A review of current criteria for the diagnosis of categories related with vascular cognitive impairment, in particular the nomenclature, diagnostic criteria, and differential clinical–radiological findings.
DevelopmentThe criteria for the diagnosis of vascular cognitive impairment have evolved, but available criteria were designed basically for differentiating between vascular dementia and dementia due to Alzheimer disease, and for research purposes. Nevertheless, in clinical practice precise elements are required for: (1) Clinical diagnosis of dementia and mild cognitive impairment; (2) Clinical and neuroimaging criteria for identification of the various cerebrovascular lesions associated with cognitive dysfunction, and (3) A formulation of the aetiogenic–pathogenic relationship between cognitive impairment and cerebrovascular lesions. For this reason, a review was carried out on the diagnostic elements of vascular cognitive impairment categories, classification, and their most relevant characteristics. It highlights the characteristic for the diagnosis of multi-infarction dementia, strategic single infarct dementia, small vessel disease with dementia, mixed dementia, and vascular mild cognitive impairment.
ConclusionsStandardisation is required, by a multidisciplinary expert team, as regards nomenclature and criteria for the diagnosis of the full spectrum associated with vascular cognitive impairment and especially for vascular dementia and its categories.
Revisar los principios actuales para el diagnóstico de las categorías de deterioro cognitivo vascular, con énfasis en la nomenclatura, los criterios diagnósticos y los hallazgos clínico-radiológicos diferenciales.
DesarrolloLos principios para el diagnóstico del deterioro cognitivo vascular han evolucio-nado, pero los criterios disponibles fueron dise¿nados básicamente para diferenciar la demencia vascular de la demencia tipo Alzheimer, y para propósitos de investigación. Sin embargo, en la práctica clínica se requieren elementos precisos para: 1) el diagnóstico clínico de la demen-cia y el deterioro cognitivo leve, 2) la identificación clínica y por neuroimagen de las diversas lesiones cerebrovasculares asociadas con la disfunción cognitiva, y 3) la formulación de una relación etiopatogénica entre el deterioro cognitivo y las lesiones cerebrovasculares. Por esta razón se revisaron los elementos diagnósticos de las categorías de deterioro cognitivo vascular, su clasificación y características más relevantes. Se enfatizó en las características que permiten el diagnóstico de la demencia multi-infarto, la demencia por infarto estratégico, la demencia por enfermedad de peque¿no vaso cerebral, la demencia mixta y el deterioro cognitivo leve vascular.
ConclusionesSe requiere de la estandarización, por un grupo multidisciplinario de expertos, de la nomenclatura y criterios para el diagnóstico del espectro completo del deterioro cognitivo vascular, y especialmente para la demencia vascular y sus categorías.
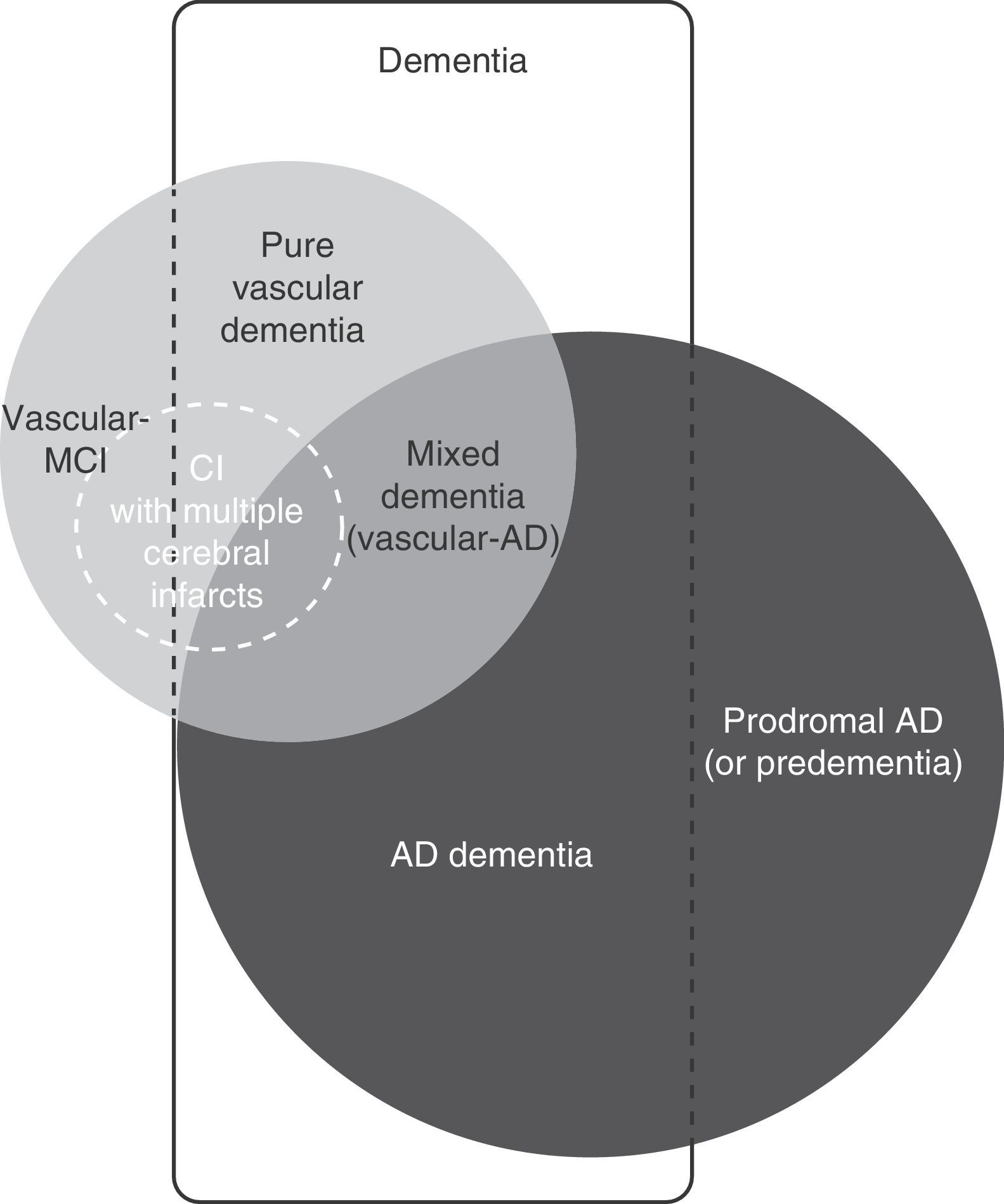
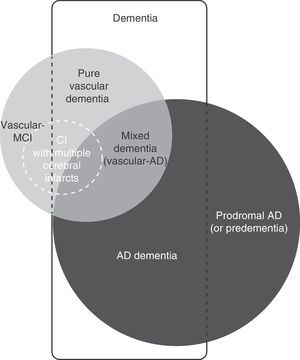
Vascular cognitive impairment (VCI) is a broad term that includes dementia and mild cognitive impairment (MCI) associated with or caused by cerebrovascular lesion (Fig. 1). Both entities are of great interest to clinicians and researchers because they refer to a problem that is both common and potentially preventable.1–7
This review provides an update of diagnostic principles for the different categories of VCI, with emphasis on their nomenclature, diagnostic criteria for each category, and their distinguishing clinical and radiological findings. Articles were mainly identified using PubMed searches for the following terms: ‘vascular cognitive impairment’, ‘vascular dementia’, ‘post-stroke dementia’, and ‘mild cognitive impairment’. We selected original research and reviews from the last 5 years that specifically assess the entities listed above. We also included references from the authors’ collections.
Diagnosing the dementia syndromeDiagnosis for a dementia syndrome is performed on a purely clinical basis, drawing specifically from the medical history and the neurocognitive examination.8–10 Defining the cognitive-behavioural syndrome that will identify the generic type of dementia, and particularly its vascular subtype, is crucial for assigning the diagnosis. The classic Hachinski Ischaemic Score and the Rosen modification do not include a definition of the cognitive syndrome.11 The criteria developed later to define cognitive syndromes are based on one of the following essential findings: (1) memory decline, (2) patchy cognitive deficits, (3) executive dysfunction, and (4) multifaceted cognitive impairment.
Declining memory is an essential or required feature in the definition of the cognitive syndrome of vascular dementia, according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN). Memory loss is also necessary for a diagnosis of the typical symptomatic form of Alzheimer disease (AD).12,13 However, identifying memory loss as the essential feature can impede proper identification of other patterns in vascular cognitive impairment. Marked memory loss is often not the most important sign of cognitive impairment associated with cerebrovascular disease, and it may not be the initial symptom of vascular dementia.3,11,14 A substantial portion of the cases with vascular dementia display severe deficits in executive function, language, and visuospatial reasoning, but relatively preserved delayed recall, upon formal examination.1,6,15,16
The World Health Organization's International Disease Classification, 10th revision (ICD-10) is an instrument reputed to be poorly defined and not particularly useful for early detection of dementia.17 In the specific case of vascular dementia, the ICD-10 requires a patchy or unequal distribution of deficits affecting the higher cognitive functions (i.e. some cognitive domains are impaired, while others are relatively intact).11,18 This ‘patchy’ distribution will only be seen in dementia patients with very few (2 to 3) cortical infarcts. This finding is most likely an ‘artefact’ caused by placing patients with different cerebrovascular neuropathological changes in the same group.11 Furthermore, a patchy or uneven pattern of cognitive function is not specific to vascular dementia. Measurements of patchy cognitive impairment do not reveal any quantitative differences between vascular dementia and dementia in AD.19 Several neurodegenerative disorders, such as Lewy body dementia, semantic dementia, primary progressive aphasia, and frontotemporal dementia affect cerebral regions differently at specific times, and cognitive deficits may seem heterogeneous.20,21
In patients with vascular dementia, executive dysfunction (slow information processing, impaired ability to change from one task to another, and impaired ability to retain and process information) is more characteristic than memory and language deficits.1,6,14–16,22–24 The DSM-IV criteria list loss of executive function as a secondary feature for diagnosis of dementia, but some researchers consider it to be essential.8 The latter approach should be regarded with caution for the following reasons:
- -
Potential errors in identifying the dementia subtype and loss of precision that may arise from combining heterogeneous patient subgroups.
- -
The difficulty of identifying a cognitive profile in patients if both memory impairment and another deficit are required to meet criteria for dementia.
- -
Use of test batteries whose psychometric properties are not yet fully understood.5,11
Stipulating that more than one cognitive domain must be impaired distinguishes dementia from single-dominion deficits due to stroke, amnesia, or Korsakoff syndrome. Diagnostic criteria established by the State of California Alzheimer's Disease Diagnostic and Treatment Centres (ADDTC) do not specify either the type of cognitive impairment or the number of deficits that have to be present. Instead, they require progressive decline in multiple higher cortical functions (more than one domain or specific category), with losses being sufficient to interfere with the patient's activities of daily living.25 This definition is less restrictive and it probably provides the best model for vascular dementia and cognitive impairment caused by vascular lesions.11 A similar perspective was recently used by McKhann et al.26 for diagnosis of dementia due to Alzheimer disease and other causes.
Based on the heterogeneous clinical presentations arising in vascular dementia, the neuropsychological profile should also be different. There are no cognitive deficit patterns that define vascular dementia. Attention, executive function, language, visuospatial abilities, memory, and learning can be affected to different extents and in various combinations depending on the size and localisation of the cerebrovascular lesion (Table 1). For example, single strategic infarcts lead to cognitive impairment and deficits in other neurological functions that depend on lesion localisation. In contrast, the neuropsychological profile in subcortical ischaemic vascular disease is frequently thought to include early loss of attention and executive function, together with slow motor performance and information processing.6,15,16
Diagnostic criteria for the main types of cognitive impairment
| Dementia |
|---|
| • Decline in cognitive and behavioural function affecting at least 2 of the following domains: |
| (a) Memory (impaired ability to acquire and recall new information, manifesting as repetitive questions and conversations, loss of personal belongings, forgetting events or appointments, or feeling lost on a familiar route) |
| (b) Reasoning, management of complex tasks, and judgement (poor assessment of danger, inability to manage finances, poor decision-making capacity, inability to plan complex or sequential activities) |
| (c) Complex visuospatial processing (inability to recognise faces or common objects, or locate objects in plain view despite having good eyesight; inability to use simple tools or put clothing on correctly) |
| (d) Language (difficulty thinking of common words while speaking, hesitant speech, and errors in spoken and written language) |
| (e) Personality, conduct, or behaviour (atypical fluctuations with agitation, decreased motivation, lack of initiative, apathy, loss of energy, social isolation, loss of interest in habitual activities, compulsive or obsessive behaviour, socially unacceptable behaviour) |
| • This decline must be (a) acquired (decrease in previous levels of function and ability, considering the patient's age and level of education); (b) detectable (impairment is diagnosed based on both the medical history provided by the patient/reliable informant and examination of the mental or neurophysiological state); (c) persistent over weeks or months; and (d) observed in a patient whose level of consciousness remains normal until terminal stages. |
| • Presence of significant difficulty with habitual activities, including social and occupational functioning, which cannot be explained by a sensorimotor disorder |
| • Impairment cannot be attributed to delirium or any other major psychiatric disorder. |
| Mild cognitive impairment |
| • Decline in one or more of the following dominions: (a) memory, (b) executive function, (c) attention, (d) language, (e) complex visuospatial processing |
| • This decline must be (a) acquired (decrease in previous levels of function and ability, considering the patient's age and level of education); (b) detectable (impairment is diagnosed based on both the medical history provided by the patient or a reliable informant and examination of the mental or neurophysiological state)*; (c) persistent over weeks or months; and (d) observed in a patient with a normal level of consciousness. |
| • The patient remains independent for functional activities of daily living (for example, bathing, dressing, and eating), with minimal need for assistance or support. The patient may experience slight difficulties with the complex functional tasks he/she used to complete (for example, delays or errors in managing finances, preparing food, shopping, and travelling). This difficulty does not result from an underlying sensorimotor disorder. |
| • Lack of significant impairment in social or occupational functioning (symptoms are insufficient for a diagnosis of dementia) |
| • Impairment cannot be attributed to delirium or any other major psychiatric disorder. |
Source: references27–31.
The disorders that most frequently must be differentiated from dementia include benign concerns (patients worried about their mental function but whose test results are normal), MCI, delirium, depression and other psychiatric disorders (obsessive-compulsive disorder, late-onset psychosis), epileptic seizures, alcohol or drug abuse, adverse drug events, and single-domain cognitive impairment (aphasia caused by stroke, amnesia in Korsakoff syndrome).27–32 In the same way, leaving aside certain dementia disorders in which apraxia is the sole initial prominent manifestation, apraxia due to stroke should not be mistaken for dementia.31
Delirium or mental confusion is a standard exclusion criterion. The NINDS-AIREN criteria offer a more rigorous framework for ruling out patients with decreased awareness, delirium, psychosis, severe aphasia, sensorimotor disorders that make mental testing difficult, and diseases such as AD that may cause impairment of cognitive functions, especially memory.12 Nevertheless, vascular dementia is often ruled out erroneously in patients with reduced sensorimotor function or aphasia caused by stroke.4 Furthermore, depressive symptoms are common in VCI.33
In addition, it is not logical to define dementia according to velocity of onset and progression, severity, reversibility, or duration. As stated by Robles et al., establishing a minimum disease duration may not be the best approach, even though the ICD-10 criteria call for a duration of 6 months. Many forms of dementia (Creutzfeldt-Jakob disease, chronic hydrocephalus in adults, chronic subdural haematoma, etc.) may develop in a shorter time period. On the other hand, some transient confusional syndromes may last several weeks, and they must be distinguished from dementia.30
Diagnosing the cerebrovascular originAccording to G. Román et al., the tendency towards overlooking vascular dementia as an entity is particularly pernicious because it gives the impression that no further research is needed. Vascular dementia is an entity commonly observed in the clinical setting, especially after stroke. This form of dementia is easily recognisable in elderly patients, and it constitutes a major cause of functional disability and institutionalisation.34 It is also inappropriate to perpetuate the impression that vascular dementia is a single pathological entity displaying different phenotypes, as is true of Parkinson's disease or AD. In fact, vascular dementia is an entity whose heterogeneous clinical manifestations are due to a substrate of multiple pathogenic and structural factors.9,15
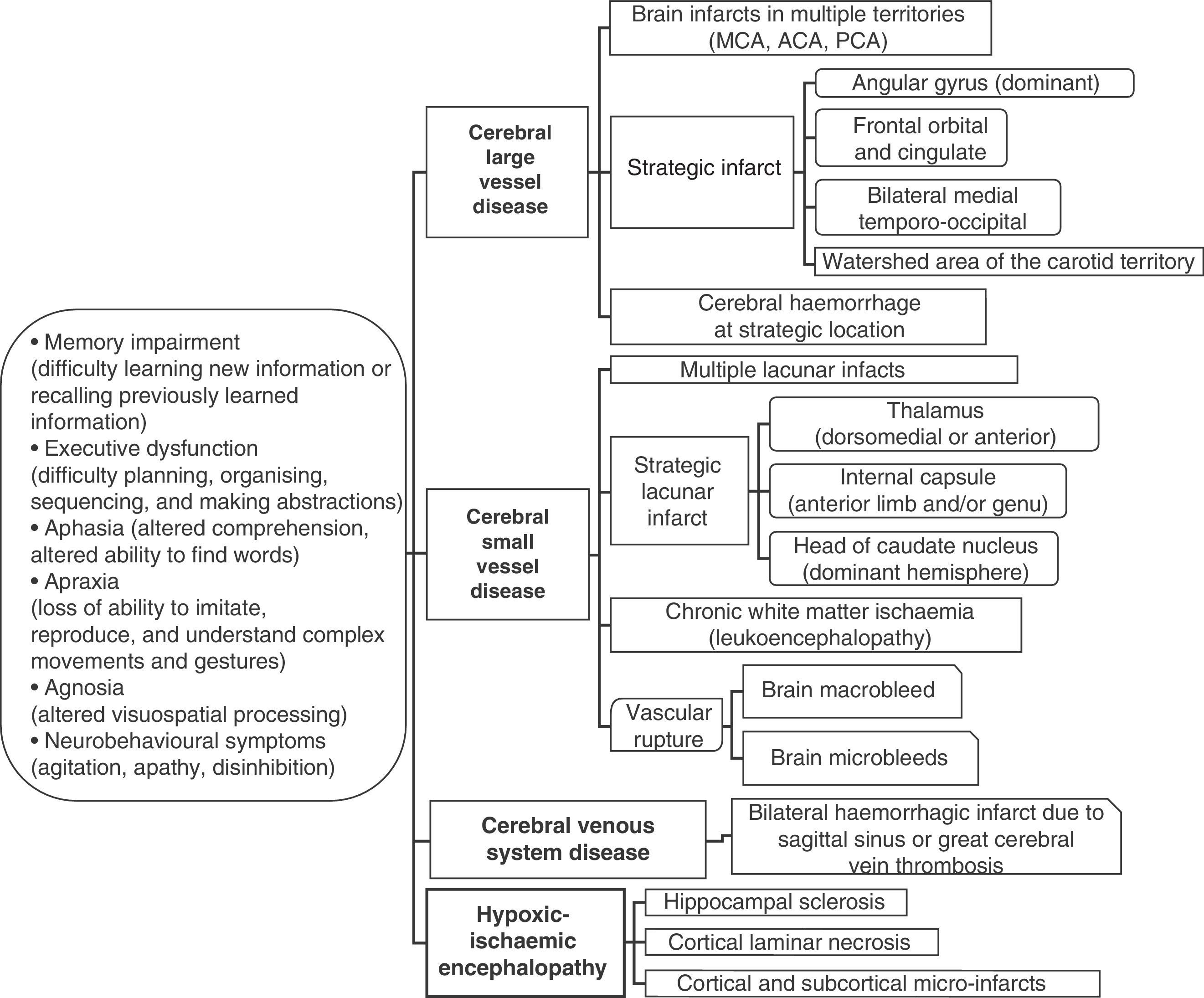
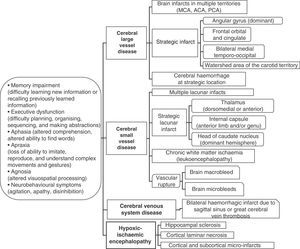
Cognitive impairment may be caused by different types of ischaemic and/or haemorrhagic brain lesions (Fig. 2). The term ‘vascular dementia’ refers to a group of entities (as in the case of stroke), and each of the entities under that umbrella term has its own definition (Table 2).9,35,36 The typical example is that of dementia due to a series of infarcts caused by occlusion of large-calibre vessels (classic multi-infarct dementia), or else by small vessel disease with multiple lacunar infarcts (lacunar state) and chronic white matter ischaemia. Likewise, dementia may result from an infarct or haemorrhage that injures a cortical or subcortical area that is strategically important to cognitive functions (for example, the angular gyrus of the dominant hemisphere or the anterior thalamus).
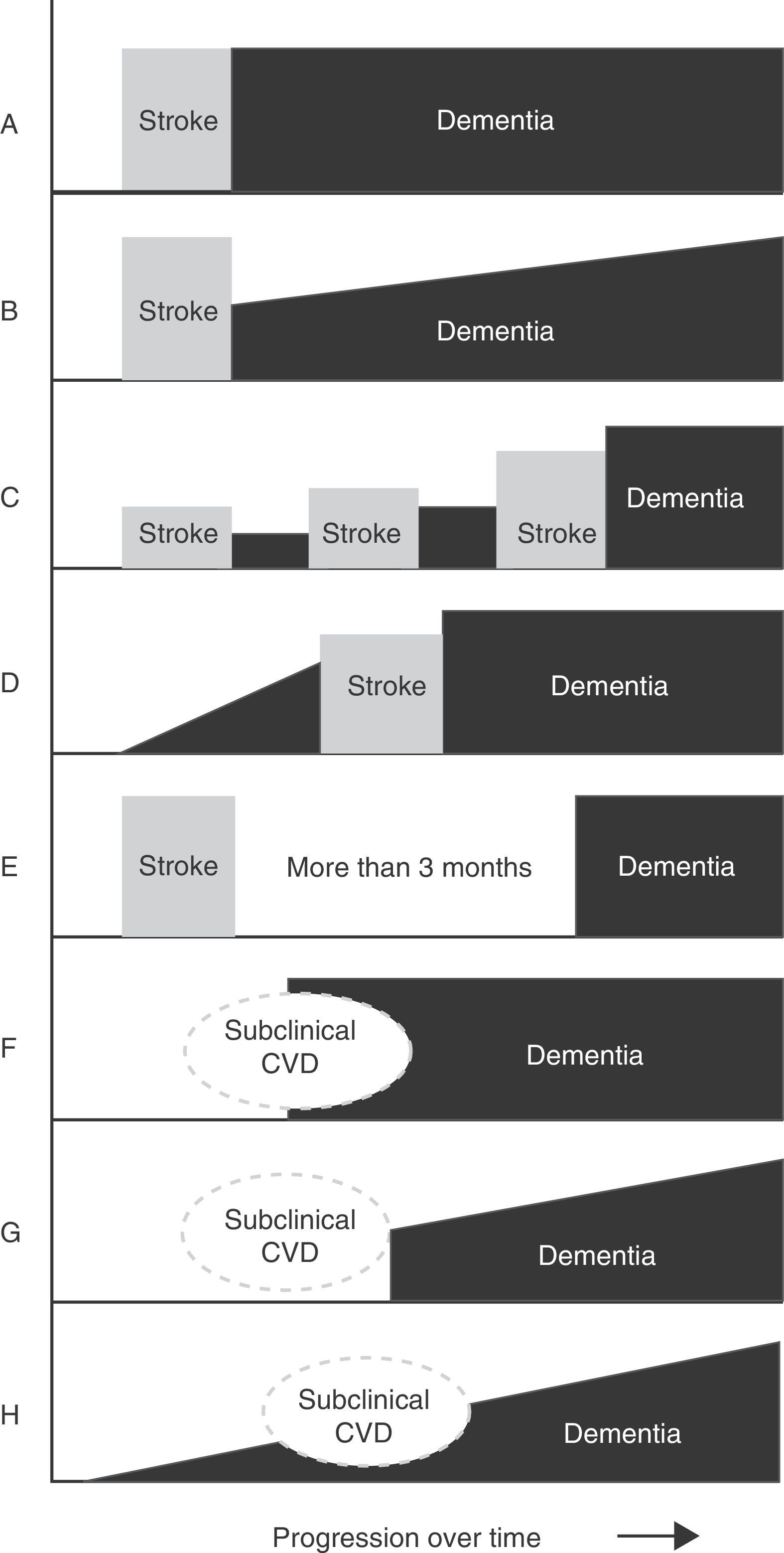
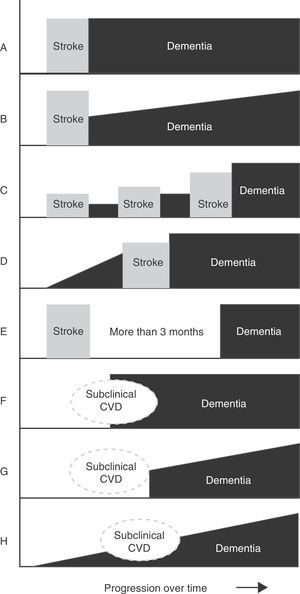
Main temporal patterns for the relationship between dementia onset and the cerebrovascular event. A. Post-stroke dementia (static form). B. Post-stroke dementia (progressive form). C. Dementia secondary to multiple strokes. D. Pre-stroke dementia. E. Dementia unrelated to stroke event. F. Dementia following subclinical cerebrovascular disease (CVD). G. Progressive dementia following subclinical CVD. H. Progressive dementia with subclinical CVD.
Main aetiopathogenic categories for vascular lesions associated with cognitive impairment
| I. Classic multi-infarct encephalopathy (or due to multiple diseased large vessels). Multiple large cerebral infarcts in cortical areas and white matter/basal ganglia due to occlusion of large to medium-calibre arteries (usually due to atherosclerotic thrombosis or cardiac embolism) |
| II. Strategic infarct (or single strategic infarct in the cortical or subcortical area). Small to medium cerebral infarct in a strategic localisation (thalamus, frontal lobe white matter, head of caudate nucleus, anterior limb and/or genu of internal capsule, angular gyrus, frontocingulate cortex, medial temporal area, and hippocampus). These infarcts are attributed to the usual causes of critical arterial stenosis/occlusion. |
| III. Ischaemic cerebral small artery disease (or cerebral small vessel disease, multi-microangiopathic disorders). Multiple lacunar cerebral infarcts in the central white matter and subcortical structures (lacunar state) or diffuse and extensive ischaemic changes in deep white matter. These two entities are caused by occlusion of small cerebral arteries and critical stenosis and hypoperfusion, respectively. |
| IV. Hypoxic-ischaemic encephalopathy. Entity including cortical laminar necrosis secondary to cardiac arrest, multiple cortical and subcortical micro-infarcts due to arterial hypotension, and hippocampal sclerosis due to a hypoxic-ischaemic event |
| V. Haemorrhagic cerebrovascular disease. Lesion to the cerebral parenchyma (macrobleed or microbleed) secondary to a hypertensive vascular rupture, or rupture due to other haemorrhagic disorders (clotting disorders, vasculitis, aneurysms, arteriovenous malformations, hereditary amyloid angiopathy [familial haemorrhagic dementia], neoplasia, cerebral venous thrombosis) |
| VI. Mixed cerebrovascular disease. Combination of the cerebrovascular lesions listed above (for example, strategic infarct with lacunar state) |
| VII. Cerebrovascular disease with Alzheimer disease. Any of the cerebrovascular lesions listed above plus clinical or laboratory evidence of Alzheimer disease |
One of the main obstacles to standardising diagnostic criteria for vascular dementia has to do with the ambiguous, heterogeneous, and overlapping features referred to in definitions of cerebrovascular lesions and vascular disorders. A well-designed array of diagnostic criteria requires specific definitions and a high level of interobserver agreement.6,8,37 In general, cerebrovascular lesions causing dementia have been defined by various researchers based only on descriptive criteria –clinical, radiological, and/or pathological– rather than from an aetiopathogenic viewpoint that recognises the essential role played by structural and functional imaging studies in diagnosis.3,18 Insistence on a description-based rather than a diagnosis-based approach is probably due to the following factors: (1) vascular and degenerative disease frequently appear together, and (2) there are no pathognomonic radioimaging signs in VCI.1
The classic types or categories that have been proposed for ischaemic vascular dementia (multi-infarct dementia, subcortical vascular dementia, strategic-infarct dementia) overlap in various ways due to the lack of clear distinguishing features, which once again reflects the heterogeneous nature of vascular dementia. None of the standard criteria given for vascular dementia include a detailed clinical and biological description of its subcategories.11,37 The Hachinski Ischaemic Score and Rosen's modified version do not address causal relationships, offer no specifics as to the location of vascular lesions required for a diagnosis of multi-infarct dementia, and only provide a rough indication of the underlying pathological process.24,36 The DSM criteria also omit mention of any type of diagnostic approach based on cerebrovascular lesions or different cerebrovascular mechanisms. The ICD-10 contains 5 poorly defined subtypes (acute onset, multi-infarct, subcortical, mixed cortical and subcortical, and other unspecified). These types do not fit well with information provided by neuroimaging studies; for example, the same patient may have subcortical vascular dementia of acute onset.18,38 The ADDTC criteria in turn are quite liberal and refer only to ischaemic vascular dementia. They only require specifying the differential findings for the infarct (for example, localisation, size, distribution, severity, and cause) for purposes of research.6,16,36 Lastly, while the NINDS-AIREN criteria are the most specific, they do not provide a detailed description of the subtypes they include, and they are very restrictive in that they require a temporal relationship between dementia and cerebrovascular disease.3,12,24 Within this framework, a hypertensive patient with slowly progressing cognitive impairment, pseudobulbar palsy, extensive leukoaraiosis, and multiple lacunar infarcts may not be diagnosed with vascular dementia, or else the entity may be assigned a different name (for example, Binswanger disease or subcortical arteriosclerotic encephalopathy).18
Multi-infarct dementiaMulti-infarct dementia is probably the most common type of vascular dementia. It is attributed to multiple large or small cerebral infarcts affecting cortical and subcortical areas. Arterial occlusion will typically be situated in large-calibre cerebral arteries, and it is usually attributed to atherosclerotic thrombosis or cardiogenic embolism.5,6
Although the pathological process in multi-infarct dementia is reasonably well-defined, there is no consensus regarding its clinical presentation and characteristic cognitive profile. On occasions, multi-infarct dementia has been used as a synonym of the larger concept of vascular dementia, or considered equivalent to post-stroke dementia (any form of dementia occurring after stroke, regardless of its cause) (Fig. 3).4–7,11 As a concept, multi-infarct dementia may refer to multiple lacunes (lacunar state), multifocal thrombotic and/or embolic infarcts, or a combination of the two.36,39 Only a few studies have linked cognitive impairment with the number of small cerebral infarcts, but this is a complex calculation in which many additional factors intervene: age, education level, volume of tissue loss, lesion location, and brain comorbidities.37 Some degree of cognitive impairment is present in virtually every patient with multiple cerebral infarcts, but most cases will not be regarded as dementia when this diagnosis is based on the score on the Mini-Mental State Examination. Although some studies report inter-individual variability in the degree of neurocognitive impairment, doctors do not generally distinguish between MCI with multiple infarcts and multi-infarct dementia.40
In a typical case of multi-infarct dementia, the patient will experience several small ischaemic strokes that may not result in a focal neurological deficit.41 Cognitive symptoms may present in early or in more advanced stages of the disease, depending on infarct location. A characteristic clinical feature is the dissimilar level of impairment in different areas of cognitive function. Impairment is often found to be irregular, with certain intellectual functions remaining intact in contrast to marked decline in others (patchy impairment). As infarcts recur, most cognitive functions will be affected and dementia will become patent. The disease follows a progressive course and the effect of the strokes is cumulative. Most patients experience clinical fluctuations in cognitive performance and follow a stepped progression; cognitive impairment is similar in severity to that occurring in AD.6,24
Gait disorders and urinary disorders, manifesting with increased frequency and urgency or incontinence, appear in early stages, even prior to cognitive decline. Lateralised sensorimotor changes are frequently present (hemiparesis, spasticity, hemisensory deficit, visual field loss, marked asymmetry of deep tendon reflexes, Babinski reflex). These findings are associated with cognitive impairment and suggest infarct due to occlusion of a large artery. Focal neurological signs may resolve completely or partially, leaving behind only slight residual symptoms. The patient may also present agnosia, apraxia, and pseudobulbar syndrome.6,42
Perfusion studies reveal patchy regions of low cerebral blood flow (CBF), especially in the distribution of both middle cerebral arteries. These ischaemic areas frequently appear with bilateral involvement of the thalamus and surrounding frontal and temporal cortex, or adjacent to both sylvian fissures. Ischaemia affects specific neural areas, but the possibility always remains that some neurons will recover. Cognition may improve spontaneously if the regional CBF increases with the development of collateral circulation and/or restoration of arterial patency. Nevertheless, prolonged periods of severe regional ischaemia will probably result in focal infarcts that will negatively impact cognition. These infarcts may appear as hypodense areas on cranial computed tomography (CT) images, or as hyperintense areas on T2-weighted magnetic resonance imaging (MRI) studies.31,43
Dementia due to strategic infarctIschaemic lesions are focal and they affect cortical or subcortical sites that may be critically important to cognitive capacity and behaviour. Single infarcts strategically located in the distribution of the carotid and cerebral arteries (anterior, middle, and posterior) will give rise to dementia and a variety of neurological and neurobehavioural findings. Dementia onset is typically sudden, or else a stepped decrease in cognitive function may be detected.6,36
Diagnosis of a strategic infarct is clearer when the patient presents symptoms of motor or sensory deficit (hemiparesis, hemianopsia, hemianaesthesia). Nevertheless, dementia may be the only sign of strategic infarct in some patients, and this may lead doctors to suspect a neurodegenerative disorder. These neurological manifestations, especially cognitive changes, depend on a complex array of factors, including lesion localisation, the volume of damaged tissue, and the brain's ability to compensate for changes.9 Since evidence in this area is limited, Leys et al.4 state that the concept of strategic infarct should be revised in long-term prospective studies, with thorough MRI studies to exclude associated lesions contributing to the observed deficiency, and with follow-up periods long enough to rule out cases of associated AD.
At present, multi-modal MRI images are an excellent means of locating lesions so that we may better understand the spectrum of cognitive disorders in stroke and achieve greater precision in diagnosing areas and strategic networks in the brain. A recent analysis by Hoffman et al. posits that cognitive syndromes are present in most stroke patients, and that they fundamentally result from lesions associated with the following major anatomical cognitive networks: (1) pre-frontal subcortical for executive function (51%); (2) left hemisphere for aphasia and Gerstmann syndrome (36%); (3) right hemisphere for anosognosia, hemineglect, and aprosody (15.3%); (4) hippocampal-limbic for memory and emotional disorders (22%); (5) occipito-temporal for complex visual processing (6%); and (6) miscellaneous networks (for example, dyscalculia, apraxia, and disconnection syndrome).44
Infarct in the region of the dominant angular gyrusInfarcts near the dominant angular gyrus cause Gerstmann syndrome, with its tetrad of typical symptoms: not recognising the fingers on both hands (finger agnosia), confusing the left and right sides of the body, losing the ability to calculate (dyscalculia), and losing ability to write (dysgraphia). These symptoms may be associated with alexia, visual hemianopsia, severe left-sided neglect, and anomic aphasia.5,31,37,45
Frontal orbital and cingulate infarctBehaviour disorders are significant in lesions of the frontopolar and callosomarginal arteries (branches of the anterior cerebral artery). Orbitofrontal lesions produce lack of inhibition, impulsive behaviour (‘acquired sociopathy’), and antisocial and sexually inappropriate behaviour (indiscreet and lewd conduct). Medial lesion to the anterior cingulate causes loss of initiative; patients predominantly display lack of motivation, apathy, passiveness, and inertia. Cognition may be intact, or patients may display impairment in temporal organisation and behaviour planning. Loss of intellectual control over conduct and dependence on the social and physical environments manifest as a tendency to imitate the conduct of others and to automatically use objects offered to them without any accompanying instructions (imitation and utilisation behaviours). Other manifestations that may be associated include akinetic mutism (bilateral medial frontal lesion), transcortical motor aphasia (with dominant hemispheric lesions), gaze deviation towards the lesion, diffuse rigidity, sucking and grasp reflexes, and sphincter incontinence.31,36,44,46
Bilateral medial temporo-occipital infarctVertebrobasilar occlusion produces bilateral lesions of the hippocampus with severe amnesia. Memory disorder is accompanied by other signs caused by infarct in the territory of the posterior cerebral arteries (for example, prosopagnosia, cortical blindness, Anton-Babinski syndrome, simultanagnosia, difficulty with coordinated gaze, metamorphopsia, and visual agnosia). Some cases may also present with delirium and psychomotor agitation.31,36,44 In contrast, it has been reported that extensive infarct of the right posterior cerebral artery may be present in individuals with intact cognitive and functional abilities.47
Thalamic and other subcortical strategic infarctsUnilateral or bilateral infarct in the territory of the paramedian arteries (mainly the dorsomedial nucleus and the intralaminar nuclei) can cause amnesia associated with behaviour disorders (‘thalamic dementia’). Decreased or fluctuating level of consciousness is a distinctive finding in the initial phase. Amnesia is also the dominant symptom in cases of infarct of the anterior thalamic nuclei due to disruption of the mammillothalamic fasciculus, as well as in cases of internal medullary laminar infarct due to lesion to amygdalo-thalamic projections. The latter 2 types of lesions are attributed to occlusion of the tuberothalamic artery, and in some cases, of the paramedian artery.31,48–51 The main feature in thalamic vascular amnesia is loss of long-term declarative anterograde memory with a less marked loss in long-term declarative retrograde memory. Implicit and short-term memory are largely preserved. Disorientation, agitation, aggressiveness, apathy, and dysexecutive syndrome are often associated with this type of amnesia.51,52 Extensive infarct in the dominant tuberothalamic territory gives rise to anomic aphasia, other aphasia (impaired fluency and comprehension; paraphasia), and acalculia. Lesion to the non-dominant hemisphere will result in visual memory impairment.53
Globus pallidus infarct in the dominant hemisphere may manifest only as acute cognitive and behavioural changes (lack of attention, decreased verbal fluency, emotional blunting, amnesia, and executive dysfunction).54 Infarction of the head of the caudate nucleus in the dominant hemisphere is caused by obstruction of the lateral lenticulostriate arteries (branches of the middle cerebral artery). Clinically, this manifests as memory disorders or general mental impairment (apathy, aggression, affective symptoms with psychotic features). Lastly, the literature reports that infarct in the anterior limb and/or genu of the internal capsule may initially manifest with dementia and absent or minimal motor signs (for example, faciolingual weakness).55–57
Dementia due to cerebral small vessel diseaseThe term ‘cerebral small vessel disease’ refers to a group of pathological processes that are caused by various agents and affect small arteries, arterioles, venules, and capillaries in the brain. Its 2 most common forms are cerebral amyloid angiopathy and small vessel disease related to age and arterial hypertension. The consequences of small vessel disease on the cerebral parenchyma are heterogeneous and primarily located in subcortical structures. They include lacunar infarcts, ischaemic white-matter lesions, and haemorrhages. The term ‘small vessel disease’ is often used as a synonym of lesions to the cerebral parenchyma since there is thought to be a causal relationship, and since viewing small blood vessels in vivo is not currently possible. Furthermore, small vessel disease is often used to refer exclusively to arterial disease without considering venous pathology; in these cases, ‘small artery disease’ would be a more appropriate term.58
L. Grinberg and H. Heinsen recently showed how the same specific type of subcortical vascular dementia might be assigned different names by neurologists, radiologists, and pathologists, or even worse, how a single radiological or clinical entity could correspond to multiple neuropathological lesions. For example, 3 different anomalies, such as arteriolosclerosis, white-matter oedema, and local myelin rarefaction, may all be called ‘leukoaraiosis’ by radiologists. Similarly, the ‘lacunar state’ described by Marie resembles ‘atherosclerotic cerebral degeneration or atrophy’ as described by Binswanger and Alzheimer. Interpreting atherosclerotic lesions in small vessels is also difficult because arteriolosclerosis is occasionally subdivided according to its most pronounced histological trait (fibrinoid necrosis, lipohyalinosis, microatheromas), and these findings may also present simultaneously. Lastly, some definitions of ‘lacune’ cite incorrect measurements (for example, a maximum volume of 15mm3), or they do not specify its cause (infarct, haemorrhage, enlarged perivascular space).9,39,58,59
The clinical characteristics of VCI associated with small vessel disease include gait, mood, and behaviour disorders, and loss of sphincter control. Signs are initially mild and only vaguely associated. As the disease progresses, the patient develops dementia, severe gait impairments resulting in frequent falling and eventual inability to walk, marked depression and apathy, urinary incontinence, and extrapyramidal motor signs.6,60–62 Non-cognitive symptoms are often overlooked and patients are mainly examined for cognitive deficit, which provides a limited perspective on the effects of cerebrovascular disease. In contrast, cognitive problems are the most prevalent in patients with AD.58,63
Unlike the dementia syndromes appearing after a major stroke, the processes that lead to dementia by small artery disease can be grouped into different stages. Their course is progressive and relatively insidious.58,63 There are 3 types of lesions involved in cognitive impairment secondary to small cerebral artery disease, and all 3 may appear at once: (1) ischaemic white-matter lesions, (2) lacunar infarcts, and (3) haemorrhages.64
Ischaemic white-matter lesionsMRI studies show more or less confluent lesions situated bilaterally and symmetrically in the periventricular or subcortical white matter. Lesions are hyperintense in T2 and FLAIR sequences (white matter hyperintensities, WMH). Lesions near the frontal and occipital horns are homogeneous, but those located in the periventricular white matter frequently appear as conglomerations of small lesions. CT shows these lesions as hypodense areas around the frontal and occipital horns of the lateral ventricles; they frequently extend to the subinsular white matter of the frontal lobe and the white matter of the centrum semiovale.65,66 By definition, these lesions are not found adjacent to areas of cortical lesion or ventricular enlargement, and this fact is useful for distinguishing them from lesions left by large white-matter infarcts. Leukoaraiosis, or rarefaction of the white matter, is a term introduced by Hachinski, Potter, and Merskey in 1986 to describe these changes, to prevent confusion with an ambiguously defined clinical and pathological entity known as ‘Binswanger disease’,39 and to discourage doctors from rushing to assign a specific meaning to radiology findings. When this neologism was introduced, most studies were based on CT findings, and some doctors still believe that the term should only be used to refer to lesions viewed using CT.58,67
The implications of leukoaraiosis and its related vascular changes have yet to be fully explained. The condition probably arises due to multiple factors,64 but it is generally believed to originate with hypoxia and ischaemia.3,14,15,68 Some cases of leukoaraiosis are likely to have occurred due to small isolated or confluent infarcts.68 Leukoaraiosis is a finding in cerebral small vessel disease, an entity that favours stroke and includes hypertensive arteriopathy, amyloid angiopathy, CADASIL (cerebral autosomal dominant arteriopathy subcortical infarcts and leukoencephalopathy), and possibly venous disease as well.15,58,69
It is currently believed that mild leukoaraiosis detectable by an MRI study is a relatively normal finding in the brains of elderly patients.5 However, cumulative evidence shows that moderate to severe changes in the white matter are not benign. These cases are linked to motor and gait disorders, depressive symptoms, urinary disorders, and specific cognitive deficits that worsen due to the influence of associated lesions (lacunar infarcts, coexisting degenerative diseases).70,71 The cognitive domains affected by leukoaraiosis are not clearly established, but they show a strong association with cognitive and functional impairment.6,19,72 In general, patients with periventricular leukoaraiosis show a more marked cognitive decline than those with deep-tissue lesions.64 While visual and volumetric quantitative measurement scales are comparable, they do not have a well-defined practical role. This is probably due to their ceiling effects which limit their use as outcome measures.19,66,73
Lacunar infarctsLacunar infarcts (or type 1 lacunae) are frequently associated with chronic ischaemic lesions of the cerebral white matter. They are typically located in the corona radiata, basal ganglia, internal capsule, thalamus, and base of the pons. Lacunar infarcts appear as hypodense areas in CT images, hypointense areas in T1-weighted MRI sequences, and hyperintense areas in proton density-weighted or T2-weighted MRI. While there is no real consensus on the size of lacunar infarcts, the commonly accepted maximum diameter is 15mm based on the original pathology description (this limit is equivalent to 20mm on CT/MRI images).58,64
Some lacunar infarcts are found within the area of diffuse white-matter lesions.71 It is difficult to use MRI to determine, in such cases, whether these lesions should be classified as pure lacunar infarcts, or rather as the end consequences of leukoaraiosis. In fact, the most severe forms of leukoaraiosis resemble cavities on neuroimaging studies, and they may therefore be interpreted as lacunar infarcts.58 Differentiating between cavitated infarcts and dilated perivascular spaces (type III lacunae) also poses considerable diagnostic challenges.74
Lacunar infarcts are widely accepted to be a sign of cerebral small artery disease.64 Following strict criteria, it is more appropriate to diagnose patients with a cerebral small artery disease after excluding sources of embolism and finding multiple lacunar infarcts, or lacunar infarcts associated with moderate to severe leukoaraiosis.58 Lacunar infarcts may present in the following forms: (1) lacunar syndrome (pure motor hemiparesis, pure sensory syndrome, sensorimotor syndrome, ataxic hemiparesis, dysarthria/clumsy hand, or atypical lacunar syndrome); (2) transient ischaemic attack; or (3) an asymptomatic finding in a neuroimaging study. Nevertheless, silent progression of cerebral small vessel disease has been demonstrated for all 3 forms, and lacunar infarcts are potential causes of sequelae and functional disability.75,76 Arterial hypertension and diabetes are risk factors that clearly indicate a predisposition to lacunar infarct caused by ischaemic small vessel disease.58
Factors associated with cognitive decline in lacunar infarct are localisation in strategic areas (for example, the thalamus, putamen, or globus pallidus) and presence of multiple lacunae (lacunar state).6,37,77,78 A strategic lacunar lesion may be associated with cognitive disorders as part of the clinical presentation of the stroke. Cognitive impairment is also frequently associated with silent lacunar infarcts (infarcts that are not clinically related to stroke and are discovered incidentally in neuroimaging studies).79,80
Mild neuropsychological disorders have been observed in more than half of all patients with an initial lacunar infarct, especially those appearing with atypical lacunar syndrome or pure motor hemiparesis.81 Furthermore, volumetric loss of grey matter studied with voxel-based morphometry, as reported by M. Grau-Olivares et al.,82 is thought to contribute to the cognitive impairment that accompanies lacunar infarct. Patients with vascular MCI show more atrophy in the middle temporal gyrus, frontal regions, and posterior occipito-parietal regions, bilaterally in all cases and including the posterior cingulate and cerebellum.
The number of infarcts in basal ganglia and the internal capsule due to occlusion of small cerebral arteries (lacunar state) is related to dementia and multifocal sensorimotor manifestations (rigidity, spasticity, pseudobulbar paralysis, hemiparesis, muscle hyperreflexia, Babinski reflex, gait with small steps, and urinary incontinence). There is typically a history of mild and transient neurological disability, and the first symptoms may resemble classic lacunar syndrome.83 Cognitive manifestations include slow information processing, memory loss, and reduced capacity for sustained attention. Behavioural manifestations are normally attributed to prefrontal lesions, and they include apathy and akinetic mutism. Lacunar lesions in the subcortical prefrontal cortex are associated with decreased verbal fluency and executive function, increased risk of stroke and dementia, and more rapid cognitive decline, even when controlling for other vascular risk factors.5,15,84
CADASIL is the most important vascular dementia model to be linked to subcortical microangiopathy. This autosomal dominant disorder is caused by a mutation in the gene coding for the transmembrane receptor Notch 3 on chromosome 19 (19p13.1). It is probably one of the most common heritable neurological diseases, and the most important hereditary cause of ischaemic stroke. Its clinical manifestations are diverse, and most patients present recurring headache (migraine, usually with prolonged or atypical aura) and focal neurological deficit (secondary to one or more cerebral infarcts). Patients in advanced stages may display progressive psychomotor impairment, including dementia. Depression is present in approximately 20% of all cases. Typical MRI findings are as follows: (1) multifocal and bilateral hyperintensities in FLAIR and T2-weighted sequences located in the deep periventricular white matter (mainly affecting the temporal anterior pole, frontal and parietal lobes, external capsule, pons, and basal ganglia), (2) focal hypointensities in T1 (lacunar infarcts), and (3) lesions suggesting microbleeds. The clinical diagnostic process, based on molecular genetic studies, or examination of blood vessels in different affected organs (usually the vessels of the dermis), has been thoroughly evaluated in the reviews by André and by Río-Espínola et al.85,86
HaemorrhagesThe description of small vessel disease is restricted to the ischaemic process (lacunar infarcts and ischaemic white matter lesions). This practice is somewhat deceptive, and overlooks the possibility of there being large haemorrhages and microbleeds (lacunar haemorrhage, type 2 lacunae). Large haemorrhagic lesions in strategic areas of the brain are easily recognisable using conventional neuroimaging, including CT, but microbleeds can only be detected with the appropriate MRI techniques, such as gradient-echo or T2-weighted sequences.58,87,88
Microbleeds are observed as small areas (usually round foci smaller than 5mm in diameter) that are hypointense in T2-weighted brain MRI sequences.64 They are frequently associated with lacunae and white matter hyperintensities. These entities represent focal haemosiderin deposits left behind by previous haemorrhagic events. Microbleeds are a marker for cerebral microangiopathy, which is usually lipohyalinosis. In patients with ischaemic cerebrovascular disease, the number and location of microbleeds may be associated with executive dysfunction.15,37,88
Mixed Alzheimer disease and vascular dementiaThe relationship between AD and VCI is not fully understood, but it is widely accepted that the entities often coexist.3,4,8,18,36,37,83,89 Vascular dementia and Alzheimer-type dementia are distinguished as concepts based on their vascular risk factors, clinical findings (such as acute onset, stepped progression, and emotional lability), and neuroimaging findings (Table 3).5,41
Useful criteria for diagnosing vascular cognitive impairment
| Essential diagnostic criterion |
|---|
| A. Presence of cognitive impairment plus cerebrovascular lesion |
| • Dementia or mild cognitive impairment (Table 1) |
| • Cerebrovascular lesion: presence may be suggested by clinical information (history of stroke, focal neurological signs with or without a history suggesting a lesion). Presence of lesions should always be demonstrated by cranial MRI/CT and/or anatomical pathology study (Table 2). |
| Criteria indicating a causal relationship |
| B. Onset of cognitive impairment occurs immediately after stroke, plus any one of the following: |
| • Cognitive impairment does not intensify or improve over timea |
| • Cognitive impairment with stepped progressiona |
| • Younger patient unlikely to show associated AD (especially early onset familial Alzheimer disease) |
| C. MRI or anatomical pathology study revealing a cerebrovascular lesion affecting a strategic area or network for cognitive functions |
| • Localisation in border zone (in dominant hemisphere or bilaterally): superior frontal (watershed between the ACA and the MCA), parieto-occipital (watershed between the MCA and PCA), and internal (between the ACA, MCA, PCA, and the area supplied by the recurrent artery of Heubner, lenticulostriate, and anterior choroid arteries) |
| • Angular gyrus (of dominant hemisphere or bilaterally) |
| • Orbitofrontal and cingulate (bilateral ACA) |
| • Anterior thalamus, dorsal-paramedian or dorsomedial (unilateral or bilateral thalamic perforating artery) |
| • Medial temporal, hippocampus (bilateral PCA) |
| • Caudate nucleus (lenticulostriate branch of the MCA in the dominant hemisphere) |
| • Other key areas of the grey or white matterb in the dominant hemisphere or bilaterally: anterior part of the putamen, anterior limb of internal capsule, genu of the internal capsule |
| D. Identification of a genetic biomarker for cerebrovascular disease that causes dementia |
| • Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: mutation in the NOTCH3 gene located on chromosome 19 |
| • Hereditary cerebral haemorrhage with amyloidosis: mutation in the gene for amyloid precursor proteins |
| Exclusion criteria |
| • Absence of cerebrovascular lesion in multimodal MRI study |
| • Evidence of a disorder severe enough to cause cognitive impairment: major depression, toxic-metabolic disorder (requires specific studies), intracranial neoplasia, subdural haematoma, chronic hydrocephalus, intracranial infection |
ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; AD: Alzheimer disease; MRI: magnetic resonance imaging; CT: computed tomography.
Implies a sufficiently prolonged progression time. History of gradually progressing cognitive decline before or after a stroke is suggestive of a neurodegenerative disorder.
Ischaemic leukoencephalopathy must be diffuse and extensive (at least 25% of the total white matter distributed arbitrarily in periventricular regions, or an area of the white matter exceeding 10cm2). In clinical practice, diagnosis of the specific form of vascular cognitive impairment is considered when the patient meets requirement A and at least one of the criteria indicating a marked causal role (for example, B, C, or D).
The diagnostic utility of this dichotomy is limited, however, and the concept of ‘mixed dementia’ is important if we are to understand the underlying pathophysiology in AD and accurately estimate the impact of VCI.8,9,18,90 In this area, standard diagnostic criteria provide an imprecise perspective. In cases of systemic or other cerebral disorders linked to declining memory and cognition (such as AD), only the ADDTC model proposes the alternative diagnosis of ‘mixed dementia’. The NINDS-AIREN criteria list a more specific term, ‘AD with cerebrovascular disease’.11,12
Evidence of both AD and cerebrovascular disease is present in the most prevalent form of mixed dementia.91 The possibility of concomitant AD often obscures the relationship between the cerebrovascular lesion and cognitive impairment. Cerebrovascular disease is able to lower the threshold at which Alzheimer disease can give rise to clinical manifestations of dementia. Degenerative or vascular lesions alone can be insufficient to cause dementia, but as they accumulate and interact with each other, they may result in impaired cognition and functional ability.9,91 Determining which of the pathological findings is the main cause of cognitive impairment may be quite difficult in vivo.14,40
In mixed dementia, evidence of cerebrovascular disease is present and typically associated with the following indicators of AD: (1) history of slowly progressive memory loss, (2) marked episodic amnesia (decreases in recent memory and learning capacity with preservation of attention and poor cued recall), and (3) atrophy of the medial temporal lobe in MRI.36,91 The impact of both cerebrovascular disease and AD increases with age.
Gorelick et al.92 recently published an interesting article on the evidence of how vascular disease contributes to cognitive impairment and dementia. This article proposes a novel classification system for the certainty of a VCI diagnosis (probable for more ‘pure’ forms, and possible when diagnostic certainty is lower or a ‘mixed’ process is present). Nevertheless, we note that the above description should be interpreted with caution because it lacks an acceptable level of consistency with regard to temporal patterns, cognitive profiles, imaging study findings, and pathological markers.93 To optimise diagnostic capability for both ‘pure’ and ‘mixed’ forms of vascular dementia, we recommend using the criteria and protocols listed here (Tables 3 and 4) in clinical practice.1,5,6,18,94
Recommendations for clinical evaluation of patients with VCI
| Medical history and clinical examination |
|---|
| • Patient's demographic data: sex, date of birth, racial/ethnic subgroup, level of education |
| • Information supplied by the informant: demographic data, length and type of contact with patient |
| • Family history: stroke, vascular disease, or dementia in first-degree family members |
| • Personal history: cardiovascular or cerebrovascular disorder, arterial hypertension, hyperlipidaemia, diabetes mellitus, tobacco or alcohol dependence, physical inactivity, sleep disorders, sickle-cell trait, hypercoagulable state or related disorders (deep vein thrombosis, pulmonary embolism, miscarriage), chronic infections, nephropathy, surgery, hormonal contraceptives, other drugs, and environmental exposure to toxins |
| • History of the disease in question: symptom onset, cognitive and behavioural symptoms, motor symptoms (gait disorder, muscle weakness, difficulty swallowing, urinary incontinence), emotional and mood changes (evaluate for depression), functional capacity (especially for instrumental activities of daily living) |
| • General physical and neurological examination: weight, height, blood pressure, nutritional state, heart rate, visual acuity, hearing, cardiovascular study, and neurological examination (items from the NIH stroke scale, gait, muscle reflexes, Babinski sign) |
| • Mental examination: Subtests on the 5-minute Montreal Cognitive Assessment (immediate and deferred recall of 5 words, orientation of 6 items, and phonemic fluency test for the letter F)*; other tasks may be added to further assess specific functions. |
| Special diagnostic tests |
| • Blood analysis |
| • Complete blood count including platelet count, erythrocyte sedimentation rate, glycaemia, renal function panel, coagulation profile, lipid profile, thyroid hormones, and serum B12 levels |
| • Depending on the clinical context, other tests may include syphilis and HIV serology, thrombophilia testing, lupus anticoagulant testing, anticardiolipin antibodies test, toxin level testing, and ceruloplasmin test |
| • 12-lead electrocardiogram |
| • Cerebral imaging tests |
| • Multimodal cranial MRI: T1-weighted 3D, T2-weighted, and FLAIR (fluid-attenuated inversion recovery) sequences to detect infarcts or other lesions (atrophy, white matter hyperintensities, malformation, extra-axial fluid collection) and gradient-echo to detect haemorrhage. MRI study should be performed in the early stages of VCI, especially if CADASIL is suspected. If the technique is available, the study should be completed with echo-planar diffusion-weighted imaging and MRI angiography (intracranial and cervicocephalic arteries). |
| • Cranial CT: ventricular dilation, medial temporal lobe atrophy, severe white matter hypodensities (number, volume, localisation), acute haemorrhage. This alternative to MRI serves the specific purpose of demonstrating brain lesions in severe cases. |
| • PET or SPECT: pattern of hypometabolism or hypoperfusion that is irregular or predominantly frontal (includes the cingulate and superior frontal gyri), with preservation of the occipital cortex. Useful for differential diagnosis with AD |
| • Vascular ultrasound: carotid duplex and transcranial Doppler studies (vasoreactivity, pulsatility index, blood flow velocity, microemboli) |
| • Cerebral angiography study: to evaluate carotid stenosis, or in suspected cerebral vasculitis |
| • CSF study: for suspicion of infectious or inflammatory origin, or chronic hydrocephalus in an adult; used in biomarker-based differential diagnosis in selected cases |
| • Genetic study: in suspected cases of hereditary cerebral arteriopathy. If there is a strong clinical suspicion of CADASIL, but genetic study results are negative or not available, ultrastructural examination of a skin biopsy specimen is performed. |
Source: references1,5,6,18,94.
Various biomarkers have been explored to aid in early detection, distinguish between neuropathological findings, assess prognosis, and monitor disease progression or treatment response in patients with VCI.5,95 In this area, markers related to genetic factors and mediators of inflammation involved in VCI aetiopathogenesis are of particular interest.
Genetic factors play a confirmed role in vascular dementia. Several monogenic forms of cerebrovascular diseases that affect cognitive function have now been identified. Scientists have shown particular interest in CADASIL and hereditary cerebral haemorrhage with amyloidosis, Dutch type (HCHWA-D). In contrast, few studies have examined the genes that affect the brain's susceptibility to lesions caused by cerebrovascular disease (for example, genes linked to AD).3,85,86
Analysis of cerebrospinal fluid (CSF) biomarkers, along with clinical and neuroimaging findings, may also be helpful in diagnosing the different brain disorders causing cognitive impairment.95 Vascular dementia is generally related to the following array of CSF biomarkers:
- -
Total tau. Total tau is a dynamic marker of the intensity of axonal degeneration and damage. Elevated levels are found in Creutzfeldt-Jakob disease, and they may also be high in post-stroke dementia, brain trauma, and AD.96–99
- -
Light subunit of the neurofilament protein. The light subunit of the neurofilament protein is the best CSF biomarker of subcortical axonal damage and degeneration. High levels are present in vascular dementia, frontotemporal dementia, and numerous inflammatory disorders (multiple sclerosis, AIDS dementia). When found in combination with an AD biomarker, it indicates mixed dementia.5,100–103
- -
CSF/serum albumin ratio. CSF/serum albumin ratio is the best-defined measure of the integrity of the blood-brain barrier. This ratio tends to be high in patients with vascular dementia, especially that caused by subcortical vessel disease.95,98,99,103
- -
Level of tissue necrosis factor alpha. Tissue necrosis factor alpha, a proinflammatory cytokine, mediates damage to myelin. High levels are measured in patients with subcortical vascular dementia, and these levels correlate to levels of sulfatide (a marker of white matter degradation).99,104
CSF findings of low levels of the 42-amino acid form of amyloid beta, high levels of hyperphosphorylated tau, or biochemical signs of neuroinflammation (elevated leukocytes, IgG or IgM production) all indicate non-vascular dementia. These biomarkers may be useful for ruling out a diagnosis of pure vascular dementia. Nevertheless, using CSF biomarkers to diagnose vascular dementia requires a standardised procedure for obtaining, storing, and measuring the samples.5,95,96,105
Diagnosis of vascular mild cognitive impairmentCriteria for diagnosing vascular dementia are not sensitive to clinical phenotypes of cognitive impairment caused by a vascular process, if symptoms are not severe enough to be considered dementia (VCI no-dementia). A lack of precise diagnostic criteria for this entity has led to ambiguous and heterogeneous use of these terms. VCI-no dementia, categorised with the VCI entities, might be described in similar terms to MCI.5,63,106–108
Based on a long list of studies and consensus statements, MCI has been defined as a syndrome of impairment of memory and/or other higher cognitive functions that is more pronounced than that seen in age- and education-matched subjects with normal ageing. MCI may or may not affect daily life, but it is not severe enough to be considered dementia (Table 1).27,109 As perspectives on MCI have evolved, the concept has become more refined and is now thought to include 4 subtypes:
- -
Amnestic single domain MCI. The sole manifestation of cognitive impairment is a slight memory deficit.
- -
Amnestic multiple domain MCI. Slight memory deficit and similar degrees of difficulty in other areas, such as problem-solving or finding words.
- -
Non-amnestic single domain MCI. No memory deficit is present, but evidence points to mild impairment limited to another cognitive area: executive functions, visuospatial ability, or language use.
- -
Non-amnestic multiple domain MCI. No memory deficit is present, but several other cognitive areas display impairment.5,28,29
The term ‘vascular predementia MCI’ is sometimes used instead of VCI no-dementia.5 Although most patients diagnosed with MCI will develop dementia, this does not always occur.28,110 MCI may be transient and reversible (unstable MCI), or continued with little variation over long periods of time, or else progressive and evolve into dementia without a clearly defined transitional stage.30,111,112 Vascular MCI symptoms may disappear in the context of other processes (for example, post-stroke recovery).
Numerous and contradictory reports describe the risk of dementia progression associated with each MCI subtype, and some address using MCI subtypes to predict different forms of dementia.113 Nordlund et al.112 demonstrated that the conversion rates for AD and vascular dementia (including multiple domain dementia) are quite similar. Only patients with MCI and multiple affected domains developed dementia; patients with a single-domain form seemed to have a more favourable prognosis.
The role of vascular disease in patients with MCI has been examined by only a few longitudinal studies, and some results are contradictory.114 The study by Di Carlo et al. shows that arterial hypertension and heart failure are consistently and significantly associated with multiple domain forms of MCI. On the other hand, prior stroke was a strong predictor of dementia, even for patients whose initial assessment did not reveal cognitive impairment.106 An evaluation of a large sample of patients with MCI identified an association between WMH and cognitive decline.72 Furthermore, the same group of researchers reported that WMHs were linked to a higher risk of vascular or mixed dementia, but not of AD.115 Smith et al. found that WMHs were associated with risk of progression from normal cognitive function to MCI, but not from MCI to dementia.116 These results may be skewed by the fact that stroke and vascular risk factors (especially arterial hypertension) may affect cognition at different time intervals after the index event or the initial diagnosis of cognitive impairment. It has also been suggested that WMHs that affect the fronto-parietal network, but not the frontal network, contribute significantly to executive function loss in MCI.117
A recent study reported that a group with subcortical vascular MCI had relatively greater success with medium-term recognition and memory tasks (verbal and visual), compared to subjects with MCI-AD.118 Nevertheless, the cognitive effects of certain specific forms of cerebrovascular disease, such as microbleeds, have scarcely been researched.64 In the Staekenborg et al.114 study of patients with MCI, deep periventricular WMHs were more severe, and microbleeds and basal ganglia lacunes were more frequent, in patients who progressed to non-Alzheimer dementia than in patients who did not exhibit that progression. These researchers also found that deep periventricular WMHs predict progression to non-Alzheimer dementia, whereas this risk is not associated with microbleeds. These results indicate that the combination of biomarkers with more precise and limited cognitive profiles may serve as a tool for achieving more precise diagnoses and predicting outcomes in VCI.
ConclusionsThe principles underlying diagnosis of VCI have advanced, but the available criteria were basically designed for differentiating vascular dementia from Alzheimer-type dementia, and for use in research. However, clinical practice requires precision in the following areas: (1) clinical diagnosis of dementia and MCI, (2) clinical and imaging-based identification of the different cerebrovascular lesions associated with cognitive dysfunction, and (3) establishment of an aetiopathogenic relationship between cognitive impairment and cerebrovascular lesions. To this end, we suggest forming a multidisciplinary group of experts to standardise the nomenclature and diagnostic criteria for the full spectrum of VCI subtypes, especially vascular dementia and its categories. Clarifying these concepts has the potential to further epidemiological and pharmacological research on an increasingly important health issue.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez García PL, Rodríguez García D. Diagnóstico del deterioro cognitivo vascular y sus principales categorías. Neurología. 2015;30:223–239.