Published data demonstrate a serious interaction between valproic acid and meropenem. However, recommendations about the management of concomitant treatment are contradictory; some experts recommend closer monitoring of valproic acid serum concentrations and others recommend avoiding concurrent therapy. The purpose of this study is to critically analyse the interaction and to evaluate the impact of pharmaceutical intervention in the use of these drugs in hospitalised patients.
Material and methodsStudy of the concomitant prescription of valproic acid and meropenem in a general hospital of 1080 beds divided in to two periods; the first period was retrospective and observational and it was followed by a prospective period involving pharmaceutical intervention. The prescription habits between both periods were compared.
ResultsA total of 26 patients received concurrent treatment with valproic acid and meropenem (13 per period) and none of them maintained therapeutic serum levels of the antiepileptic drug. Pharmaceutical intervention modified prescription habits, reducing by half the number of days of concomitant treatment, changing the antibiotherapy and/or monitoring serum concentrations more often.
ConclusionsThe interaction between valproic acid and meropenem is serious, especially because of the dramatic decrease in the antiepileptic serum concentrations. The concomitant use of both drugs should be avoided, replacing the antibiotherapy empirically, or according to the resistance profiles of the microorganism and maintaining the same the anti-epileptic treatment.
Existen referencias en la literatura acerca de la gravedad de la interacción entre el ácido valproico y el meropenem. Sin embargo, las recomendaciones en cuanto a su manejo son contradictorias, recomendándose en algunos estudios la monitorización más estrecha del antiepiléptico si se emplean juntos y en otros contraindicando su uso concomitante. El objetivo de este trabajo es analizar la interacción entre el ácido valproico y el meropenem y evaluar el impacto de la intervención farmacéutica sobre la utilización de estos fármacos en pacientes hospitalizados.
Material y métodosEstudio de la prescripción concomitante de ácido valproico y meropenem en un hospital de tercer nivel de 1.080 camas dividido en dos periodos: uno retrospectivo y observacional, el otro prospectivo y con intervención farmacéutica. Se compararon los hábitos de prescripción entre ambos periodos.
ResultadosUn total de 26 pacientes recibieron ácido valproico y meropenem simultáneamente (13 en cada periodo), no alcanzando ninguno niveles terapéuticos del antiepiléptico durante el tratamiento. La intervención farmacéutica cambió los hábitos de prescripción, disminuyendo a la mitad los días de tratamiento concomitante, cambiando la antibioterapia y/o monitorizando más estrechamente el antiepiléptico.
ConclusionesLa interacción entre el ácido valproico y el meropenem es grave, especialmente por la rapidez con la que disminuyen los niveles del antiepiléptico. Se debe evitar el uso concomitante de ambos fármacos, sustituyendo la antibioterapia de manera empírica o según los patrones de resistencia del microorganismo para mantener el mismo tratamiento anticomicial.
Pharmaceutical intervention (PI) in pharmacotherapeutic monitoring aims to identify and resolve medication-related problems (MRPs). Drug interactions are one of the MRPs in which pharmacists can advise on clinical decision-making to ensure the safest and most effective therapy. In the case of patients with epilepsy, drug interactions have proven to be a major challenge for the maintenance of therapeutic concentrations of anticonvulsant drugs.1
Valproic acid (VPA) is one of the most frequently used drugs in the treatment of epilepsy and should be monitored because it has a narrow therapeutic blood margin (50–125μg/ml).2 On the other hand, meropenem is a bactericidal antibiotic with a broad spectrum of activity that is used for a variety of infections in hospitals, for example, in patients with neurological base pathologies, who present respiratory infections requiring broad spectrum antibiotics. These are two drugs whose coincidence in the drug therapy of a single patient is not exceptional and whose interaction is reflected in specific bibliographic sources on this topic.3
This interaction is characterised by a rapid decline in VPA levels within 1–7 days after the start of coadministration with meropenem and their slow recovery after treatment discontinuation (from 3 days to 2 weeks). There are published case series and retrospective studies confirming these data.4–9 Recent studies point to a combination of the mechanisms of VPA absorption, distribution and metabolism that would explain the interaction.10 Avoiding coadministration is the most recent recommendation11 and has been included in the meropenem data sheet.12 Meanwhile, Mancl and Gidal13 recommend in a recent review that, in the absence of an alternative antibiotic therapy, VPA levels should be monitored more frequently and the use of higher doses of VPA should be considered.
After an exhaustive literature review, we found no prospective studies with such a large number of patients that evaluated the interaction between meropenem and VPA. Neither did we find the PI performed in this respect.
The aim of this study was to analyse the interaction between VPA and meropenem and to evaluate the impact of the PI on the use of these drugs in hospitalised patients.
Patients and methodsThe study was conducted at a tertiary care hospital with 1080 beds in 2 periods: a retrospective and observational study without PI (January to November 2007) and a prospective study with PI (March 2008 to January 2009).
Prescriptions for both drugs were checked daily and a PI was performed in cases of concomitant treatment (during the prospective period). This consisted of contacting the treating physician through online messages and/or telephone calls. The PI included suggestions to change to antibiotic therapy and, if this was not possible, to monitor VPA levels. In addition, we created an electronic alert within the Prescriwin® prescribing module of the Hospiwin® program, which consisted of having an automatic warning appear when both drugs were prescribed for the same patient, thus reporting about the interaction in real-time.
In both periods we selected admitted patients with assisted electronic prescription, recording age, gender, indication, duration, dose and date of prescription of both drugs, as well as reviewing medical records for the presence of seizures during admission. We recorded VPA levels before, during and after treatment with meropenem. We calculated the drug interaction probability scale (DIPS)14 for each of the individuals.
We analysed the impact of the PI and the electronic warning for the prescription of both drugs between the two study periods. We compared the number of patients who were prescribed the 2 drugs in each period, the number of days of concomitant treatment and the number of requests for plasma VPA levels, as well as the acceptance of the PI by the physician.
The data were analysed using the “Evaluation of treatments” program, version 1.0.1, developed by the hospital clinical biostatistics unit (http://www.hrc.es/investigacion/bioest/otras_calculadoras.html), which enabled relative risk reduction and absolute risk reduction. We used a 95% confidence interval.
ResultsThe main treatment indications for VPA were different types of epilepsy and control of seizures, whereas it was the empirical treatment of aspiration pneumonia in severely ill patients for meropenem. The interaction variables collected between the 2 study periods are shown in Table 1.
Demographic and pharmacokinetic variables of the interaction.
| Variables | Retrospective | Prospective |
| Number of patients | 13 | 13 |
| Percentage of males | 54% | 54% |
| DIPS14 | ||
| Highly probable | 0 | 1 |
| Probable | 6 | 9 |
| Possible | 1 | 1 |
| Not calculated | 6 | 2 |
| Number of study months | 11 | 11 |
| VPA range during concomitant treatment | 2.14–22.76μg/ml | 1.63–43.87μg/ml |
| Decrease in VPA levels | 73.6–96.3% | 49.1–88.4% |
| Number of requests for VPA levels during concomitant treatment | 10 | 12 |
| Number of days to recover VPA levels after suspension of meropenem | 13 | 14 |
During the prospective period, pharmacists performed a total of 13 PI and all were reported to the physician responsible for the interaction. In 4 cases, the physician decided to monitor the VPA levels more closely, in 3 the antibiotic was changed to piperacillin-tazobactam, in 2 to levofloxacin and in 4 the prescription of both drugs was maintained.
As a result of the PI, the number of days of concomitant treatment with meropenem was halved (from 10 to 4.7 days) and VPA levels were requested 2.6 times more often per day of concomitant treatment compared with the retrospective period. These differences were statistically significant (95% confidence interval).
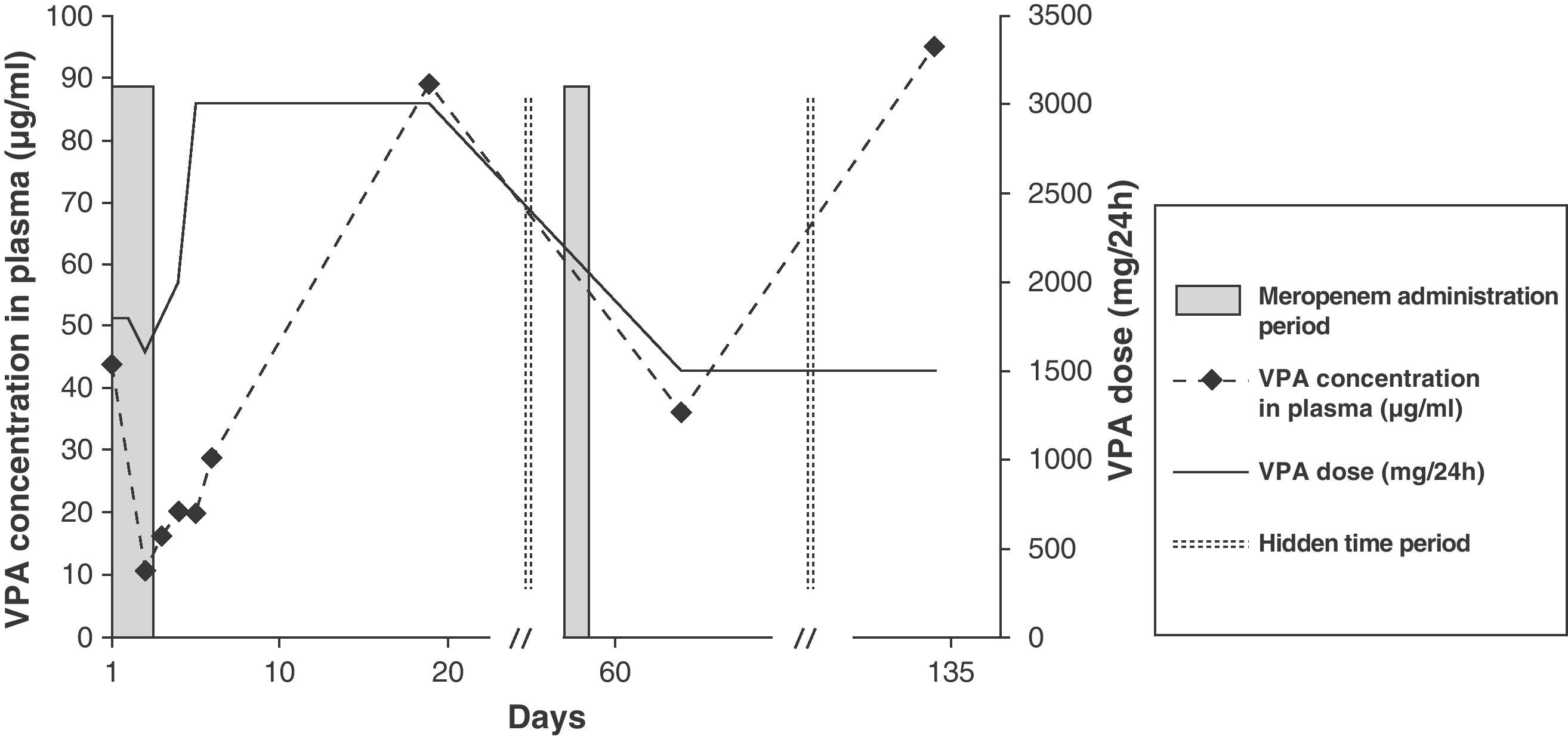
Fig. 1 shows the drug therapy prescribed for the patient who obtained a DIPS score above 8 (very likely). While in hospital, he received meropenem on 2 occasions; in both cases the VPA levels decreased to less than therapeutic values, even though higher doses of the antiepileptic agent were prescribed during those days. On the first occasion, after 2 days of meropenem treatment, it was decided to change the antibiotic to levofloxacin, and on the second to piperacillin-tazobactam, followed in both cases by PI. Once the effect of meropenem was over in this patient, the dose of VPA could be reduced to the initial value and the therapeutic dosage levels were recovered.
Following administration of a single dose of meropenem, 1 of the patients presented seizures that were directly related to the interaction, since less than 24h after the administration of antibiotics, the VPA levels were 28.80μg/ml (infratherapeutic). The antibiotic was then changed to piperacillin-tazobactam.
DiscussionFrom a pharmacokinetic standpoint, the interaction between VPA and meropenem is very complex, since it seems to involve numerous mechanisms that lower antiepileptic agent concentrations.13 A decrease in the absorption of VPA,15 decrease in its enterohepatic recycling,16 a change in distribution volume reducing the free drug proportion17 and, finally, increase in VPA glucuronidation18 could all justify such abrupt reductions in plasma concentrations of the antiepileptic agent.
Coinciding with other authors, our study found that the interaction between meropenem and VPA was potentially serious, especially due to the speed with which plasma concentration of the antiepileptic agent decreased and the consequences this entailed.19,20 In our study, 1 of the patients presented epileptic seizures after administration of a single dose of meropenem. Monitoring the antiepileptic revealed a decrease in VPA values to less than therapeutic levels within 24h, so the interaction appears to have a very rapid onset. De Turck et al.21 found that the half-life of VPA decreased from 15 to 4h, 24h after administration of meropenem.
Haroutiunian et al.11 found that, during concomitant therapy with meropenem, mean VPA levels were 9.9μg/ml. None of the patients in our study maintained therapeutic levels of VPA and, in addition, the decrease in VPA levels after administration of the first dose of meropenem ranged between 49.1% and 96.3%. Different studies have reported similar declines (from 66% to 90%).6,11,21,22
For some authors the recovery of VPA levels after discontinuation of the antibiotic is extended from 3 days to 2 weeks.13 However, we were only able to determine it for 2 patients, who recovered their levels 13 and 14 days after discontinuation of meropenem, because the levels were not requested until much later for the remaining patients.
At present there is no consensus regarding the therapeutic approach to be followed with this interaction and, consequently, no agreement as to the PI that must be carried out. Several authors recommend closer monitoring of the antiepileptic agent when both drugs are to be administered, as in those cases where it is not possible to replace either of them.6,9,13,23 This recommendation also appears in the technical data sheet of VPA.2 However, other authors are more categorical and suggest that good clinical practice would mean not to administer both drugs together.22,24 In some countries, such as Japan, health authorities have banned the administration of both drugs at the same time.25 The meropenem technical data sheet also reflects that the coadministration of VPA and carbapenem agents is not manageable and should therefore be avoided.12 In 2010 the Spanish Agency for Medicines and Health Products issued a warning on the prescription of these drugs, contraindicating their simultaneous use.26 In our case, after the PI some physicians chose to carry out more comprehensive monitoring, while others opted for suspending the antibiotic.
As a limitation of the study, we must indicate that we did not include patients admitted to units that did not have assisted electronic prescription (cardiovascular surgery ICU, coronary care unit and paediatric cardiology unit).
Given the results obtained and the literature reviewed, and considering that the effectiveness of anticonvulsant therapy is directly proportional to therapeutic drug levels and the vast antibiotic arsenal at our disposal, we believe the first choice should be not to administer both drugs concomitantly. The declines in VPA levels of up to 90% in different patients in our study, as well as in other published studies, make it very difficult to manage VPA to achieve therapeutic levels without exceeding maximum doses. Therefore, we conclude that the concomitant use of both drugs should be avoided, replacing the antibiotic therapy empirically or according to the resistance patterns of the microorganism being treated in order to maintain the same anticonvulsant therapy.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Vélez-Díaz-Pallarés M, et al. Análisis de la interacción ácido valproico-meropenem en pacientes hospitalizados. Neurología. 2011;27:34–38.