Focal dystonia is the most frequent form of primary dystonia, with a prevalence of 110 cases per million population.1 Cervical dystonia and blepharospasm are the most frequent forms of focal dystonia.
The treatment of choice for focal dystonia consists of periodic injections of botulinum toxin (BTX).2,3 In clinical practice, BTX is administered at fixed intervals, generally longer than 3 months. For more than 2 decades, these intervals have been recommended with a view to reducing the risk of the patient developing neutralising antibodies against BTX. However, although the highly purified type-A botulinum toxins currently used present practically no association with the development of clinically relevant neutralising antibodies,4 these prolonged intervals are maintained. The duration of the clinical improvement induced by BTX may be shorter than 3 months in patients with focal dystonia.5,6
In this letter, we present the results of a study conducted at our centre to evaluate the duration of the effect of BTX in patients with focal dystonia. From September 2015 to March 2016, we evaluated 90 consecutive patients diagnosed with blepharospasm and cervical dystonia and treated with BTX. Using a structured questionnaire, we collected data on the duration of the effect of the last injection of BTX, as well as the actual duration of the intervals between the last 3 treatment sessions. We also collected data on disease duration, duration of treatment with BTX, the dose used, the type of BTX, and patients’ demographic data (sex and age).
Data were analysed using descriptive statistics and the one-way ANOVA test was used to compare means.
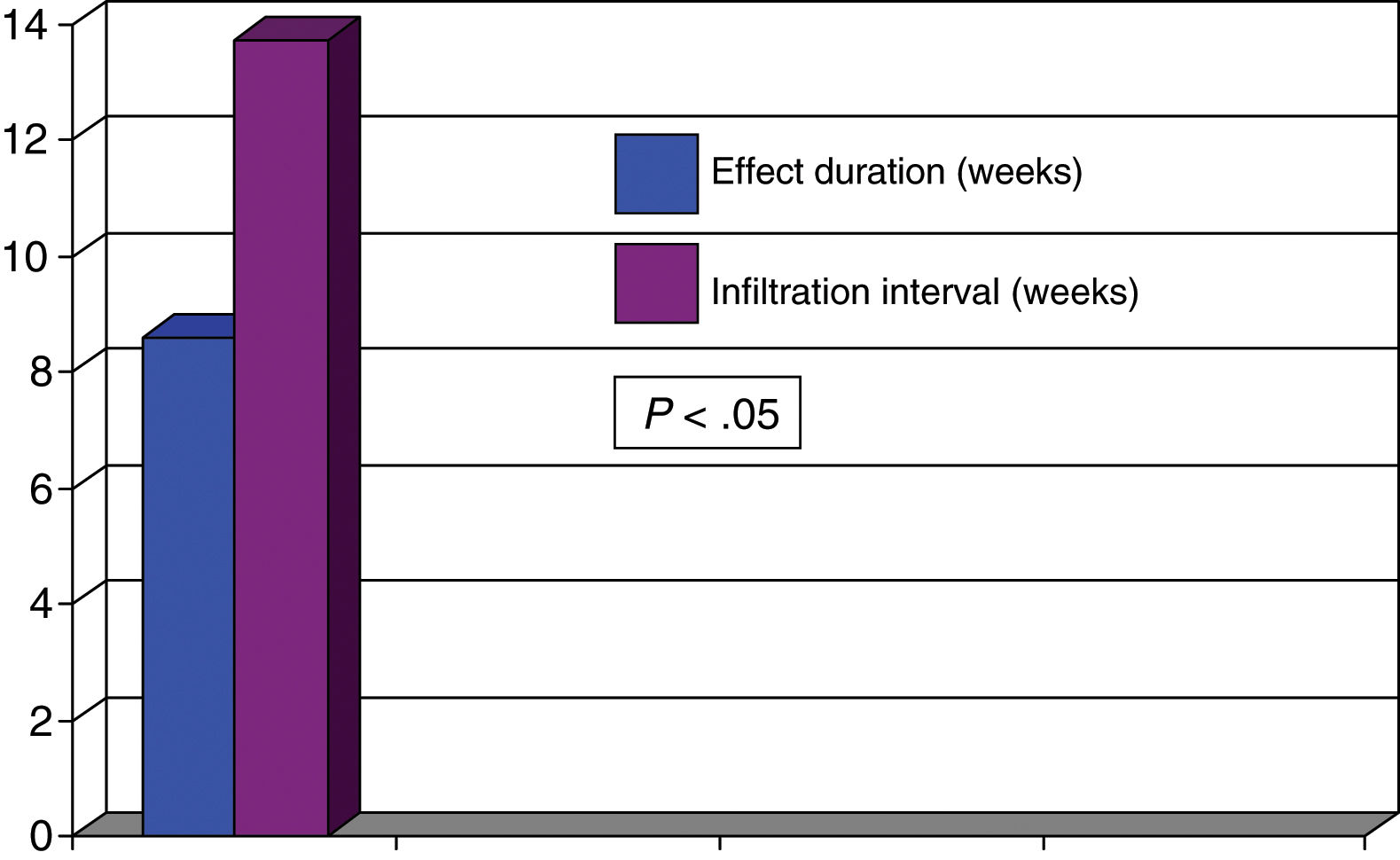
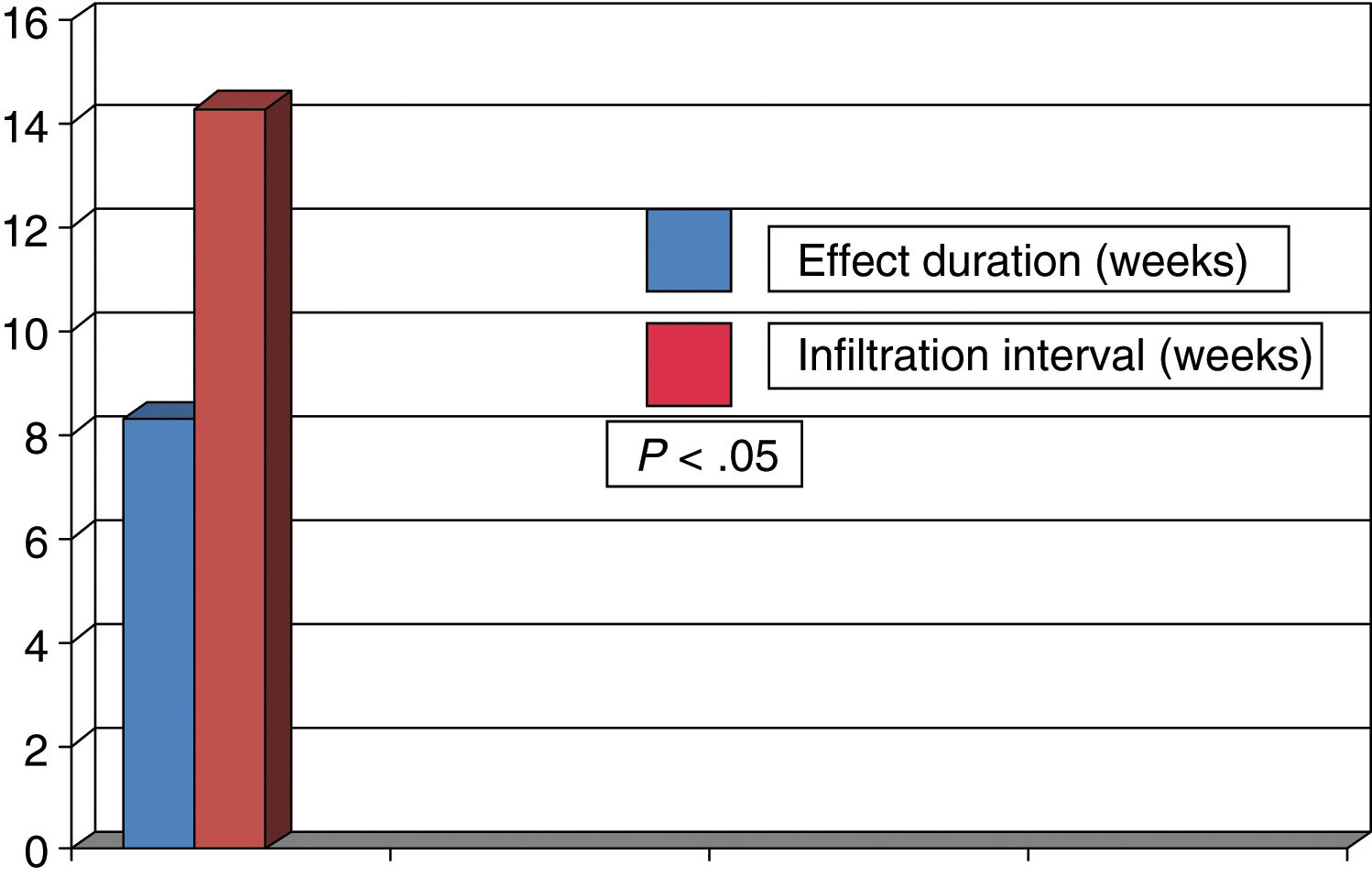
Women accounted for 73% of the patients, and mean age (standard deviation) was 67.8 years (14.0); 31 patients presented cervical dystonia and 59 blepharospasm. Mean disease duration was 11.5 years (8.4). Botulinum toxin A was administered to 77.8% of patients and 22.2% received incobotulinumtoxin A. The mean dose was 46.3 units (11.8) in patients with blepharospasm and 191.3 units (62.1) in patients with cervical dystonia. The duration of the effect of BTX was 11.6 weeks (4.0), with actual infiltration intervals being 15.3 weeks (3.2) (P<0.02). Overall, symptoms reappeared before 3 months in 38 patients (42.2%). In these patients, the mean duration of the effect of BTX was 8.4 weeks (2.1), with the actual infiltration interval being 14.2 weeks (2.7) (P<0.05). Symptoms reappeared before 3 months in 44% of the 59 patients with blepharospasm and in 42% of the 31 patients with cervical dystonia. After analysing the duration of the effect in patients presenting symptom reappearance before 12 weeks, we observed that the mean duration of the effect was 8.3 weeks (1.7) in patients with blepharospasm and 8.6 weeks (2.1) in patients with cervical dystonia. The mean actual infiltration interval was 14.3 weeks (2.3) in patients with blepharospasm and 13.7 weeks (3.4) in patients with cervical dystonia, with statistically significant differences (P<0.05) found in both groups in the comparison against duration of the clinical effect (Figs. 1 and 2). Differences between the different toxins were not studied.
In summary, in approximately 40% of patients with focal dystonia, the duration of the clinical effect of BTX was less than 3 months whereas injections of BTX were administered at intervals that generally exceeded 12 weeks.
We should note that the duration of the clinical effect was assessed by directly asking the patient, rather than with objective, scale-based measures, as our aim was to study patients’ subjective assessment of their symptoms. We did not assess other objective factors that may cause a delay in the response to treatment, such as the degree of muscle atrophy in patients with cervical dystonia.
It seems evident that the current therapeutic approach, with fixed, prolonged intervals between treatment sessions, is inappropriate as it does not control dystonic symptoms throughout the time between BTX injections in a high percentage of patients.
To optimise the therapeutic effect of BTX in focal dystonia, the toxin should be administered at flexible intervals, adapted to the duration of the clinical response in each patient.4,5
Please cite this article as: Contreras Chicote A, Miguel Velázquez J, Sainz Amo R, Grandas F. Evaluación de la duración del efecto de la toxina botulínica en la práctica clínica. Neurología. 2020;35:347–348.