Drug-induced parkinsonism is a major type of parkinsonism in our setting. Symptoms usually disappear after discontinuation of the drug. However, they may persist in patients with a variant known as subclinical drug-exacerbated parkinsonism; early identification of this entity has important prognostic and therapeutic implications. The most widely used complementary test in this diagnosis is single-photon emission computed tomography with ioflupane (123I), also known as 123I-FP-CIT SPECT. The aim of our study is to verify its diagnostic accuracy.
MethodsWe designed a prospective study of patients with drug-induced parkinsonism in which, after discontinuing the drug and undergoing a 123I-FP-CIT SPECT scan, patients would be monitored for at least 6 months. Patients were categorised as having iatrogenic parkinsonism if symptoms disappeared, or as having subclinical drug-exacerbated parkinsonism if they persisted. Lastly, we verified concordance between the clinical diagnosis and results from the 123I- FP-CIT SPECT scan.
ResultsThe sample included 19 patients. The most commonly prescribed drug class was neuroleptic agents. For the diagnosis of both subgroups, 123I-FP-CIT SPECT showed a sensitivity of 66.7%, specificity and positive predictive value of 100%, a negative predictive value of 86.7%, and a negative likelihood ratio of 0.33.
ConclusionsAlthough the study needs to be repeated in a larger sample of patients, 123I-FP-CIT SPECT is useful in the diagnosis of drug-induced parkinsonism since it is a very precise tool for identifying patients with that illness.
El parkinsonismo relacionado con fármacos es una de las principales causas de parkinsonismo en nuestro entorno. La clínica suele desaparecer tras la retirada del fármaco, sin embargo puede persistir en un grupo de pacientes que presentan lo que se conoce como parkinsonismo subclínico exacerbado por fármacos y cuya identificación precoz es importante por las implicaciones pronósticas y terapéuticas. La prueba complementaria más utilizada para ello es la tomografía por emisión de fotón simple con 123Ioflupano, también conocida como SPECT [123I]FP-CIT. El objetivo de nuestro estudio es corroborar su utilidad para tal fin.
MétodosSe diseñó un estudio prospectivo de pacientes con parkinsonismo relacionado con fármacos a los que, tras retirada del mismo y realización de SPECT [123I]FP-CIT, se les siguió durante un mínimo de 6 meses. Los pacientes se catalogaron como parkinsonismo iatrogénico si había desaparecido la clínica o parkinsonismo subclínico exacerbado por fármacos si la misma persistía. Finalmente se comprobó la concordancia entre el diagnóstico clínico y los resultados del SPECT [123I]FP-CIT.
ResultadosLa muestra quedó constituida por 19 pacientes. El grupo terapéutico mayoritario fueron los neurolépticos. Para el diagnóstico de ambos subgrupos el SPECT [123I]FP-CIT mostró una sensibilidad del 66,7%, una especificidad y un valor predictivo positivo del 100%, un valor predictivo negativo del 86,7% y una razón de verosimilitud negativa de 0,33.
ConclusionesAunque es necesario comprobarlo con un número mayor de pacientes, el SPECT [123I]FP-CIT es una técnica útil en el diagnóstico de parkinsonismo relacionado con fármacos ya que identifica con gran precisión a los enfermos.
After idiopathic Parkinson's disease (IPD), drug-induced parkinsonism (DIP) is the second leading cause of parkinsonism in both the elderly1 and the general population.2,3 It is also the most common cause of secondary parkinsonism in the Western world.4 Spanish medical literature retains the concept of ‘induced’ by translating the English term as parkinsonismo inducido por fármacos. In this article, however, we have opted to use the term ‘drug-related parkinsonism’ (DRP). We believe that the word ‘related’ is more suitable for defining this group of patients. It expresses a link or an association between a drug and the patient's clinical condition without suggesting any aetiological implications.
DRP is secondary to the intake of a wide number of drugs, mainly dopamine antagonists (such as neuroleptics and benzamides), and to a lesser extent, antidepressants and calcium channel blockers.4–7 Parkinsonian symptoms have also been described with a wide variety of drugs, including amiodarone,8 valproic acid,9,10 lithium,11 and trimetazidine.12 Drug-induced parkinsonian symptoms usually disappear within 6 months after the drug is discontinued,6,7,13 but symptoms persist in between 10% and 48% of all patients.2,4,5,7,14–16 These patients present latent parkinsonism which first manifests with the intake of certain drugs. The entity is known as subclinical drug-exacerbated parkinsonism (SDEP). Many of these patients are finally diagnosed with DIP.7 Some studies have demonstrated that levodopa treatment, in addition to discontinuing the problematic drug, yields good results,8,17 which means that identifying the entity in an early stage is crucial. Unfortunately, the clinical similarity between iatrogenic parkinsonism (IP) and neurodegenerative parkinsonism,8,18–20 including SDEP, makes it more difficult to perform differential diagnosis. Therefore, a series of complementary tests are usually required to establish an accurate diagnosis.
Single-photon emission computed tomography with 123ioflupane, also known as 123I-FP-CIT SPECT or DaTSCAN, is currently considered the test of choice for differential diagnosis of DRP and other types of parkinsonism that are neurodegenerative.8,18,19,21–23 IP appears as a consequence of the dopamine receptor blockade induced by drugs. It does not affect the striatonigral pathway, and therefore, radiotracer uptake remains unchanged.17–19,24–26 On the contrary, neurodegenerative forms of parkinsonism, including SDEP, do exhibit altered radiotracer uptake.17–19
According to the literature search, the total number of patients with DRP who have undergone a 123I-FP-CIT SPECT scan and clinical monitoring is quite low,8,17,22,25,27 and very few studies provide data about validity and safety of this diagnostic technique.22,25,27 These findings have led us to design the current study, which aims to verify the diagnostic accuracy of 123I-FP-CIT SPECT for early identification of patients with IP or SDEP, in the context of DRP. A secondary objective is to compare clinical characteristics between the 2 groups.
Patients and methodsA prospective, blinded study of patients referred by general neurology departments was performed in accordance with guidelines established by the Ethics Committee at Hospital Torrecárdenas (Almería, Spain). The inclusion criteria for this study were as follows:
- •
Legal adulthood.
- •
Non-idiopathic Parkinson's disease, defined as the presence of bradykinesia and at least 1 of the following clinical features: resting tremor (4–6Hz) and/or extrapyramidal rigidity.
- •
Treatment with potential inducers of parkinsonism.
Exclusion criteria were as follows:
- •
History of parkinsonism prior to starting treatment with potential inducers of parkinsonism.
- •
Cognitive impairment simultaneous to onset of parkinsonian symptoms, or within 1 year of symptom onset.
- •
Neuroimaging tests (MRI and/or CT) showing lesions that could explain parkinsonism.
- •
Findings strongly indicative of non-pharmacological parkinsonism, such as ophthalmoparesis, pseudobulbar syndrome, cortical focality, symptomatic dysautonomia that cannot be explained by other causes, or history of early and recurrent falls.
- •
Obstacles to discontinuing the parkinsonism-inducing drug.
- •
Not meeting the inclusion criteria according to a specialist in movement disorders.
After providing their informed consent, the patients included in the study were evaluated by the lead researcher (a neurologist with several years of experience supervising the movement disorders unit in a tertiary hospital), using UPDRS (United Parkinson's Disease Rating Scale) as a motor subscale. The parkinsonism-inducing drug was discontinued. Researchers completed functional and structural image studies (123I-FP-CIT SPECT or MRI/CT, respectively) and patients returned for a check-up within 6 months at the most. At that time, they were re-evaluated by the same researcher using UPDRS, as described before. Patients whose parkinsonian syndrome had resolved were categorised in the IP group, whereas those who continued to experience symptoms were placed in the SDEP group. The gold-standard diagnostic method was clinical evaluation performed by the specialist in movement disorders. Once patients had been categorised in the appropriate groups, the specialist checked the result from SPECT and its concordance with the reference or clinical diagnosis.
The methodology for performing a 123I-FP-CIT SPECT scan followed the protocol established by the department of nuclear medicine at Hospital Torrecárdenas: 123I-FP-CIT injection with the standard dose; brain SPECT scan at 3hours; acquisition of 64 frames, 30seconds per frame, 128 x 128 matrix; and subjective evaluation of the uptake pattern and intensity by 2 nuclear medicine specialists and comparison with the normal pattern (obtained when the Department started using the technique in 2004). No semi-quantitative analyses of the images were carried out.
A descriptive analysis of the qualitative variables was performed using a frequency table and percentages. Quantitative variables were described using the mean, standard deviation, median, confidence interval, and range from the groups constituted by the variable ‘final diagnosis’.
To calculate the sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), and likelihood ratios (LR), we defined the following assumptions:
- -
TP: Clinical diagnosis of SDEP and altered striatonigral uptake.
- -
TN: Clinical diagnosis of IP and no changes in striatonigral uptake.
- -
FP: Clinical diagnosis of IP and altered striatonigral uptake.
- -
FN: Clinical diagnosis of SDEP and no changes in striatonigral uptake.
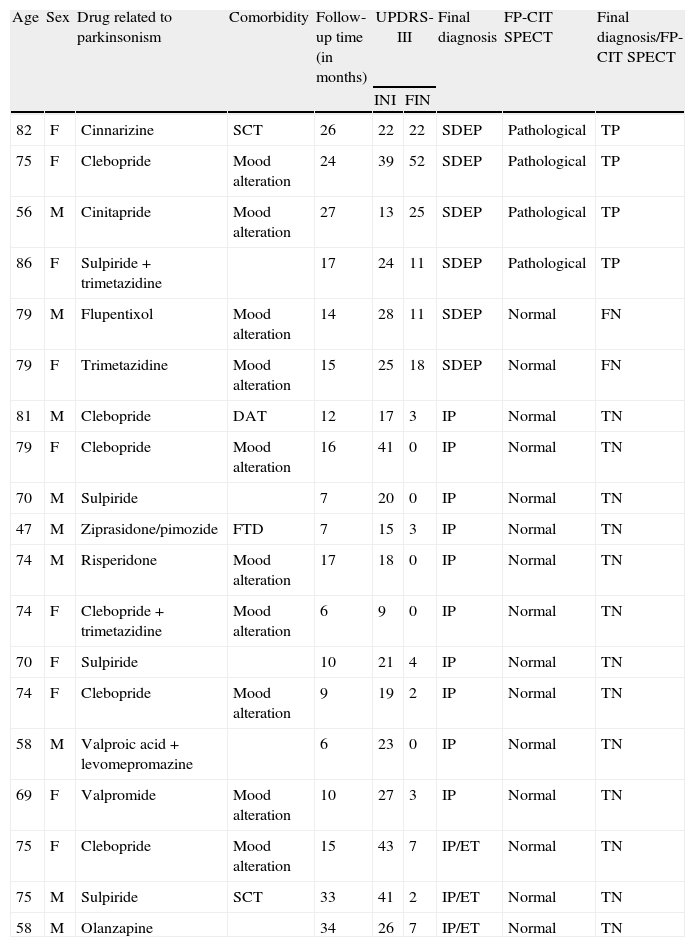
Between 2008 and 2011, 28 patients were recruited to participate in the study. Of these 28, there were 9 exclusions (32%) due to different reasons: three patients refused to undergo 123I-FP-CIT SPECT; 2 patients left the study; and MRI and/or cranial CT scans for the remaining 4 patients revealed vascular lesions explaining the parkinsonian symptoms. The final sample used for the statistical analysis therefore consisted of 19 patients; demographic and clinical characteristics and results from the 123I-FP-CIT SPECT scan are shown in Table 1.
Demographic and clinical characteristics of patients and results from the 123I-FP-CIT SPECT scan in patients with drug-related parkinsonism.
| Age | Sex | Drug related to parkinsonism | Comorbidity | Follow-up time (in months) | UPDRS-III | Final diagnosis | FP-CIT SPECT | Final diagnosis/FP-CIT SPECT | |
| INI | FIN | ||||||||
| 82 | F | Cinnarizine | SCT | 26 | 22 | 22 | SDEP | Pathological | TP |
| 75 | F | Clebopride | Mood alteration | 24 | 39 | 52 | SDEP | Pathological | TP |
| 56 | M | Cinitapride | Mood alteration | 27 | 13 | 25 | SDEP | Pathological | TP |
| 86 | F | Sulpiride+trimetazidine | 17 | 24 | 11 | SDEP | Pathological | TP | |
| 79 | M | Flupentixol | Mood alteration | 14 | 28 | 11 | SDEP | Normal | FN |
| 79 | F | Trimetazidine | Mood alteration | 15 | 25 | 18 | SDEP | Normal | FN |
| 81 | M | Clebopride | DAT | 12 | 17 | 3 | IP | Normal | TN |
| 79 | F | Clebopride | Mood alteration | 16 | 41 | 0 | IP | Normal | TN |
| 70 | M | Sulpiride | 7 | 20 | 0 | IP | Normal | TN | |
| 47 | M | Ziprasidone/pimozide | FTD | 7 | 15 | 3 | IP | Normal | TN |
| 74 | M | Risperidone | Mood alteration | 17 | 18 | 0 | IP | Normal | TN |
| 74 | F | Clebopride+trimetazidine | Mood alteration | 6 | 9 | 0 | IP | Normal | TN |
| 70 | F | Sulpiride | 10 | 21 | 4 | IP | Normal | TN | |
| 74 | F | Clebopride | Mood alteration | 9 | 19 | 2 | IP | Normal | TN |
| 58 | M | Valproic acid+levomepromazine | 6 | 23 | 0 | IP | Normal | TN | |
| 69 | F | Valpromide | Mood alteration | 10 | 27 | 3 | IP | Normal | TN |
| 75 | F | Clebopride | Mood alteration | 15 | 43 | 7 | IP/ET | Normal | TN |
| 75 | M | Sulpiride | SCT | 33 | 41 | 2 | IP/ET | Normal | TN |
| 58 | M | Olanzapine | 34 | 26 | 7 | IP/ET | Normal | TN | |
The mean age was 71.6 years (SD, 10.1) with a range of 47 to 86. Females accounted for 53% of the population. While neuroleptics were the most frequently prescribed drugs (39%), the drug most likely to provoke parkinsonian effects was clebopride (26%). Of the patient total, 31.5% had a final clinical diagnosis of SDEP. The mean follow-up period was 16 (8.8) months, with a range of 6 to 34 months.
Table 2 displays values of SE, SP, PPV, NPV, and LR obtained in this study for purposes of diagnosing DRP using 123I-FP-CIT SPECT.
Reliability and safety data (95% confidence interval) for 123I-FP-CIT SPECT scan as a method for diagnosing drug-related parkinsonism in clinical follow-up studies.
| n | SE | SP | PPV | NPV | PLR | NLR | |
| Our study | 19 | 66.7% (20.6–100) | 100% (96.1%–100) | 100% (87.5%–100) | 86.7% (66.1–100) | NC | 0.33 (0.11–1.03) |
| Vlaar et al.22 | 5 | 80% | 100% | 100% | 15% | – | – |
| Booij et al.25 | 3 | – | – | 100% | 92% | – | – |
| Jennings et al.27 | 1 | 96% | 80% | – | – | – | – |
SP: specificity; n: DRP patients; NC: not calculable because of 100% SP; NLR: negative likelihood ratio; PLR: positive likelihood ratio; SE: sensitivity; PPV: positive predictive value; NPV: negative predictive value.
The clinical characteristics of the subgroups determined after clinical follow-up are listed in Table 3.
Clinical characteristics of DRP patients.
| Diagnosis | Bradykinesia | Rigidity | Tremor | Postural reflexes | Symmetry |
| SDEP | +++ | ++ | – | Altered | Yes |
| SDEP | ++ | + | +++ | Altered | No |
| SDEP | ++ | + | ++ | Normal | No |
| SDEP | ++ | ++ | ++ | Normal | No |
| SDEP | ++ | ++ | – | Normal | Yes |
| SDEP | +++ | + | + | Altered | Yes |
| IP | ++ | + | +++ | Altered | Yes |
| IP | + | + | + | Normal | Yes |
| IP | ++ | + | + | Normal | No |
| IP | ++ | + | – | Normal | No |
| IP | ++ | + | +/++ | Normal | No |
| IP | ++ | + | ++ | Normal | No |
| IP | ++ | + | ++ | Altered | No |
| IP | ++ | + | + | Normal | Yes |
| IP | ++ | +/++ | + | Normal | No |
| IP | +/++ | + | +/++ | Normal | No |
| IP | + | + | ++ | Normal | No |
| IP | +/++ | ++ | – | Normal | Yes |
| IP | ++ | + | +/++ | Altered | Yes |
SDEP; subclinical drug-exacerbated parkinsonism; IP: iatrogenic parkinsonism: +: mild; ++: moderate; +++: severe.
Results from our study demonstrate that 123I-FP-CIT SPECT is highly precise for detecting patients with DRP (100% specificity [96.1–100]). An abnormal result justifies monitoring the patient, who is very likely to have neurodegenerative parkinsonism (100% positive predictive value [87.5–100]). Sensitivity is not very high (66.7% [20.6–100]) due to the presence of 2 false negatives, one of which may be caused by the patient's intake of selective serotonin reuptake inhibitors (SSRIs) at the time when the 123I-FP-CIT SPECT scan was being performed. SSRIs can inhibit ioflupane uptake through competition, resulting in higher levels of ioflupane binding to dopamine transporters, which in turn increases the probability of obtaining images showing less affectation in cases of degenerative parkinsonism.28,29
We must be mindful of the fact that results may be affected by the low prevalence of the disorder (approximately 2% to 3%). This being the case, the right way to interpret results would be to use likelihood ratios, since these statistical values are not affected by prevalence. In our study, the absence of false positives delivers a specificity of 100%. Consequently, the positive likelihood ratio (PLR, sensitivity/1−specificity) cannot be calculated because of the zero in the denominator. The negative likelihood ratio (NLR) is 0.33, meaning that the probability of a negative test result is approximately 3 times greater in healthy subjects than in patients.
The literature search yielded no follow-up studies using exactly the same methodological design, although a number of studies were similar.22,25,27 They all included patients who presented a parkinsonian syndrome that had not yet been classified, underwent a baseline 123I-FP-CIT SPECT scan, and were diagnosed using the gold standard method after the follow-up period. Only a few of the patients in other studies were diagnosed with DRP, and their numbers are therefore lower than in our study (Table 2). In any case, our results resembled those from other articles (Table 2). In the study by Vlaar et al.,22 248 patients were monitored over a mean of 18 months, and 223 received a clinical diagnosis. Of that subgroup, 127 were diagnosed with IPD and 5 with DRP; 123I-FP-CIT SPECT was able to distinguish between the two groups with a specificity of 100%. In the series presented by Booij et al.,25 33 patients were monitored for several years. Out of 19 cases in which the baseline 123I-FP-CIT SPECT scan was normal, only 3 were diagnosed with DRP. However, none of the 22 patients with abnormal radiotracer uptake abnormalities were given this diagnosis. Lastly, in the study by Jennings et al.,27 35 patients were monitored for six months. Ten of them were identified at the end of the study as having non-parkinsonian syndrome, and only 1 was diagnosed with DRP. The 123I-FP-CIT SPECT scan identified non-parkinsonian syndrome very precisely. Other prospective studies8,17 have different methodological approaches and offer no information about the validity and safety of this technique for diagnosing DRP. However, by reading these articles we may deduce that DRP patients presenting a normal 123I-FP-CIT SPECT scan improve significantly after the problem drug is discontinued8 and do not respond to levodopa treatment.17 Moreover, findings from their functional imaging studies remain unchanged after the follow-up period.17 In contrast, patients who present an altered 123I-FP-CIT SPECT scan at baseline do improve with anti-parkinsonian drugs,8,17 and experience decreased striatonigral uptake over time.17
Analysis of the clinical characteristics of the subgroups that were designated after clinical follow-up (Table 3) revealed no significant differences between the IP and SDEP groups with regard to bradykinesia, rigidity, tremor, or symmetry of symptoms. The SDEP group, however, showed a slight tendency to present more severe bradykinesia. Altered postural reflexes were a more frequent finding in the SDEP subgroup (50%) than in the IP subgroup (23%), but this difference was not statistically significant (Fisher exact test, 0.21). At present, there is no unanimous opinion regarding this subject. While some studies have found differences regarding clinical symmetry and presence of orofacial dyskinesia between IP and degenerative parkinsonisms,19,30 others, including our study, have not.8,18
The percentage of associated mood changes in the SDEP group was higher than that observed in the IP group (66.6 vs 46.1). Although the finding was not statistically significant (Fisher exact test, 0.28), it may possess clinical value since it resembles results from studies associating depression and risk of developing IPD.31,32
One of the main limitations of the study is its reduced sample size, which resulted from the high percentage of excluded patients and, more particularly, from the difficulty of patient selection. While our exclusion criteria were very restrictive, this was essential in order for the study to be carried out properly. The most limiting criterion was the one requiring exclusion of patients for whom discontinuing the drug was not an option; keep in mind that the narcoleptic drug group was the one most frequently involved in inducing parkinsonism. Narcoleptic agents could not be discontinued in a sizeable number of eligible patients, whether by doctor's orders or because the patient or family feared recurrence or aggravation of psychiatric symptoms.
Furthermore, the follow-up duration may be considered insufficient since the minimum was established at 6 months; several published articles have reported that parkinsonian symptoms may recur in up to 7% of all patients16 a year after they had apparently resolved. Only 7 out of the 19 patients included in our study were monitored for less than 12 months; 3 in this group were completely asymptomatic (UPDRS III, score 0), and 4 were functionally asymptomatic (their score on UPDRS III was caused by dyspraxia and anhedonia rather than parkinsonian symptoms), at the time of the evaluation (Table 1).
Another important limitation is that the established reference diagnosis was in fact the result of the lead researcher's clinical evaluation. For IP cases, clinical monitoring over time may be considered the gold standard diagnostic method because no diagnostic criteria have been established. For SDEP cases, however, only a diagnosis based on anatomical pathology may be considered definitive.
Although the study needs to be repeated using a larger sample of patients, it is possible to conclude that 123I-FPCIT SPECT is a useful tool in diagnosing DRP since an abnormal result confirms the existence of the disorder (excellent specificity and PPV). Obviously, in terms of efficiency, performing SPECT scans on all patients may not be recommendable. The technique should be reserved for those with clinical findings suggesting SDEP (presence of asymmetry and/or alteration of postural reflexes).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Manuel Guerrero Ortiz, José Ramón Gómez Fuentes, and Luis Bañuelos Andrío (specialists in nuclear medicine at Hospital Torrecárdenas). We would also like to thank Pablo Garrido Fernández for designing the methodology.
Please cite this article as: Olivares Romero J, Arjona Padillo A. Utilidad de la tomografía por emisión de fotón simple con 123Ioflupano en el diagnóstico del parkinsonismo relacionado con fármacos. Estudio prospectivo. Neurología. 2013;28:276–82.