Trichosporon spp. has gained importance as the cases of immunosuppressed patients increase. The genus Trichosporon includes 6 species of clinical relevance that may cause superficial infections, such as white piedra and onychomycosis, or deep and invasive infections with high mortality rates. These microorganisms have a broad geographical distribution and some species are resistant to antifungal drugs in vitro. The present paper is a review on the virulence factors, associated infections, and in vitro susceptibility of the species with the highest incidence as pathogenic agents in humans.
Introduction
Trichosporon spp. was first described by Beigel in 1865 as the agent responsible for a benign hair infection called white piedra (meaning "stone" in Spanish). Beigel classified the microorganism as algae and placed it in the genus Pleurococcus. It wasn't until 1890 when Behrend identified the microorganism causing white piedra as a fungus which he called Trichosporon ovoides.1 Although generally associated with this superficial infection, Trichosporon spp. has gained importance as one of the most opportunistic systemic infections since the first case of invasive trichosporonosis involving the brain, reported in 1970.2
Trichosporon spp.
Trichosporon spp. belongs to the phylum Basidiomycota. It is a yeast-like fungus macroscopically characterized by forming radially symmetrical colonies; these colonies may be white or yellowish, with a smooth texture, creamy, cerebriform, powdery or moist. Microscopically, Trichosporon spp. forms hyaline septate hyphae, with abundant arthroconidia and blastoconidia, and in some cases presents appressoria. Regarding physiological tests, they assimilate several carbohydrates and other carbon sources, yet they do not possess fermentation capabilities. A major characteristic of the Trichosporon spp. genus is their ability to hydrolize urea.1,3-5
In 1902 Vuillemin grouped the species described for this genus under the Trichosporon beigelii denomination, a practice that remained until the late 90's.5,6 Nevertheless, posterior classifications at morphological, biochemical, and molecular levels provided evidence of the existence of more than one species in the genus.
Approximately 50 species of the genus Trichosporon have been characterized, 16 of which are associated with diseases in humans. Guého et al. (1994) typified species that had displayed a major adaptation to the human body: T. asahii, T. asteroides, T. cutaneum, T. inkin, T. mucoides and T. ovoides. These 6 species, reclassified from the Trichosporon beigelii taxon, remain the most relevant clinical species to date.7
Virulence factors
There is little information regarding virulence factors produced by this microorganism.
Hemolysins, proteases, and lipases allow protein degradation and destabilization of the membranes of the host cell, increasing fungus pathogenicity. Dað and Cerikçioðlu analyzed the production of proteases, phospholipases, and esterases of 48 T. asahii clinical isolates, and did not detect protease nor phospholipase activity in any of the isolates analyzed; however, all the evaluated strains displayed esterase activity.8 Similarly, Sun et al. evaluated protease, phospholipase, and hemolysine production in 23 T. asahii clinical isolates, and observed no protease nor phospholipase activity, yet 100% of isolates showed hemolysin activity. This activity, however, varied for the different T. asahii genotypes identified.9 It is important to underline that there are no reports about the production of lytic enzymes for Trichosporon non-asahii species.
Phenotypic switching is a fast and reversible change of colonial morphology and/or microscopic features. These changes may occur as a response to different environmental stimuli such as oxidative stress or lack of nutrients, or intrinsic factors such as a mutation in the deoxyribonucleic acid (DNA) repair systems.10 When phenotypic switching occur in vivo, they involve modifications in the expression of virulence factors, or alterations in the microorganism-host cell interactions, thus provoking an increment in the pathogenicity and evasion of the immune response, as observed in Candida albicans and Cryptococcus neoformans.11-13 Lee et al., reported phenotypic switching in vitro for the first time for T. beigelii. The isolated microorganism, recovered from a patient with a systemic infection, was capable of producing two different morphologies when cultivated at 30°C. Such morphotypes were accompanied by their respective structural microscopic changes. Thus, a rough, cerebriform, moist colony with irregular edges was accompanied microscopically by hyphae while a second powder morphotype, with a more regular topography and borders, showed microscopically a greater proportion of arthroconidia or blastoconidia.4 The phenotypic switching was also reported in vivo by Karashima et al., who performed experiments involving repetitive inoculation of T. asahii in mice. They observed macro- and microscopic morphological differences when comparing the fungi before being inoculated and the same fungi recovered after 3 repetitive passages in 8-week-old ICR mice. Similar to the results obtained by Lee et al., Karashima observed that the rugged colony was accompanied microscopically by a greater proportion of hyphae, while a powdery colony was accompanied by a greater amount of conidia.14
In the same paper, Karashima et al. reported the production of a cell wall component similar to the glucuronoxylomannan (GXM) of C. neoformans. They observed that a larger amount of GXM was present in the recovered microorganism after repeated inoculations in mice. GXM has been proposed to offer C. neoformans protection against phagocytosis by neutrophils and monocytes.14
Another factor providing protection to the fungus is the formation of biofilms. Some microorganisms have the ability to produce an extracellular matrix, formed by polysaccharides, proteins, and DNA, which allow conglomeration of cells and their adherence to both organic and inorganic surfaces.15 Due to the association of invasive infections to the use of catheters and similar devices, the microorganism's ability to adhere itself and form a biofilm could play a very important role in the pathogenesis during trichosporonosis. In 2006, Di Bonaventura et al. assessed the biofilm growth kinetics in vitro for some clinical isolates of T. asahii. They found that T. asahii cells were capable of quickly adhering to polystyrene after incubation for 30 minutes, displaying a low metabolic activity during the first 4 hours. The biofilm's complexity increased after 6 to 8 hours of incubation, period during which it was possible to observe a monolayer formed by filamentous structures. At 72 hours, the cells showed both yeast-like and filamentous structures embedded in an extracellular matrix, forming a mature biofilm ranging from 20 to 40 μm in thickness. Biofilm formation was accompanied by a consequent increase in the resistance to voriconazole (up to 16,000 times more compared to planktonic cells).16 That same year, Dað and Cerikçioðlu reported a moderate to weak production of biofilms in 58% of the clinically isolated T. asahii species that were analyzed.8
Associated infections
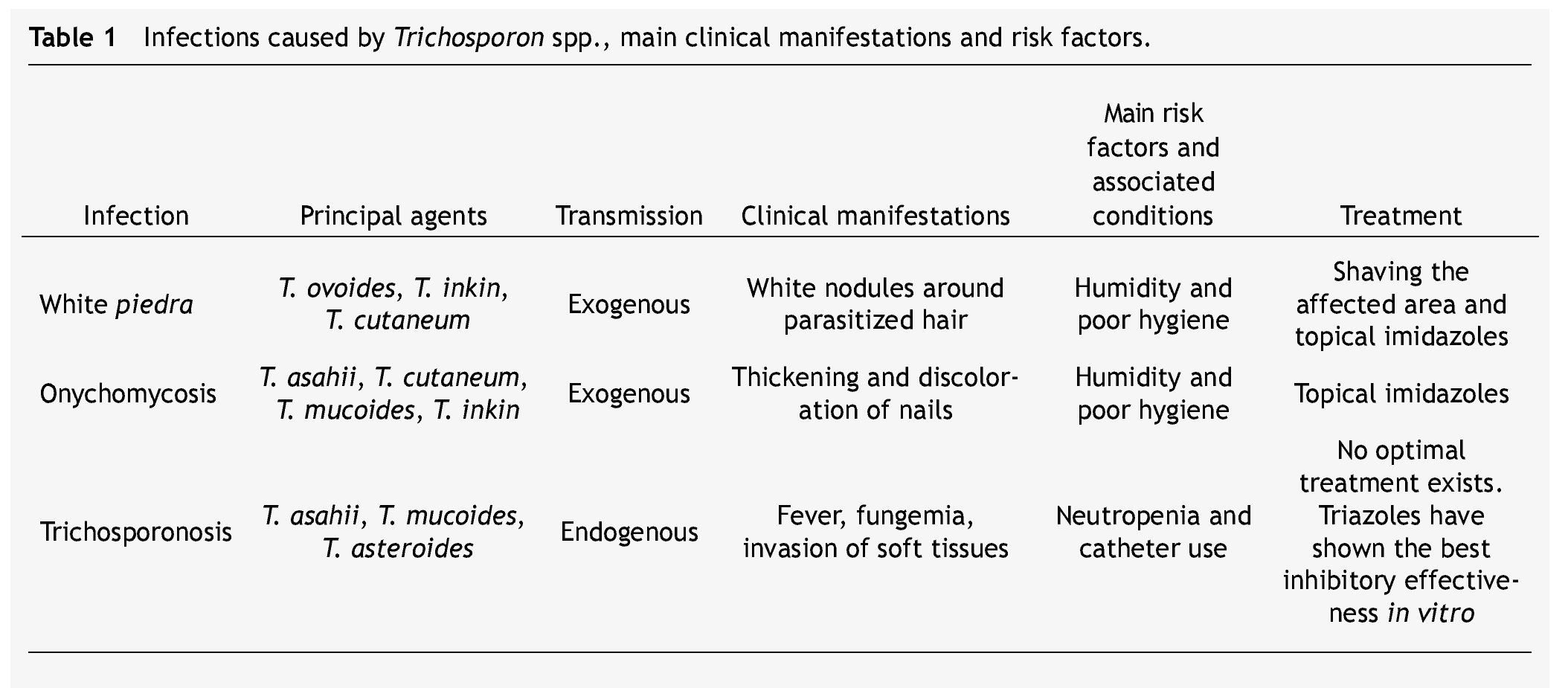
Table 1 summarizes the main clinical manifestations and risk factors in infections caused by Trichosporon spp.
White piedra
White piedra is an infection in which white nodules are formed due to the aggregation of conidia around the hair shaft. These nodules are soft, whitish, have a defined border, and measure approximately between 1 and 3 mm of diameter. White piedra mainly affects capital hair; however, the infection may present itself in the hair of armpits, pubis, and in a lesser degree moustaches and beards. It is a chronic and asymptomatic disease. It is a superficial infection in which fungi remain in contact with the cuticle, without invading neither the hair medulla, scalp, nor skin.3
White piedra is a cosmopolitan and exogenous mycosis occurring more frequently in tropical and temperate climates. Humidity and poor hygiene are among the risk factors for the attainment of this pathology. Women and children are the groups mainly affected by capital white piedra.17,18
Most cases of genital white piedra, second in incidence, involve males between 15 and 44 years of age.19,20T. asahii, T. inkin, T. cutaneum, T. mucoides, and T. ovoides have all been associated with white piedra, T. ovoides being the one with the highest incidence.1,21
Trichosporonosis
When Trichosporon spp. develops as an invasive infection, the disease is known as trichosporonosis. This infection has a mortality rate between 50% and 80%,22 and it is the second or third cause of fungemia in immunocompromised patients just after Candida spp.23-25 Trichosporonosis is considered an endogenous disease because the microorganism is commonly found as a part of the flora in the gastrointestinal tract, lungs, and skin.1,3
These opportunistic systemic infections have gained clinical importance due to their growing prevalence in certain groups of patients. Neutropenia is the main risk factor. A relationship between trichosporonosis and having undergone an invasive clinical procedure (e.g., probes and catheters) has been established as well.
Kontoyannis et al. (2004) evaluated risk factors associated with the infection caused by Trichosporon spp. in 17 cancer patients. They observed that out of the 10 patients who displayed fungemia, 41% developed trichosporonosis subsequent to the use of catheters. The underlying disease in most patients was acute leukemia, and in 65% of the cases neutropenia was observed. Some patients had received chemotherapy (76%) or high doses of corticosteroids (89%) 1 month prior to the trichosporonosis diagnosis.26 Girmenia et al. (2005) performed a retrospective study including 287 cases of trichosporonosis worldwide. They found that 62.8% of the patients had suffered from a hematologic disease previous to the trichosporonosis diagnosis. Acute leukemia had the highest incidence (68%). Other conditions of risk associated with the development of trichosporonosis were solid tumors, transplants, peritoneal dialysis, and human immunodeficiency virus.27 Ruan et al. (2009), based on a study of 19 cases of invasive trichosporonosis, reported the use of central catheters as the main risk factor (90%).22 Suzuki et al. (2010) analyzed 33 patients with hematological diseases; the results were similar to the ones previously reported: acute leukemia (82%) and neutropenia (85%) were the main risk factors for trichosporonosis. Moreover, it was observed that most patients were undergoing chemotherapy when diagnosed with this mycosis.28
The main trichosporonosis manifestations are fever and fungemia. However, tissue invasion may develop. Cases of inflammation and abscesses in different organs and tissues (heart, brain, liver, spleen, esophagus, urinary tract, joints, peritoneum) have been reported. T. asahii, T. asteroides, T. cutaneum, T. inkin and T. mucoides are the 5 most common species associated with trichosporonosis cases.9,22,29-32
Tissue invasion and damage
There are reports using murine models for evaluating tissue damage in invassive infections by Trichosporon spp.. Mice require cyclophosphamide-induced immunosuppression prior to high doses of fungi inoculation, which emulates the neutropenia in patients who develop trichosporonosis.
Studies in animal survival have proven that the establishment of the infection is dose dependent. However, both the inoculum size causing mortality and time elapsed until tissue invasion is detected vary in different reports. Gokaslan and Anaissie determined that an inoculum of ≥ 2 x 107 colony forming units (CFU) causes 100% mortality and invasion of organs in immunosupressed mice 6 days after infection.34 On the other hand, Hospenthal et al. reported that an inoculum of ≥ 7 x 106 CFU caused 100% mortality with tissue invasion 6 hours after infection.35 These models were developed with infection by T. beigelii, therefore the observed differences in infectivity and progress of the disease could be a result of an induced infection by an unidentified species of the genus Trichosporon. Yamagata et al. utilized an inoculum of 3 x 106 CFU of T. asahii in a model in which mice were immunosuppressed at week 1, showing a significant difference compared to the animals which were not immunosup-pressed or those receiving a second cyclophosphamide dose at week 3 (20% of mortality). They concluded that the first 3 weeks are critical for the development of systemic trichosporonosis by T. asahii.29
Fungi presence in the affected organs is generalized. Persistence of the microorganism in kidneys of immunosuppressed mice as well as of immunocompetent ones has been reported. In animal groups where mortality is induced via inoculation of lethal doses, the initial inoculum has been recovered as much as 36-fold from brain, heart and kidney. A resolution of the infection in re-immunosuppressed animals has not been observed. The presence of Trichosporon spp. has been confirmed by molecular methods, even when the cell count is under the detection limits.29,34,35
Hyphae and conidia have been observed in different proportions depending on the organ and the stage of infection. Yeast-like structures are often found in earlier stages, while hyphae and arthroconidia are present in later stages of the infection. Predominance of yeast-like structures in the lungs of the infected animals has been reported.35
One of the most prevalent signs of invasive trichosporonosis is the granulomatous inflammation reported in lungs, liver, lymph nodes, and spleen in immunosuppressed and immunecompetent mice, as well as a fungal invasion of the blood vessels' lumen or periphery.34-36
Other findings include kidney, spleen, lung, and heart hyperemia. Tubular edema, focal parenchymal destruction, cortical tubular necrosis, and chronic inflammation with fibrosis have been observed in the kidneys of mice surviving infection. In the liver, hepatic sinus dilation and congestion have been reported, as well as degeneration and necrosis of some hepatocytes and proliferation of Kupffer cells. Splenic sinuses dilation and splenomegaly have also been observed. In lungs, edema, hemorrhage, and alveolar ectasia have been described. In the heart, evidence of hemorrhage and cardiomyocytes necrosis have been reported. 34-37
Onychomycosis
In the last few years, cases of onychomycosis associated with infections caused by Trichosporon spp. have increased. Around the world, this genus is the agent responsible for 1.3% to 10% of onychomycosis cases, being T. asahii, T. mucoides, and T. inkin the most frequently involved microorganisms.38-40 There have been discrepancies between cases reported in Mexico and other countries. In a study performed in pediatric patients from a rural area, Archer-Dubon et al. isolated T. cutaneum in 42% of the patients with onychomycosis and athlete's foot, a percentage higher than the estimated for infections by dermatophytes and Candida spp., microorganisms typically responsible for foot infections.41
Laboratory diagnosis
Clinical laboratory diagnosis varies in cases of superficial and systemic infections. In the case of white piedra, the hair sample is submerged in a potassium hydroxide solution for observation under the microscope of the nodules formed by the aggregated spores around the hair shaft, which allows differential diagnosis of other parasitizations such as pediculosis or trichomycosis. Similarly, onychomycosis diagnosis requires the nail scrape to be submerged in potassium hydroxide for microscopic observation of conidia and hyphae. In both cases, precise etiologic diagnosis requires a culture. Trichosporonosis diagnosis is made routinely by the recovery of fungi from blood and/or biopsies.3
Cultures obtained from diverse clinical samples are used for identifying the etiologic agent through its microscopic morphology by confirming the presence of the 3 characteristic structures of the genus Trichosporon: hyphae, arthrocondia, and blastocondia. The biochemical profile may be obtained by auxanography, which evaluates the ability to assimilate different sources of carbon. However, the complete biochemical characterization of species of the genus Trichosporon is based on the assimilation of approximately 50 carbon compounds and requires anywhere between 5 and 15 days for the readings; consequently, it is not practical to carry out this battery of tests for the precise identification of pathogenic species. There are commercial methods which systematize the application of assimilation tests and reduce the required identification time. The API 20C AUX system by bioMérieux is a commercial micromethod, which has turned into the most used in clinical laboratories. This system evaluates the assimilation ability of 19 carbohydrates and allows identification within a timeframe of 24 to 72 hours of incubation. However, a limitation of this test is that it only allows the identification of 3 species of the genus: T. asahii, T. inkin, and T. mucoides. This last characteristic prevents its generalized use.
Different molecular methods have been developed as fast and effective alternatives for the precise identification of many pathogens at the species level. Ribosomal DNA is widely used in the systematic identification of microorganisms. Molecular identification of fungi is typically performed by sequencing of the internal transcribed spacer (ITS) region. One of the inconveniences of this region is the fact that it is highly homologous between the different species in the genus Trichosporon; therefore, the analysis of other genes or regions with higher heterology is needed in order to obtain a more accurate identification. Sugita et al. analyzed 84 strains corresponding to 25 species of the genus Trichosporon to evaluate the efficacy of sequencing the ITS region and the intergenic spacer 1 (IGS1) region for the precise identification at a species level. They found a higher heterology between IGS1 regions in comparison to ITS. This makes the IGS1 sequencing analysis superior compared to ITS for the differentiation between species of the genus. In addition, this method allows genotypification.42
A growing number of reports reveal the effectiveness of molecular methods in the identification of this microorganism. These publications show the lack of specificity of the conventional identification methods based in morphologic and biochemical criteria.43-46
Treatment
Superficial infections caused by Trichosporon spp. may be treated with galenic solutions such as 1% mercuric chloride, 1% iodine solution, and 30% salicylic acid. They may be treated with topical antifungals, mainly imidazoles like econazole, isoconazole, miconazole, and ketoconazole. In patients with white piedra, the trimming or shaving of parasitized hairs or affected areas prior to topical treatment is recommended.1,3,17
Management of deep infections has been a bigger challenge. Trichosporon spp. has displayed variable resistance to the most common antifungal treatments; consequently, there is no optimal treatment for trichosporonosis cases.
In vitro susceptibility
Trichosporon spp. was first reported as an amphotericin B-resistant pathogen by Walsh et al. in 1990, after evaluating 2 T. beigelii isolates obtained from patients with systemic infections.47 Since its taxonomic revision, several studies have assessed the efficacy of different antifungals against the species of the genus, mainly T. asahii.
It is important to mention that to this day there is not an established method to determine in vitro susceptibility of species belonging to the genus Trichosporon. The most widely used protocol is an adaptation of the method used for Candida spp. and C. neoformans described in documents M27 and M44 of the Clinical and Laboratory Standards Institute.
One of the most evaluated antifungals has been amphotericin B (AMB). This is a polyenic derivate that destabilizes the fungal cytoplasmic membrane; it is the treatment of choice for systemic infections. The resistance of Trichosporon spp. to AMB has been reported. T. asahii consistently exhibits a MIC of ≥ 2 μg/mL (Minimal Inhibitory Concentration, which prevents visible growth of 90% of isolates).22,44,48,49 There are similar MICs for other clinical isolates including T. asteroides, T. cutaneum, and T. ovoides.22 Rodríguez-Tudela et al. (2005), contrary to the previous study, reported low MICs for T. cutaneum (0.25-0.5 μg/ml) and T. ovoides (0.37 μg/ml). T. mucoides showed great variability regarding susceptibility to AMB, with MICs ranging between 0.015 and 16.0 μg/ml.44T. inkin is the species which has displayed greater in vitro susceptibility to the inhibitory activity of AMB, with MICs from 0.14 to 1.0 μg/ml.44,48
Triazolic compounds are inhibitors of the ergosterol synthesis, the main component of the fungal cytoplasmic membrane. In addition, they interact with enzymes at a mitochondrial level causing the accumulation of free radicals inside the cell and provoking the inhibition of fungal growth. Triazolics include fluconazole (FLC), voriconazole (VRC), itraconazole (ITR), and posaconazole (POS). These agents have displayed better in vitro activity against Trichosporon spp., with relatively low MICs for T. asahii (FLC 1.27 to ≤ 10.3 μg/ml, ITR ≤ 1.4 μg/ml, POS 0.25 μg/ml), T. asteroides (FLC 0.8 μg/ml, ITR 0.06 μg/ml), T. inkin (FLC ≤ 4 μg/ml, ITR ≤ 1 μg/ml), T. cutaneum (FLC ≤ 32 μg/ml, ITR ≤ 1 μg/ ml), and T. ovoides (FLC 1.74 μg/ml, ITR 0.1 μg/ml).22,44,48,49
Voriconazole is particularly effective for fungal inhibition in vitro, with MICs of ≤ 0.28 μg/ml for the 6 more frequently isolated Trichosporon species causing human pathology.44,49
Terbinafine (TER) is a synthetic antifungal derived from allylamines that inhibits ergosterol synthesis. TER requires high concentrations in order to inhibit T. asahii (12.6 μg/ ml), as opposed to the inhibition of T. cutaneum o T. inkin (1 μg/ml).48
5-Flucytosine (5-FC) is an inhibitor of the synthesis of nucleic acids; it interacts with the fungal RNA disrupting the synthesis of proteins. 5-FC has shown poor antifungal activity against the most clinically relevant species, with MICs ≥ 2 μg/ml and reaching concentrations of up to 128 μg/ml for T. mucoides.9,22,44,49
Caspofungin (CAS) is an echinocandin that inhibits the synthesis of the fungal cell wall. CAS MICs are high for T. asahii (≥ 4 μg/ml) and T. asteroides (16 μg/ml).49,50
Immunotherapy
Since neutropenia is the main condition associated with the development of trichosporonosis, the use of cytokines has been considered for the treatment of these infections. Some of the factors used are the granulocytes colony stimulating factors (G-CSF), which increase polymorphonuclear cell count, and the granulocytes-macrophages colony stimulating factors (GM-CSF), which activate monocytes and macrophages functions. Muranka et al. (1997) used a murine model with disseminated T. asahii trichosporonosis for the evaluation of G-CSF and GM-CSF as therapy against systemic infection. They observed that the use of G-CSF prior to fungal infection increased the survival in mice from 25% to 100%, decreasing the fungal load and tissue damage in affected organs, with the most notable improvement in the lungs. The increase in neutrophils by GM-CSF was lower than the one caused by G-CSF, so the use of GM-CSF therapy did not generate any major improvements in animals. In the same study, they observed an increase of tumor necrosis factor alpha (TNF-α) in bronchoalveolar lavage fluid in mice infected with lethal doses of T. asahii and treated with GMCSF compared to non-immunosuppressed animals or animals treated with G-CSF. In addition, a negative correlation between TNF-α production and the presence of leukocytes in peripheral blood was observed, decreasing as mice recuperated from the neutropenia. Anti-TNF-α therapy did not favor infection resolution. The investigators concluded that neutrophil count was the most critical factor in the development of trichosporonosis; however, other factors, such as TNF-α in lung, played an important role in the resolution of the infection.51
Similarly, the use of macrophages colony stimulating factor (M-CSF) has been evaluated as an activator of phagocytosis in a trichosporonosis murine model with T. asahii. M-CSF increased fungicidal activity in mononuclear cells, with a notable increase in survival and a decrease in fungal load. In addition, an increase in TNF-α in lungs and plasma in animals treated with M-CSF was noticeable. Different from the results reported by Muranaka et al., anti-TNF-α treatment did increase survival in mice. Results showed how M-CSF exponentially increased fungal activity by mononuclear phagocytes, helped partly by the production of TNF-α.52
Similar results regarding the effect of G-CSF, GM-CSG, and M-CSF on leukocytes and their role in trichosporonosis resolution were reported by Roilides et al. (2002).53
Conclusion
Vast distribution, antifungal resistance, and an increase in the number of patients presenting risk factors have made relevant the study of species belonging to the genus Trichosporon as opportunistic fungal agents relevant. High mortality rates in patients with systemic infections as well as a lack of an optimal treatment generate the need to increase the knowledge of this particular pathogen, in a way that allows us to understand its virulence mechanisms, and consequently, to obtain the necessary tools for the development of effective therapies against these microorganisms.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Received: September 2013;
Accepted: December 2013
* Corresponding author:
Department of Microbiology,
Faculty of Medicine,
Universidad Autónoma de Nuevo León, Monterrey, N. L., Mexico.
E-mail address: alexandra.montoya@aol.com (A. M. Montoya).