The cerebellar response has been studied for years with different models of alteration of other brain structures to understand its complex functioning and its relationship with the rest of the body. Studies in patients with Parkinson's disease (PD) showed that the cerebellar function is modified by deficit of the basal ganglia; which supports the hypothesis that both structures are related anatomically and functionally.
MethodsIn our study, the ventrolateral striatum (VLS) of the basal ganglia was altered by an electrolytic lesion, in order to produce a similar jaw frequency of jaw tremor movements presented in parkinsonism, thereafter we analyzed the effect of the lesion on the expression of multiunit activity (MUA) of the cerebellum.
ResultsWe found cerebellar activation during mandibular movements and increment during oral jaw tremor movements. In addition, the amplitude of baseline MUA registered in animals with alteration of the VLS decreased with respect to the intact group.
ConclusionsAccordingly, we conclude that cerebellar changes in MUA may be due to a decrease in the cerebellar inflectional or as a possible compensatory function between cerebellum and basal ganglia.
La respuesta cerebelar se ha estudiado durante años con diferentes modelos de alteración de diferentes estructuras cerebrales, para comprender su complejo funcionamiento y su relación con el resto del organismo. Los estudios en pacientes con la enfermedad de Parkinson mostraron que la función cerebelar se modifica por el déficit de los ganglios basales, lo que apoya la hipótesis de que ambas estructuras están relacionadas anatómica y funcionalmente.
MétodosEn nuestro estudio, el estriado ventrolateral (EVL) de los ganglios basales fue alterado por una lesión electrolítica, con el fin de producir una frecuencia mandibular similar a los movimientos de temblor mandibular que se presentan en el parkinsonismo. Posteriormente analizamos el efecto de la lesión sobre la expresión de actividad multiunitaria (AMU) del cerebelo.
ResultadosEncontramos activación cerebelar durante los movimientos mandibulares e incremento durante los movimientos de temblor mandibular. Además, la amplitud de la AMU basal registrada en animales con alteración del EVL disminuyó con respecto al grupo intacto.
ConclusionesEn consecuencia, concluimos que los cambios cerebelares en la AMU, pueden deberse a una disminución de la modulación cerebelar o como una posible función compensadora entre el cerebelo y los ganglios basales.
Within the central nervous system, the cerebellum has been classically associate with the control of fine movements, balance and proprioception. However, different experimental approaches have shown its role in some types of memory,1,2 planning and sensory discrimination,3–5 language,6,7 reward,8,9 sexual behavior10–12 and motor control during sexual performance.13,14 Accordingly, it has been argued that an anatomical and functional relationship must exist between the cerebellum and the basal ganglia, which would help explain some mechanisms behind motor disorders such as Parkinson's disease,15 Huntington,16 attention deficit disorder,17 depression,18 and obsessive-compulsive disorder, among others.19
One former study carried out on macaques showed an anatomical relationship between the basal ganglia and the cerebellum through a disynaptic connection of the cerebellar dentate nucleus (DN) that crossed from the thalamus to the striatum.20 Similarly, another study reported a communication pathway from the subthalamic nucleus through the pontine nuclei to the cerebellar cortex.21,22 Accordingly, these studies are examples of a bidirectional anatomical relationship between both structures.
As mentioned above, the functional relationship between the cerebellum and the basal ganglia is essential to understand the mechanisms that underlie disorders like parkinsonism, which is a clinical syndrome that involves bradykinesia, tremor, and dyskinesia.23 The use of animal models of parkinsonism has facilitated the study of functional responses of structures that might be involved in this kind of condition. For instance, some disorders can be modeled in the laboratory by pharmacological, toxicological, and genetic manipulations24–27 or via electrolytic lesions of certain brain areas.28 For example, one popular model requires the administration of haloperidol, a dopaminergic antagonist that causes jaw tremor movements (JTMs) in rats.28 Another model uses electrolytic lesions, in which direct current passes through a specific area of the striatum (ventrolateral striatum-VLS) causing permanent damage and the corresponding JTMs. Interestingly, one study from our laboratory showed that that type of electrolytic lesions in the striatum caused activation of the cerebellar hemispheres (as observed with Fos).29 That study pointed out to a functional modification of cerebellar activity in animals with parkinsonian symptoms. Accordingly, the present study was designed to characterize patterns of multiunit activity (MUA) in cerebellar regions following electrolytic lesions of the ventrolateral striatum during the presence of the JTMs.
Many connections between sensory information, basal ganglia, and cortex could be responsible for the cerebellar response to striatal lesions. We hypothesized an increase of MUA amplitude due to the final calculation of cerebellar inputs during JTMs.
Material and methodsAnimalsFor this study, Wistar male rats (n=54) were used (300–350g). They were maintained in acrylic boxes, under conditions of a reverse light-dark cycle of 12×12h with ad libitum access to rat chow and water. Our experimental protocols were in accordance with the Official Mexican Norm (NOM-062-ZOO-1999) for the care and use of the laboratory animals. In addition, we followed the policies for use of animals as stipulated by The Society for Neuroscience (The U.S. Public Health Service's Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals). All animals were housed in the animal facilities at the Brain Research Institute at Universidad Veracruzana, Mexico.
ElectrodesElectrodes were prepared with stainless steel bars [monopolar electrodes (FHC, Inc., USA. 250μm θ, 3MΩ)]. The rods were cut according to the depth of each structure to be implanted and assembled to a pin and exposed 0.5mm at the tip. Simultaneously, stainless steel screws (Stoelting, Co., USA) were implanted on the skull to provide a reference electrical signal.
Stereotaxic surgeryFor the intracerebellar implants, we used the methodology previously published by Tamariz et al.30 The rats were anesthetized with an intraperitoneal injection of Ketamine (100mg/kg) and Xylazine (10mg/kg). Once the subject was deeply anesthetized it was placed in ventral recumbency and gently fixed with ear bars in a stereotactic frame (Stoelting, Co. USA). Then, a longitudinal skin incision was made in the midline of the head, and the skull was exposed and then sprayed with additional vasoconstrictor plus local anesthesia containing epinephrine-procaine (Adrecaine, Aranda Lab® Mexico). For the implant, a trephine was made in the skull (Dremel rotary tools®, USA) at the point indicated by the coordinates of the stereotactic atlas.31 The electrode [stainless steel monopolar (FHC, Inc. USA. 250μm θ, 3MΩ)], was lowered to the expected site (see below)expected site and then fixed to the skull with acrylic resin (NicTone®). Simultaneously, stainless steel screws (Stoelting, Co., USA) were implanted on the skull as a reference.
The coordinates for the ventrolateral struatum (VLS) was Bregma: −.40mm, lateral: ±4.4mm and ventral: −6.6mm; and for lobule Sim B, Bregma: −12.4mm, lateral: −3.4mm and ventral: −2.8mm; for crus II lobe was Bregma: −14mm, lateral: 3.4mm and ventral: −5mm and dentate nucleus (DN) was Bregma: −11.3,lateral: −3.4, and ventral: −6.4. At the end of the surgery, the rats were placed in a recovery chamber before returning them to our colony room.
Experimental modelElectrophysiological recording of MUA of cerebellar neurons was made at the Sim B lobes, Crus II on its layer granular, as well as in the DN of male rats. Subjects were randomly divided into three groups: (1) intact group, which just underwent the implant recordinggistration electrode for the basal activity of the regions to monitor; (2) the sham group, in which electrodes were bilaterally descended into the ventrolateral striatum; and (3) the experimental group of animals that received direct current to cause an electrolytic lesion (900±100μM) of the VLS in both hemispheres.
MUA recordingThe recordings were carried out 48h after stereotaxic surgery. All sessions were video recordedfilmed under red light conditions. Implanted animals were placed in transparent acrylic cages (30cm×30cm×30cm) and before the test they had a habituation period of 5min. Then, the recording and reference electrodes were connected to an interface that sent the signal to an amplifier (Grass Technologies®, Inc. 15A54, 15LT) and simultaneously to an audio monitor (AM9, Grass Technologies®, Inc.). The amplified signal was transferred to the Polyview system (Grass Technologies®, Inc. Polyview adaptor system, PVA-16) where it was digitalized and sent to a computer to be displayed by the software, where the traces of each animal were computed in real-time through the Polyview recording function (Grass Technologies®, Inc. v 2.5.). During recording, we used the mark events function to indicate when each rat displayed basal or JTMs activity.
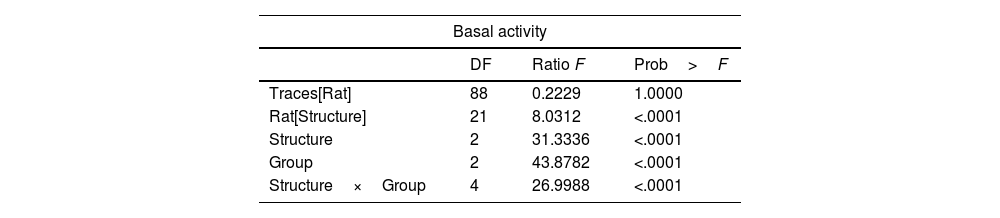
Statistical analysisFor each recording session, 10 traces of 5s each were selected for baseline activity in the intact, sham, and lesioned groups and in the same way in the sham and lesioned groups during the JTMs. The data were nested in the factor structure and group, that is, n=6 animals for each structure in each of the groups (N=54). The response variable was analyzed independently with Generalized Linear Models (LGM) in a design of fixed and nested (hierarchical) factors in analysis with pseudoreplication: y=G+E+R [E]+E [G]+Ps [R]+G*E+error. Where y is the response variable (amplitude of the recorded activity), G is the group (intact, sham, and lesioned), E corresponds to the structure (Sim B, Crus II, and the DN), R is the rat and Ps is the pseudoreplication of the traces; the brackets represent nests and the asterisk the interaction. All analyses were performed in the statistical package “JMP 6 SAS Inc. Cary, NC USA 2005”.
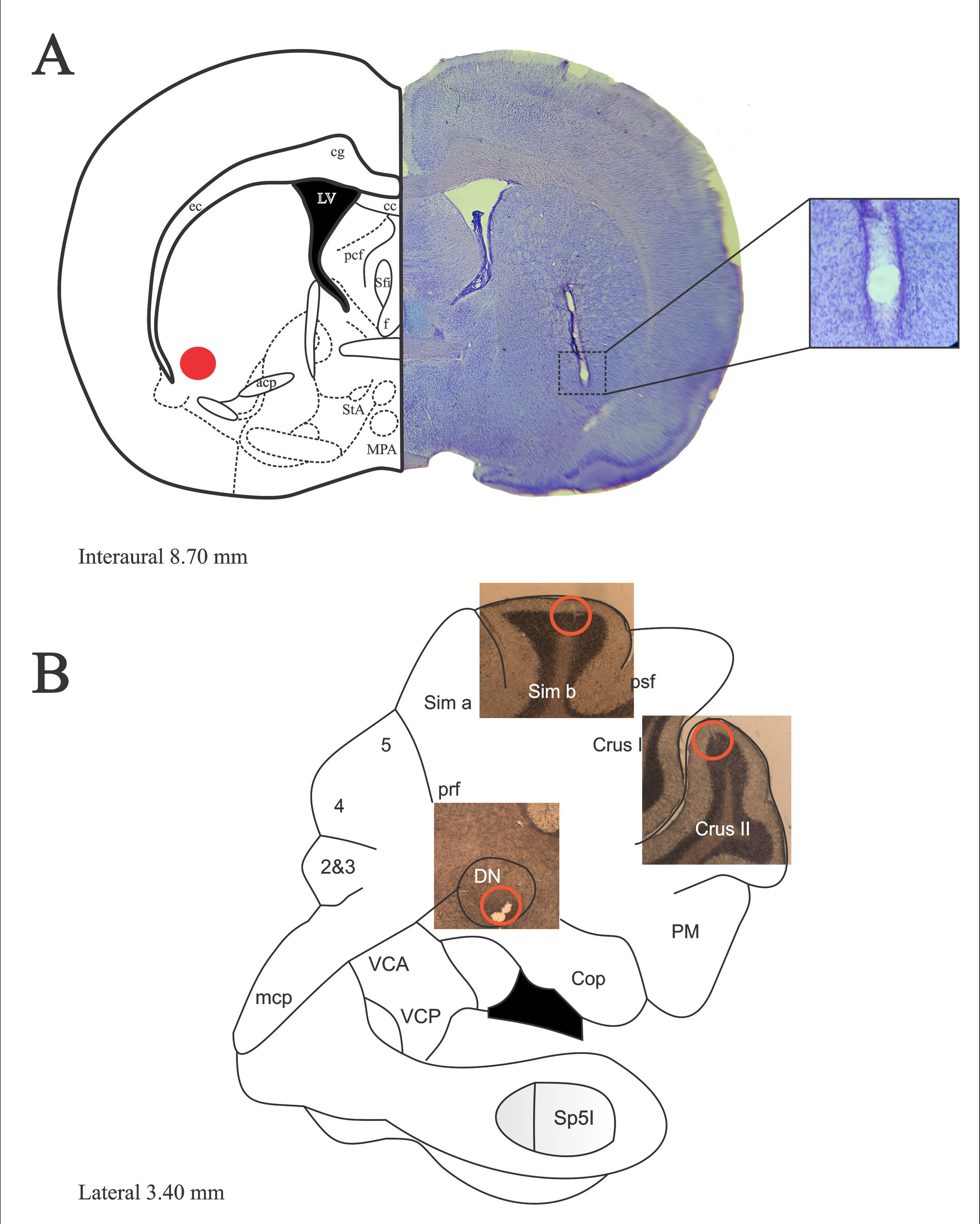
ResultsRats whose surgical implants were not located in the structures analyzed were discarded from the analysis. Fig. 1 depicts A) sites of bilateral lesion in VLS. B) sites where electrodes were located following histological verification. Thus, multiunit activity was recorded in the granular layer of Sim B and Crus II lobe and DN, and the maximum amplitude of baseline and during JTMs was compared.
(A) Representative diagrams of the descent of the electrode and its respective bilateral injury in the VLS (left orange dot). Photomicrograph of sagittal section of the VLS (right), stained with cresyl violet. Nissl staining allows differentiating the electrolytic lesion. (B) Representative diagram of the cerebellar hemisphere showing photomicrographs of sagittal sections of Sim B, Crus II and the DN, depiction the site where the tips (orange circles) of the electrodes implanted were located. The surgical implants of Sim B, Crus II and the ND were made in independent groups. Magnification ×1.25.
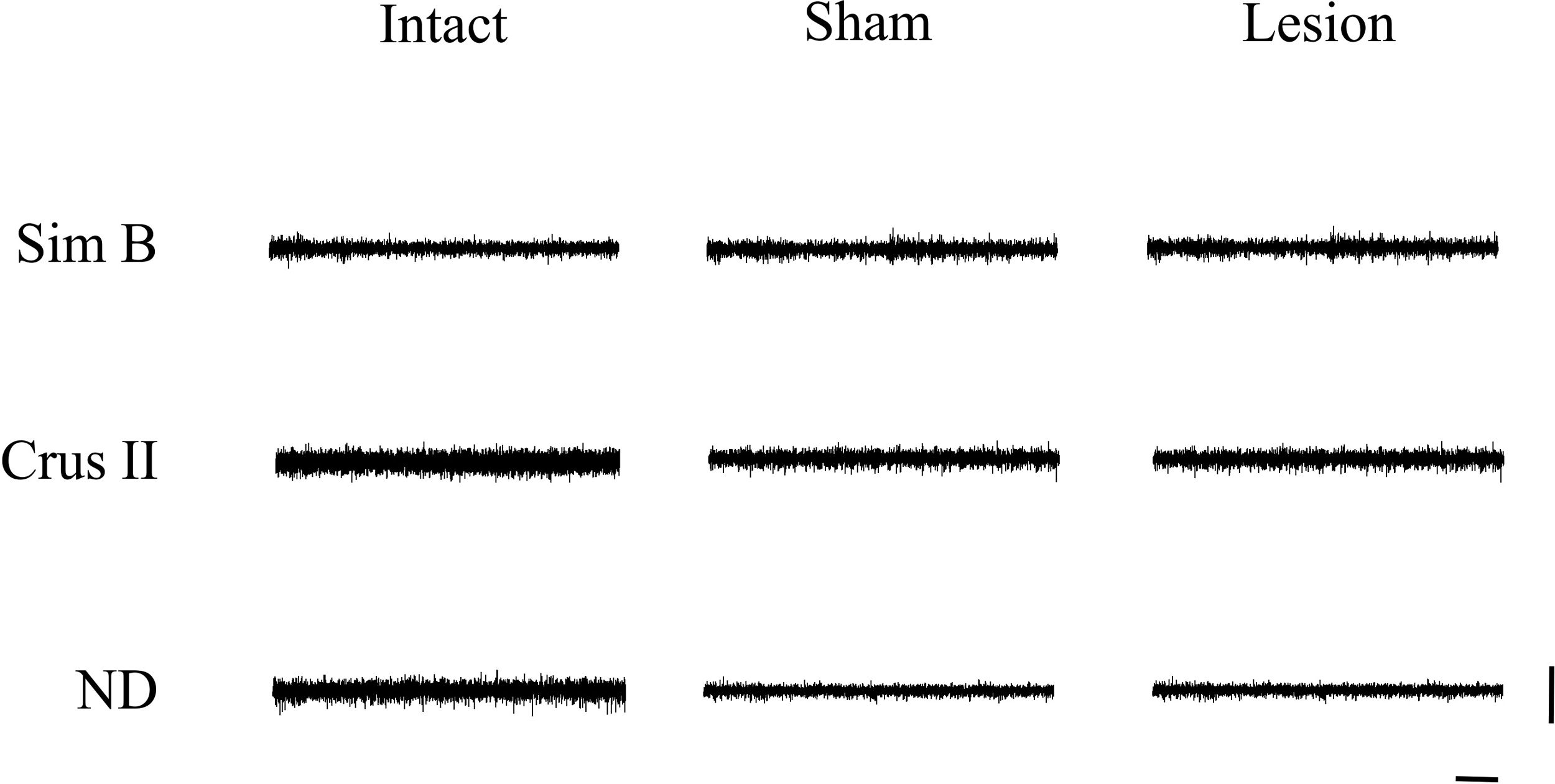
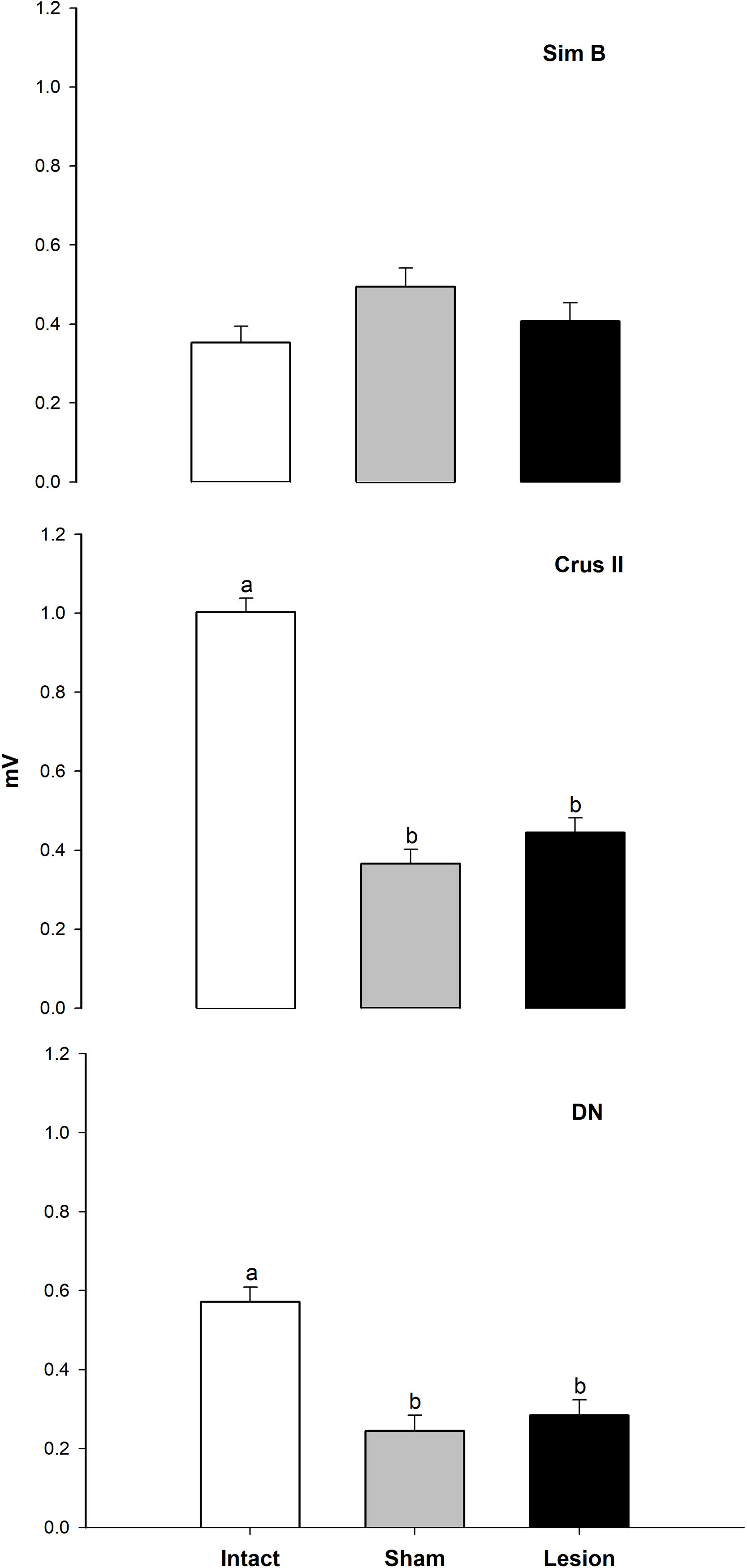
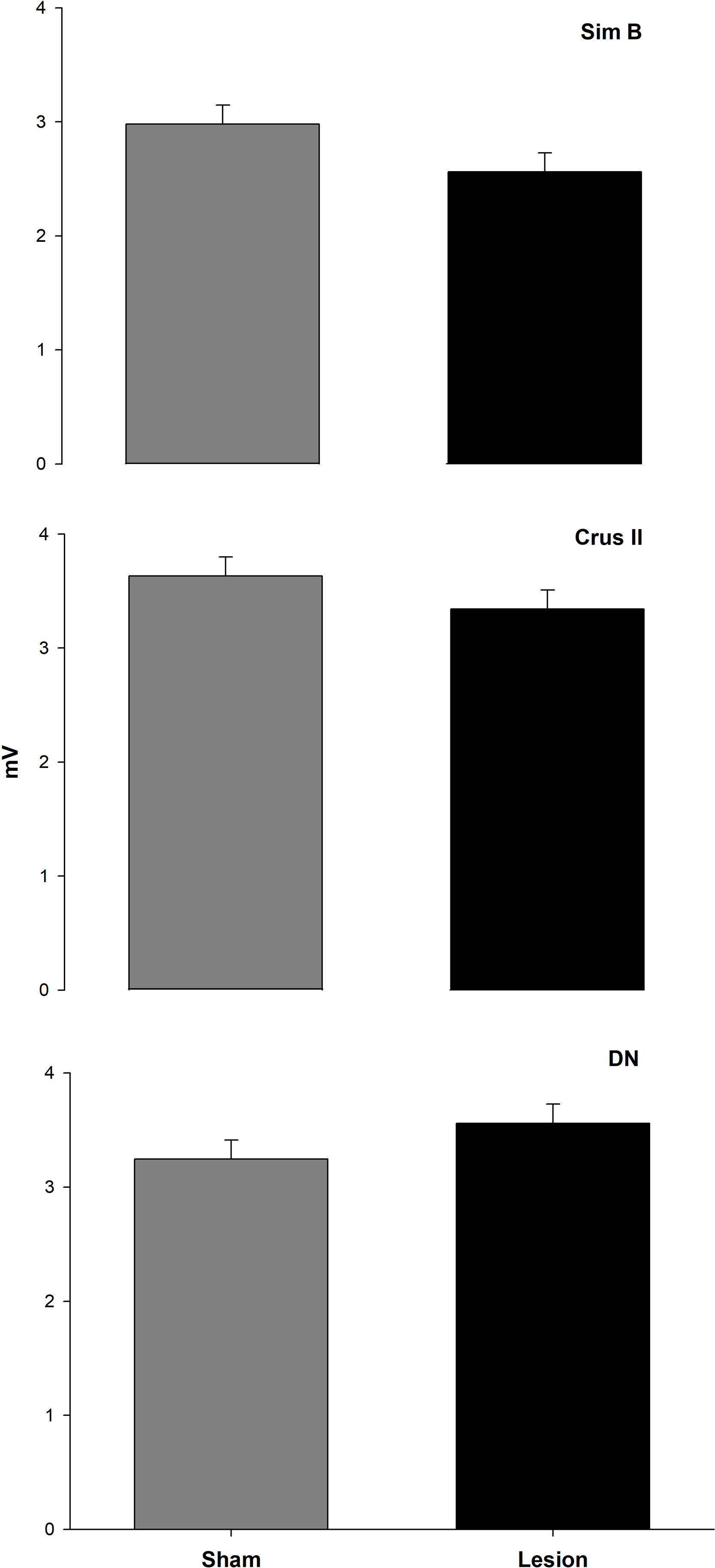
We found that the analysis of the maximum amplitude (mV) of the baseline activity between the Intact, Sham, and Lesioned groups recordgistered in the Sim B lobe, did not show significant differences. The average values of the amplitude at baseline between groups were 0.3 and 0.4mV, respectively. Nevertheless, in the intact group, the Crus II lobe and the NDN showed a greater amplitude (between 0.5 and 1mV) compared to that observed in Sim B (0.3mV). On the other hand, in the Sham and Lesioned groups, there was a decrease in the amplitude of the Crus II lobe and the NDN (between 0.3 and 0.2mV) compared to the intact group (Figs. 2 and 3, Table 1).
Multiunit activity traces of basal activity (resting state) obtained of the record in the granular layer of the lobes Sim B and Crus II, as well as of the DN of the cerebellum for every experimental group; the intact, sham, and lesion. Horizontal calibration: 500ms. Vertical calibration: 1mV.
Total mean value±S.E.M. of the amplitude of MUA (mV) recorded in the granular layer of the Sim B, Crus II lobe, and DN of the cerebellar hemisphere for the intact (white bar), sham (gray bar), and lesion groups (black bar) during the basal activity, n=6, bars connected by different letters represent significant difference, P<0.0001.
Results of effect test of statistical analysis, ‘traces of recording’ nest in ‘rat’, ‘rat’ nest in ‘structures’, our fixed factor's ‘structure’ and ‘group’ and finally the interaction between ‘structure’ with ‘group’. The animals used were made in independent groups and structures.
| Basal activity | |||
|---|---|---|---|
| DF | Ratio F | Prob>F | |
| Traces[Rat] | 88 | 0.2229 | 1.0000 |
| Rat[Structure] | 21 | 8.0312 | <.0001 |
| Structure | 2 | 31.3336 | <.0001 |
| Group | 2 | 43.8782 | <.0001 |
| Structure×Group | 4 | 26.9988 | <.0001 |
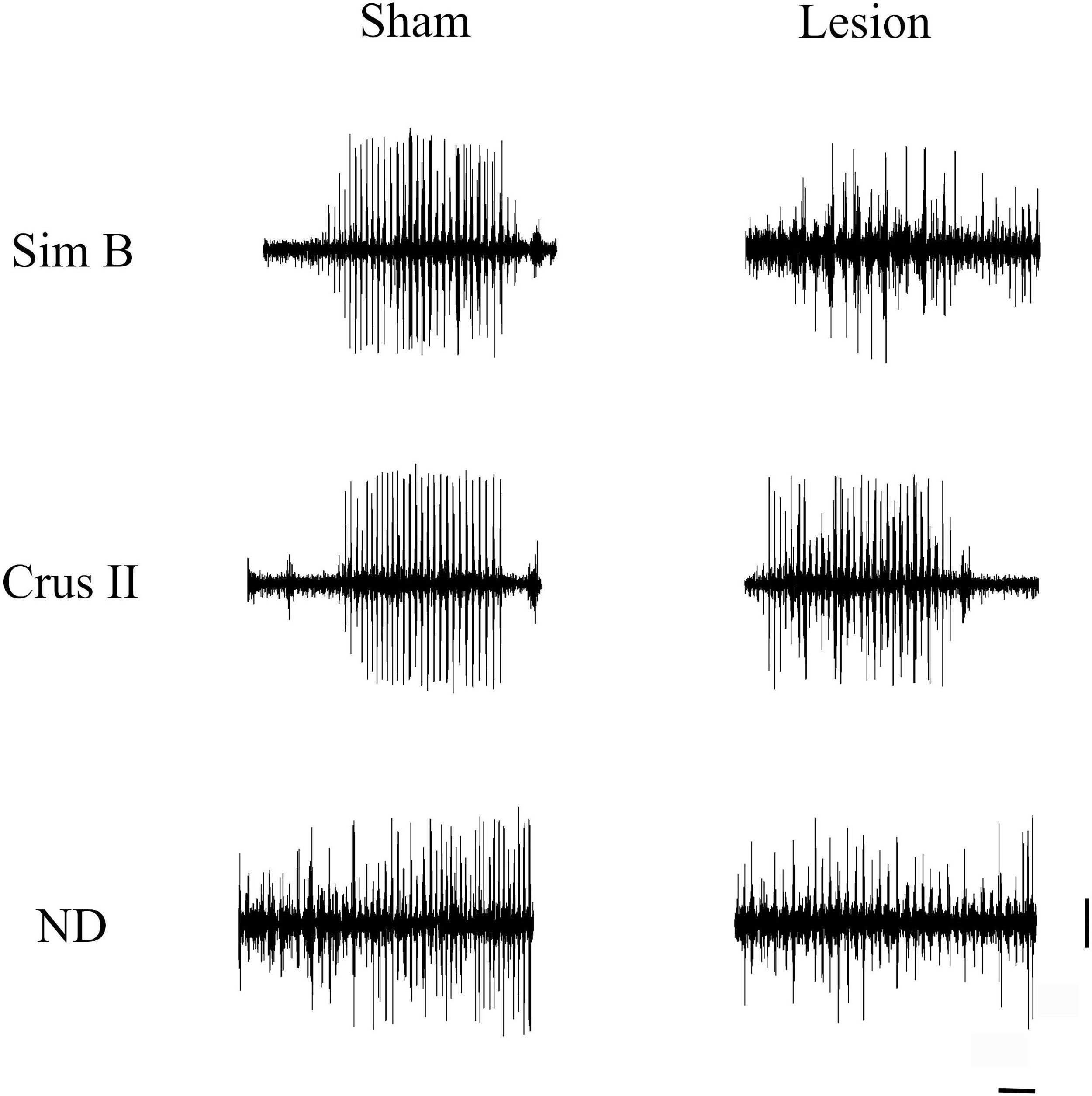
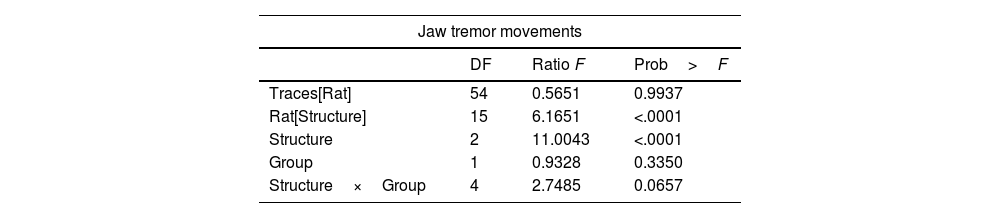
Lesion of the VLS (lesioned group) induced JTMs in all animals after recovery from anesthesia. Thus, we aimed at characterizing the electrophysiological pattern of the cerebellum in animals with electrolytic lesions in the VLS. Interestingly, we noticed JTMs in the Sham group after recovery from anesthesia (as seen in Figs. 4 and 5). Such findings indicated that mere placement of an electrode within the VLS caused similar effects to an electrolytic lesion. Accordingly, we decided to perform a comparative analysis between both groups to characterize electrophysiological traces in each implanted structure: Sim B, Crus II, and DN (Figs. 4 and 5, Table 2).
Results of effect test of statistical analysis, GLM, for the effect of ‘traces of recording’ nest in ‘rat’, ‘rat’ nest in ‘structures’, our fixed factor's ‘structure’ and ‘group’ and finally the interaction between ‘structure’ with ‘group’. The animals used were made in independent groups and structures.
| Jaw tremor movements | |||
|---|---|---|---|
| DF | Ratio F | Prob>F | |
| Traces[Rat] | 54 | 0.5651 | 0.9937 |
| Rat[Structure] | 15 | 6.1651 | <.0001 |
| Structure | 2 | 11.0043 | <.0001 |
| Group | 1 | 0.9328 | 0.3350 |
| Structure×Group | 4 | 2.7485 | 0.0657 |
The present study was designed with the objective of determining whether the MUA of specific regions of the cerebellum were altered in rats with JTMs, caused by an electrolytic lesion. Thus, the MUA of the granular layer of the Sim B, Crus II lobe, and the DN of male rats that presented JTMs was analyzed. The analysis of multiunit activity observed in the cerebellum of animals during the baseline activity showed lower amplitude values in the recordings of the Crus II lobe and DN of Sham and Lesion groups compare to the intact group (Figs. 2 and 3).
With regard to the reduction of baseline amplitude, it could be the result of altered entry pathways to the cerebellum from the basal ganglia; apparently, the damage caused by the electrolytic lesion in this zone produced an alteration of the information projected to the cerebellum.20,22,32 The cerebellum has an internal regulatory function, which could be responsible for the reduction of MUA during baseline activity observed in the cerebellum, which could involve cerebellar interneurons.33,34 Thus, when abnormal information from the basal ganglia reaches the cerebellum, the granule cells are stimulated by the mossy fibers that in turn excite the Golgi cells (glomerular complex) resulting in the regulation of cerebellar cortex.35,36 Golgi cells also mediate the received excitatory information, which resulted in a decrease of activity in the lobe Crus II in animals of the sham and lesion group (Fig. 3). In the case of DN, that regulation may be a consequence of Purkinje cells, which provide a normal bioelectric pattern on cerebellar outputs.37 The exception of this response on the Sim B lobe could be due to the natural physiology of the own structure.35,38 So far, we do know yet which part of the cerebellum is the most important for the correction of incorrect movement but a full circuit in the crus II lobe is necessary for understanding the cerebellar responses.
On the other hand, we know mossy fibers are not the only cerebellar afferents, we also find climbing fibers, coming from the inferior olive (IO) and responsible for transmitting both motor39 and sensory information40 that in turn comes from the red nucleus. Indeed, IO neurons can excite Purkinje cells to release GABA onto the DN40 which, according to our results, is found to decrease in its amplitude during the basal state in Crus II and DN respect intact group (Fig. 3).
Hence, previous studies using immunohistochemistry to Fos protein by means of chronic dopaminergic antagonism showed a similar effect.29 The Fos protein study characterized the cerebellar activity, although such expression could be presented in many ways, for that reason in our study we analyze cerebellar modification on time, specifically during JTMs.
Jaw tremor movements presented in sham and lesion groups showed a similar behavioral pattern of JTMs bursts described in an electromyographic contrast of parkinsonian rats,41,42 where vertical jaw movement could be observed, without direction to any specific stimulus.24
However, in the sham group, we found similar JTMs to those observed in animals from the lesion group, considering the dimensions of our electrode (250μm) and according to the somatotopic characterization of the striatum.43,44 Thus, the specific region where the electrode was placed might be responsible for the observed phenomenon. Nevertheless, we consider it is necessary to implement more research to characterize the decrement of cerebellar response and induction of JTMs by the destruction of VLS like a more specific parkinsonism model. The analysis of the amplitude in the recordings obtained in the sham group during the JTMs over a week did not show significant differences. We consider that more studies are further research needed to determine whether the lesion is chronic or acute in this group.
Therefore, the red nucleus is an important relay center that receives information from the trigeminal nucleus, the internal pale globe, and the DN.39,45,46 Sensory and motor information from the chewing muscles travels through the trigeminal nerve until it reaches the bulb in the trigeminal nuclei to be sent to the motor cortex by means of the red nucleus. The final computation of the information of the internal pale globe and DN,39 in the red nucleus, can lead to the cerebellar modification in response to the alteration caused in the VLS, which places this nucleus as a key relay structure for the alterations found in our study.
Our results have shown an increase in MUA during JTMs, which confirms the functional relationship between basal gangliaBG and cerebellum. We assume that the input from the motor cortex and the basal ganglia causes excitation of the granular layer in recorded structures registered.
ConclusionsOur findings demonstrate that the cerebellar responses changed following an insult to the basal ganglia. Male rats that were exposed to the descent of an electrode in VLS decreased the amplitude of their basal MUA compared to the control group. This may be due to the decrease in afferences of the basal ganglia to the cerebellum, as well as to the participation of inhibitory neurons in the cerebellar cortex that regulates the information to be processed.34,36
Finally, during the presence of JTMs cerebellum showed bursts of high amplitude spikes, which we can interpret as cerebellar activity increased in the three recordgistered structures, which could involve a functional compensation cerebellar in response to the damage on basal ganglia or also as a response to motor activity involving the JTMs. The above tells us about the important cerebellar participation in response to an alteration of the basal ganglia.
Conflict of interestThe authors declare no conflict of interest.
This work was carried out thanks to the grant from the Consejo Nacional de Ciencia y Tecnología (CONACyT) with number 575913 of LVC, as well as the resource aimed at the body academic neuroscience UV-CA-28 of the Doctorado en Investigaciones Cerebrales, Universidad Veracruzana.