerable reduction in venous stasis and venous hypertension is accomplished, considerably improving the condition of patients who were considered inoperable.
II. Surgery to completely eliminate VMs. This type of surgery is indicated in peripheral lesions secondary to extra-troncular VM, generally obtaining acceptable results and it is indicated before suggesting a reconstructive orthopedic surgery. This is a radical surgery, following the Malan method applied in oncologic surgeries.108
III. Reconstructive surgery. This technique is rarely applied in VMs due to its infiltrative nature and the pathological structure of the vessel wall. It may be indicated in venous defects localized in an organ or lower limb, causing obstruction of the aneurismal vessels.109,110
Lymphatic malformations
Treatment for troncular LM clinically presented as primary lymphoedema is usually conservative. It consists of what is known as lymphatic decongestive therapy which includes lymphatic drainage, either manual or with the use of pneumatic compressors, physical therapy exercises, profilactic measures in order to prevent complications and the use of graded compression measures.111 When LM anatomy is adequate, reconstructive surgeries can be performed, like venolymphatic or lymph-lymphatic anastomoses or a lymph node transplant using microsurgery techniques, which improve the volume of the affected limb and the patient’s quality of life.112-114
In the case of extra-troncular LM, most appear as independent lesions called lymphangiomas. Treatment is indicated in cases where the LM is located in areas which compromise a vital function, or if there is bleeding, recurrent infections, lesions with lymph drainage or lesions with VM components. The first line of treatment for these types of malformations is the use of sclerosants via endovascular treatment. OK-432 sclerosant, also known as Picibanil, is the option to start extratroncular LM therapy. OKK-432 is the lyophilized exotoxin of the Streptococcus pyogenes type III of group A.115 This lymphatic sclerosant acts over the congenital malformation through a cascade of antitumor agents secreted by neutrophils, macrophages, natural “killer” lymphocytes (CD56+) and T cells (CD3+) which activate the immune system, causing an increase of the endothelial permeability, drainage and lymph flow, resulting in the reduction of the lesion by contraction of the cystic spaces.115,116 Macrocystic lesions are easy to inject, with excellent results and a low morbidity (figure 6). On the other hand, microcystic lesions in many occasions are impossible to inject. Furthermore, this type of lesion usually drains into the normal lymphatic system, possibly damaging it. In both cases more than one therapeutic session is needed. In case OK-432 is ineffective, ethanol will be used. Ethanol is an excellent sclerosant with few recurrences, but with a higher morbidity. It is recommended only for macrocystic lesions.102 Bleomycin is an antitumor cytotoxic antibiotic with a double effect: on the one hand it induces ADN15 degradation and on the other it displays a sclerosing effect over the vascular endothelium. Because of this, it has been given an intralesional use in LM.119,120 Posterior to the sclerotherapy session, hospitalization is recommended in pediatric patients. Also, there have been reported cases using sildenafil (orally) with good results.121
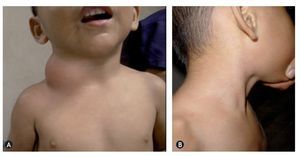
Figure 6A: male patient of 6 years with a macrocystic lymphatic malformation on the side of the neck (cystic hygroma). B: 2 months after treatment with OK-432.
Surgical treatment of LM is limited to cases where sclerotherapy was unsuccessful, or in the case of microcystic lesions associated with other CVMs (mainly venous) too difficult to treat with sclerosant.54,122 Ideally, radical surgery should be performed, in order to avoid recurrences. However, in areas involving important structures, a partial re-section is acceptable. The ideal time to perform surgery is as yet unclear, but it is recommended to delay the procedure as much as possible.
Laser treatment of LM as endovascular or transdermal procedures with a laser with a wave length of 1470 nm, has been reported by some authors,123 being a minimally invasive procedure, as a valid therapeutic alternative against conventional therapies, performed as a single procedure or associated with other therapies.
Arterio-venous malformations
Out of all CVMs, AVMs are the ones with the more complex treatment and when presented in combination with other types of CVM, are the ones with priority in treatment. The objective of the treatment is to completely eradicate the malformation, or at least reduce its hemodynamic effects. Available alternatives for AVM treatment include: embolization, sclerotherapy, surgery and laser therapy. The treatment of choice for AVMs nowadays is still surgery; nevertheless, surgical therapy only works in an incomplete treatment linked to a high morbidity. Thus, unless the AVM is localized and easily removed, surgery should be combined with an endovascular approach, using sclerotherapy or embolectomy.124,125 Endovascular techniques were at first introduced by the treatment of lesions not curable with surgery, but once good results were shown in this type of lesion, they decided to combine it in most AVM cases, either in the preoperative (in most cases), trans-operative or postoperative period, obtaining better results and fewer recurrences and morbidities.126 The utilized sclerosants are the same as in VMs and in the same manner, ethanol is the most effective yet risky (see venous malformations).
The moment of surgery is important because the more mature the lesion is, the more difficult it is to complete a full excision. Therefore, resection should take place as soon as possible and should be as complete as possible. Resection margins should be wide regarding the limits of the lesion. The use of preoperative magnetic resonance and digital angiography, especially evaluated dynamically, are particularly useful to establish these margins.
Capillary malformations
Treatment is indicated in all symptomatic lesions and those that are visible that may cause emotional stress. Among the CM treatments are: a) laser photocoagulation, which is first choice of treatment; the objective of the laser therapy is selective thermolysis with the wavelength which produces the most lightening with the least number of sessions and lower morbidity; it is useful as long as the lesion is flat and better results are obtained when the patient is younger and with a lighter skin; greater amounts of melanin will reduce the possibility of a response to the laser; b) camouflage; makeup techniques may play a significant role as well; it is useful as long as the lesion is flat; c) cryosurgery: this is reserved for those cases where the lesion presents hypertrophy and skin with a nodular aspect or presence; in these cases, laser loses its effectiveness while cryosurgery increases it in reducing the thickness of hypertrophied skin and eliminates nodules, and d) other treatments employed in flat lesions, like steroids, radiotherapy, cryotherapy and electrocoagulation, have had limited success.
Mixed malformations
In this CVM group, the priority is to first treat the AVM component (as mentioned throughout the article) if there is one. A coexistence of VM and LM is the most typical combination.128 When dealing with mixed lesions we are able to apply the previously mentioned principles for venous and lymphatic malformations, modifying them according to the degree of implication of each of the systems. The venous component is treated first because an alteration in the functioning of the lymphatic system can occur because of the interrelation of both systems; in other words, to treat the venous component of mixed malformations like Klippel-Tranaunay syndrome vigorously, the patient, which depends on its lymphatic component, may worsen notably. Hence, the most appropriate treatment choice becomes relevant. In order to plan the therapeutic strategy, the physician must know in detail the type of malformation, anatomic location and hemodynamic composition, in order to prevent complications and ensure positive results.
Conclusions
The Hamburg Classification was a major advance in the understanding and management of CVMs. However, it is far from perfect and it will be modified in the future according to the advances in knowledge of etiology, anatomy, embryology, histology, pathology, physiology, hemodynamics and genetics.
Diagnostic and therapeutic approach must be performed by a multidisciplinary group and not just by the surgeon. Ultrasound and MRI are the diagnostic tools of choice for the initial study of CVMs, subsequently complemented by a diagnostic approach according to each particular case. Surgical treatment of lesions is currently considered the gold standard. However, it is recommended to combine it with endovascular techniques like sclerotherapy or embolotherapy. Extra-troncular CVMs are the most difficult to treat, with more recurrences and a poorer prognosis.
Received: March 2014;
Accepted: June 2014
*Corresponding author:
Servicio de Cirugía Torácica y Cardiovascular,
Hospital Universitario “Dr. José Eleuterio González”,
de la Universidad Autónoma de Nuevo León,
Ave. Madero y Gonzalitos s/n,
CP 64460 Monterrey, Nuevo León, México.
E-mail address:drfrendon@gmail.com (F.G. Rendón Elías).