Last year the European Society of Echocardiography published recommendations for the use of echocardiography in identifying potential sources of embolism as a cause of ischaemic stroke in the absence of other cerebrovascular disease. Both transthoracic echocardiography (TTE) and transoesophageal echocardiography play a fundamental role in the assessment, diagnosis and management of the embolic source. Due, in part, to the increased longevity of the population and improved survival of cardiac patients, we are now seeing a gradual increase in the application of echocardiographic studies as a diagnostic test. This has led us to critically analyse their performance in the various pathologies.
ObjectiveOur aim was to analyse the diagnostic yield of TTE in patients with cerebrovascular accident in a tertiary hospital.
Materials and methodsFor this, we retrospectively analysed all echocardiographic studies during 2010 requested from the Neurology Department with a diagnosis of stroke. We have studied the diagnostic yield of the test and its contribution to the etiological diagnosis based on major and minor echocardiographic criteria as recommended by the European Society of Echocardiography.
ResultsWe found major echocardiographic criteria in 6 patients (5%) with embolic stroke and in 2 (0.7%) non-embolic stroke, P=.005. In view of our results, the performance of TTE in patients with embolic stroke has a low diagnostic yield, which leads us to consider the systematic use of this technique.
El pasado año la Sociedad Europea de Ecocardiografía publicó las recomendaciones para el empleo del ecocardiograma en la identificación de las potenciales fuentes embolígenas como causa de accidente isquémico cerebral en ausencia de otra enfermedad cerebrovascular. Tanto el ecocardiograma transtorácico como el ecocardiograma transesofágico desempeñan un papel fundamental en la evaluación, el diagnóstico y el manejo de la fuente embolígena. Debido en parte a la mayor longevidad de la población y a la mejor supervivencia de los pacientes cardiológicos, asistimos, actualmente, a un incremento progresivo de la solicitud de estudios ecocardiográficos como prueba diagnóstica; esto nos ha llevado a analizar críticamente el rendimiento de los mismos.
ObjetivoAnalizar la rentabilidad diagnóstica del ecocardiograma transtorácico en pacientes con diagnóstico de ictus isquémico en un hospital de tercer nivel.
Material y métodosHemos analizado retrospectivamente todos los estudios ecocardiográficos solicitados durante el año 2010 desde el servicio de neurología con diagnóstico de ictus isquémico. Se ha estudiado la eficacia diagnóstica de la prueba y su aportación al diagnóstico etiológico en función de los hallazgos ecocardiográficos mayores y menores, según las recomendaciones de la Sociedad Europea de Ecocardiografía.
ResultadosSe encontraron criterios ecocardiográficos mayores en 6 pacientes (5%) de los catalogados como de perfil embólico y en 2 (0,7%) de los no embólicos, siendo la diferencia estadísticamente significativa, p=0,005. A la vista de nuestros resultados, la realización de ETT en pacientes con ictus no embólicos tiene un bajo rendimiento diagnóstico, lo que nos lleva a plantearnos la rentabilidad del uso sistemático de esta prueba.
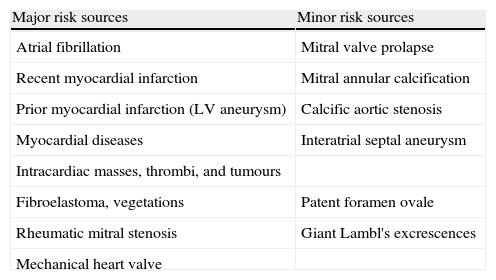
Emboli of cardiac origin are responsible for 15% to 30% of all cerebral ischaemic events.1,2 Both transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) play a vital role in the evaluation, diagnosis, and management of the source of the embolus. In 2010, the European Association of Echocardiography published its recommendations for using echocardiograms to identify potential sources of the emboli that cause cerebral ischaemia in the absence of other cerebrovascular diseases.3 According to EAE recommendations, sources of emboli may be classified as major or minor (Table 1). Sources may also be grouped into 3 categories according to their pathophysiology: cardiac conditions prone to forming thrombi (such as atrial fibrillation and appendicular thrombosis); cardiac masses; and paradoxical embolism, generally through a patent foramen ovale (PFO).3
Potential cardioembolic sources.
| Major risk sources | Minor risk sources |
| Atrial fibrillation | Mitral valve prolapse |
| Recent myocardial infarction | Mitral annular calcification |
| Prior myocardial infarction (LV aneurysm) | Calcific aortic stenosis |
| Myocardial diseases | Interatrial septal aneurysm |
| Intracardiac masses, thrombi, and tumours | |
| Fibroelastoma, vegetations | Patent foramen ovale |
| Rheumatic mitral stenosis | Giant Lambl's excrescences |
| Mechanical heart valve |
Modified from Pepi et al.3
Echocardiography plays a fundamental role in the diagnosis and study of these patients. Cardioembolic stroke is defined as an infarct of the cortex that may be medium-sized (1.5–3cm) or large (>3cm). Symptom onset occurs while the patient is awake and presentation of the neurological focus may be instantaneous (in minutes) or acute (in hours). It is true, however, that it is often difficult to distinguish cardioembolic stroke from other causes of embolism. Diagnostic techniques such as computed tomography and magnetic resonance imaging are also helpful in classifying the mechanism.4
On the other hand, given the ageing population and the rise in comorbidities, it is not uncommon to find patients presenting several pathogenic mechanisms.
Due, in part, to increased longevity in the population and better survival among patients with heart disease, we are witnessing a continuous increase in requests for echocardiography as a diagnostic test. This leads us to critically analyse the benefits of echocardiographic studies for a number of diseases. We therefore present an article which analyses the diagnostic utility of TTE in patients diagnosed with a cerebrovascular event in a tertiary hospital.
Materials and methodsWe perform a retrospective analysis of all the echocardiography studies ordered by our hospital's neurology department in 2010 for diagnoses of cerebrovascular accidents. We examine the diagnostic efficacy of this type of study and its contribution to the aetiological diagnosis based on the European Echocardiography Association's classification of major and minor echocardiographic findings.
Transthoracic and transoesophageal echocardiograms were taken using an iE 33 system (Philips, the Netherlands). Patients in whose cases there was a firm suspicion of embolism (mainly young patients with no other comorbidities) and those in whom an interatrial septal aneurysm was detected underwent a saline echo study to rule out any early appearance of bubbles in the left chambers that would suggest intracardiac shunt. TTE was performed when the transthoracic images were not sufficient to provide a diagnosis, or when endocarditis or prosthetic valve thrombosis was suspected.
Findings from patients’ cardiology examinations, results from the echocardiogram and any other diagnostic tests that were ordered, plus the definitive diagnoses upon discharge from the neurology department, were gathered retrospectively.
We used SPSS software version 15 for Windows (Chicago, IL) for data analysis. Discrete variables are expressed as percentages and continuous variables as mean±standard deviation (SD).
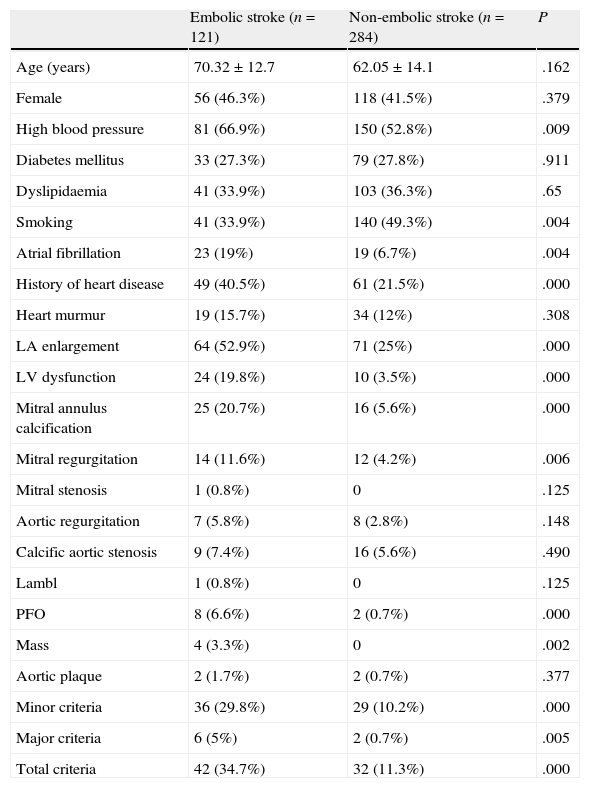
ResultsA total of 590 patients were admitted to the neurology department with a diagnosis of stroke; our study includes the 405 for whom echocardiograms were ordered. Males made up 57% of the total and mean age±SD was 64.5±14.2 years. Cardiovascular risk factors and percentages of patients with known atrial fibrillation and a prior diagnosis of heart disease (mainly hypertensive and ischaemic heart disease) are listed in Table 2.
Cardiovascular risk factors and major and minor criteria for both groups.
| Embolic stroke (n=121) | Non-embolic stroke (n=284) | P | |
| Age (years) | 70.32±12.7 | 62.05±14.1 | .162 |
| Female | 56 (46.3%) | 118 (41.5%) | .379 |
| High blood pressure | 81 (66.9%) | 150 (52.8%) | .009 |
| Diabetes mellitus | 33 (27.3%) | 79 (27.8%) | .911 |
| Dyslipidaemia | 41 (33.9%) | 103 (36.3%) | .65 |
| Smoking | 41 (33.9%) | 140 (49.3%) | .004 |
| Atrial fibrillation | 23 (19%) | 19 (6.7%) | .004 |
| History of heart disease | 49 (40.5%) | 61 (21.5%) | .000 |
| Heart murmur | 19 (15.7%) | 34 (12%) | .308 |
| LA enlargement | 64 (52.9%) | 71 (25%) | .000 |
| LV dysfunction | 24 (19.8%) | 10 (3.5%) | .000 |
| Mitral annulus calcification | 25 (20.7%) | 16 (5.6%) | .000 |
| Mitral regurgitation | 14 (11.6%) | 12 (4.2%) | .006 |
| Mitral stenosis | 1 (0.8%) | 0 | .125 |
| Aortic regurgitation | 7 (5.8%) | 8 (2.8%) | .148 |
| Calcific aortic stenosis | 9 (7.4%) | 16 (5.6%) | .490 |
| Lambl | 1 (0.8%) | 0 | .125 |
| PFO | 8 (6.6%) | 2 (0.7%) | .000 |
| Mass | 4 (3.3%) | 0 | .002 |
| Aortic plaque | 2 (1.7%) | 2 (0.7%) | .377 |
| Minor criteria | 36 (29.8%) | 29 (10.2%) | .000 |
| Major criteria | 6 (5%) | 2 (0.7%) | .005 |
| Total criteria | 42 (34.7%) | 32 (11.3%) | .000 |
All patients in the group were studied with electrocardiography. Pathological findings, such as atrial fibrillation, signs of left ventricular enlargement, or prior myocardial necrosis, were found in 17% of the patients; 13.1% had a heart murmur. All patients underwent TTE during their hospital stay (6±2 days after the stroke). An additional saline echo study was performed in 11.4%, and a TOE in 9.6%. According to the neurology department's working diagnosis recorded in the patients’ admission reports, patients in the sample fell into 2 categories: embolic stroke profile (121 patients, 29.9% of the total) and non-embolic stroke profile. Major risk sources, as defined by the European Association of Echocardiography, were detected by echocardiogram in 6 patients (5%) first assigned to the embolic profile (1 case of rheumatic mitral stenosis, 2 cases of complex aortic plaques, 2 cases of thrombus in the ventricular apex, and 1 case of thrombus in the left atrial appendage). Major sources were also found in 2 patients (0.7%) with a non-embolic profile (2 cases of aortic atheromatous plaque); the difference was statistically significant (P=.005). Minor risk sources were discovered in 36 patients (29.8%) with an embolic profile (25 cases of mitral annulus calcifications), 8 with PFO, 1 case of Lambl's excrescences, and 9 cases of calcified aortic stenosis. They were also found in 29 patients (10.2%) with a non-embolic profile (16 mitral annulus calcifications), 2 PFOs and 16 calcified aortic stenoses. All patients in the sample were monitored over 24hours with a Holter monitor or another method. Atrial fibrillation was detected in 80 patients in the embolic group (66.1%) and 37 in the non-embolic group (13%). After excluding those patients revealed to have atrial fibrillation, we found major risk sources in 6 patients with embolic stroke (14.6%) and 2 with non-embolic stroke (0.8%). Minor risk sources were present in 16 patients in the embolic group (39%) and 22 in the non-embolic group (8.9%).
Factors predicting embolic stroke in our sample are recorded in Table 2.
DiscussionAccording to our results, performing TTEs on patients with non-embolic ictus yields poor diagnostic results, which causes us to question whether or not systematic use of this method can be considered an efficient use of resources.
Few studies have analysed the cost-effectiveness of running routine echocardiograms for patients with cerebrovascular accidents. The review completed by Kapral et al. concluded that there was not enough evidence to advise either for or against systematic echocardiography in patients with no clinical signs of heart disease. However, the review did recommend it in patients in whom heart disease was suspected, and suggested TOE as the initial diagnostic test based on its higher sensitivity and better diagnostic performance.5 Nevertheless, further studies concluded that evidence did not support routine use of echocardiography.6 Some recent studies, like the study by Morris et al.,7 examine the effectiveness of a number of cardiac tests (including electrocardiogram and Holter monitor) in patients with cerebrovascular accident and propose an interesting diagnostic algorithm. According to this algorithm, based on experts’ opinions, only those patients with signs or symptoms of heart disease, abnormal ECG, abnormal chest radiography or alterations on the Holter or other telemetry monitor should undergo an echocardiography study. This also applies to patients with cryptogenic cardiovascular accident or a suspected source of embolism. On the other hand, patients with no clinical signs of heart disease, those with lacunar infarcts, or those shown to have carotid stenosis ipsilateral to the infarct, require no further cardiac studies. In our sample, echocardiographic studies of stroke patients with a non-embolic profile had a low diagnostic utility, especially with regard to major echocardiographic risk criteria. Minor risk criteria were found in 12% of the non-embolic group vs 35.5% of the embolic group, which was somewhat helpful for determining a pathophysiological diagnosis.
In light of these results, diagnostic utility of the echocardiographic study was low in patients with a non-embolic profile. This suggests a need for more precise and efficient diagnostic algorithms. Longer series and prospective studies will help us provide clearer definitions of the patients who will benefit from an echocardiogram. More specifically, they will indicate which diagnostic algorithm is the most appropriate for this type of disease.
Conflict of interestThe authors have no conflict of interest to declare.
Secades S, et al. Rendimiento diagnóstico del estudio ecocardiográfico en el accidente cerebrovascular: ¿debemos mejorar la selección de los pacientes? Neurología. 2013;28:15–8.