Update of Acute Ischaemic Stroke Treatment Guidelines of the Spanish Neurological Society based on a critical review of the literature. Recommendations are made based on levels of evidence from published data and studies.
DevelopmentOrganised systems of care should be implemented to ensure access to the optimal management of all acute stroke patients in stroke units. Standard of care should include treatment of blood pressure (should only be treated if values are over 185/105mmHg), treatment of hyperglycaemia over 155mg/dl, and treatment of body temperature with antipyretic drugs if it rises above 37.5°C. Neurological and systemic complications must be prevented and promptly treated. Decompressive hemicraniectomy should be considered in cases of malignant cerebral oedema. Intravenous thrombolysis with rtPA should be administered within 4.5hours from symptom onset, except when there are contraindications. Intra-arterial pharmacological thrombolysis can be considered within 6hours, and mechanical thrombectomy within 8hours from onset, for anterior circulation strokes, while a wider window of opportunity up to 12–24hours is feasible for posterior strokes. There is not enough evidence to recommend routine use of the so-called neuroprotective drugs. Anticoagulation should be administered to patients with cerebral vein thrombosis. Rehabilitation should be started as early as possible.
ConclusionTreatment of acute ischaemic stroke includes management of patients in stroke units. Systemic thrombolysis should be considered within 4.5hours from symptom onset. Intra-arterial approaches with a wider window of opportunity can be an option in certain cases. Protective and restorative therapies are being investigated.
Actualización de la guía para el tratamiento del infarto cerebral agudo de la Sociedad Española de Neurología basada en la revisión y análisis de la bibliografía existente sobre el tema. Se establecen recomendaciones en base al nivel de evidencia que ofrecen los estudios revisados.
DesarrolloLos sistemas de asistencia urgente extrahospitalaria se organizarán para asegurar la atención especializada de los pacientes y el ingreso en unidades de ictus (UI). Deben aplicarse cuidados generales para mantener la homeostasis (tratar la tensión arterial sistólica >185mmHg o diastólica>105mmHg, evitar hiperglucemia >155mg/dl y controlar la temperatura, tratando con antitérmicos cifras>37,5°C), y prevenir y tratar las complicaciones. La craniectomía descompresiva debe ser considerada en casos seleccionados de oedema cerebral maligno. La trombólisis intravenosa con rtPA se administrará en las primeras 4,5 horas en pacientes sin contraindicación. La trombólisis intraarterial farmacológica puede indicarse en las primeras 6 horas de evolución y la trombectomía mecánica hasta las 8 horas. En el territorio posterior la ventana puede ampliarse hasta 12–24 horas. No hay evidencias para recomendar el uso rutinario de los fármacos denominados neuroprotectores. Se recomienda la anticoagulación en pacientes con trombosis de senos venosos. Se aconseja el inicio precoz de rehabilitación.
ConclusionesEl tratamiento del infarto cerebral se basa en la atención especializada en UI, la aplicación urgente de cuidados generales y el tratamiento trombolítico intravenoso en las primeras 4,5 horas. La recanalización intraarterial farmacológica o mecánica pueden ser útiles en casos seleccionados. Terapias de protección y reparación cerebral están en desarrollo.
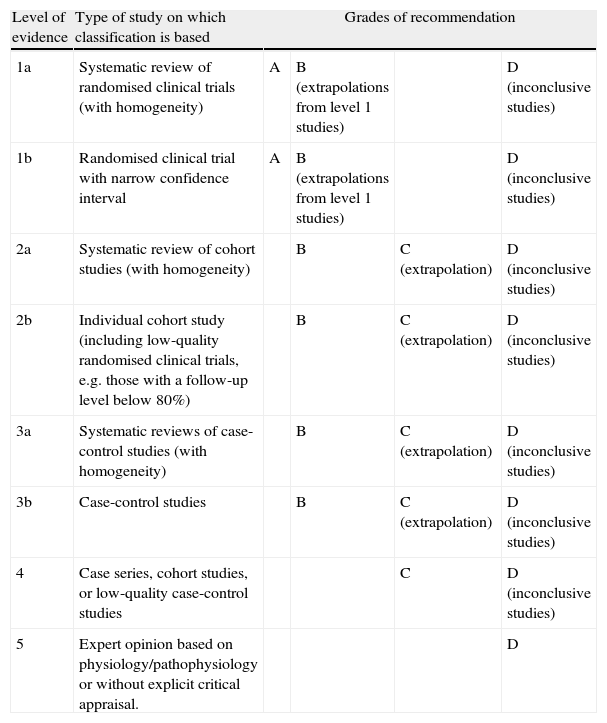
Since the publication of the most recent recommendations by the SEN Study Group for Cerebrovascular Diseases (GEECV/SEN),1 there have been substantial improvements in some aspects of acute management of patients with cerebral ischaemia. This article provides updated recommendations on the care framework, general care for patients with acute-phase stroke, and specific treatment for ischaemia or for cerebral venous sinus thrombosis. Grades of recommendation and the scientific evidence supporting them are classified according to Centre for Evidence-Based Medicine (CEBM) criteria (Table 1).2
Levels of evidence and grades of recommendation.
| Level of evidence | Type of study on which classification is based | Grades of recommendation | |||
| 1a | Systematic review of randomised clinical trials (with homogeneity) | A | B (extrapolations from level 1 studies) | D (inconclusive studies) | |
| 1b | Randomised clinical trial with narrow confidence interval | A | B (extrapolations from level 1 studies) | D (inconclusive studies) | |
| 2a | Systematic review of cohort studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 2b | Individual cohort study (including low-quality randomised clinical trials, e.g. those with a follow-up level below 80%) | B | C (extrapolation) | D (inconclusive studies) | |
| 3a | Systematic reviews of case-control studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 3b | Case-control studies | B | C (extrapolation) | D (inconclusive studies) | |
| 4 | Case series, cohort studies, or low-quality case-control studies | C | D (inconclusive studies) | ||
| 5 | Expert opinion based on physiology/pathophysiology or without explicit critical appraisal. | D | |||
Grade A recommendation: supported by level 1 studies.
Grade B recommendation: supported by level 2 or 3 studies (or extrapolation from level 1 studies).
Grade C recommendation: supported by level 4 studies (or extrapolation from level 2–3 studies).
Grade D recommendation: supported by level 5 studies only or inconclusive studies of any level.
Stroke is a neurological emergency because the injury mechanisms following cerebral ischaemia or haemorrhage progress very quickly and treatments may only be effective during a short period of time. Highly effective and specific treatments are available, but their risk/benefit margin is narrow. With this in mind, organisational frameworks must be optimised and hospitals must be equipped and prepared to care for stroke patients.
The Helsingborg Declaration establishes the goal that all stroke patients will have easy access to diagnostic techniques (Table 2), as well as to treatments with demonstrated efficacy during the acute phase of the disease, referring specifically to access to neurological care and techniques used in stroke units (SU).3,4 Given that these resources are expensive and it is not possible to provide them in all hospitals within a public health system with limited means, we must organise our care systems in such a way that all patients will have access, according to the characteristics of each health district.5 This situation, plus the fact that most available treatments have a narrow therapeutic window, requires coordination between various levels of care to guarantee a minimum response time that will allow the patient to be evaluated and treated rapidly, in a hospital and by neurology specialists. To this end, implementing ‘code stroke’, the protocol for coordinated action by non-hospital emergency services and the hospitals that will care for the patient, has been a useful initiative. Pre-hospital ‘code stroke’ is the procedure that includes implementation of protocols developed by consensus, recognition of the emergency situation, and organisation of transport to suitable hospitals (those with an on-call neurologist, SU, and ability to provide specific treatments such as thrombolysis) after those hospitals have been alerted.6–8 Pre-hospital ‘code stroke’ has been proved to decrease both care and treatment delays. Hospital emergency services should also organise care for these patients in order to reduce delays as much as possible. Action protocols created for this purpose are known as in-hospital code stroke, and they are also very effective (level of evidence 2a).9–12
Thrombolytic treatment with rtPA.
| Inclusion criteria |
| Patients with acute ischaemic stroke at less than 4.5hours after onset and none of the following exclusion criteria. |
| Exclusion criteria |
| 1. Intracranial haemorrhage in CT scan. |
| 2. Elapsed time since onset>4.5hours or time of onset unknown. |
| 3. Symptoms less pronounced or evolving favourably after starting rtPA infusion. |
| 4. Severe stroke according to clinical assessment (NIHSS>25) or neuroimaging criteria. |
| 5. Symptoms suggesting subarachnoid haemorrhage although CT findings appear normal |
| 6. Treatment with heparin in the preceding 48hours and high aPTT level, or LMWH at anticoagulant doses in the preceding 12hours. |
| 7. Stroke in the preceding 3 months. |
| 8. Platelet count below 10000 |
| 9. Untreated glycaemic level below 50mg/dL or above 400mg/dL |
| 10. Systolic blood pressure>185mmHg, diastolic blood pressure>105mmHg, or when aggressive means are required to bring blood pressure below these thresholds. |
| 11. Known bleeding diathesis |
| 12. Treatment with oral anticoagulants. Treatment with rtPA may be considered if INR≤1.7. |
| 13. Recent or manifest severe bleeding |
| 14. History of intracranial haemorrhage. |
| 15. History of SAH due to ruptured aneurysm. |
| 16. History of central nervous system lesion (aneurysms, neoplasia, intracranial or spinal surgery). |
| 17. Haemorrhagic retinopathy (ex. diabetic retinopathy). |
| 18. History of cardiac massage, childbirth, or punctured inaccessible blood vessel in the preceding 10 days. |
| 19. Bacterial endocarditis, pericarditis. |
| 20. Acute pancreatitis. |
| 21. Documented ulcerative gastrointestinal disease in the 3 preceding months. Oesophageal varices. Known intestinal vascular malformations. |
| 22. Neoplasia with an increased risk of haemorrhage. |
| 23. Severe liver disease (liver failure, cirrhosis, portal hypertension, active hepatitis). |
| 24. Major surgery or significant trauma in the 3 preceding months |
| Regimen for administering rtPA |
| – Administer 0.9mg/kg with 90mg as the maximum dose; 10% of the total dose is delivered as a bolus over one minute and the rest of the dose will be infused continually over one hour. |
| Recommendations for general management and concomitant treatments |
| – Heparin and oral anticoagulants should not be administered in the 24hours following rtPA treatment as they may increase risk of cerebral haemorrhage. |
| – Patients should be monitored, preferably in a stroke unit. |
| – A neurological examination should be performed every 15minutes during the infusion, at the 2-hour mark, at the 24-hour mark, and any time the patient's condition deteriorates. |
| – Infusion should be continuous if there are clinical signs of bleeding (intense headache, vomiting, decreased level of consciousness, increased disability). In this case, an emergency CT will be performed. |
| – Avoid or delay placement of urinary catheters, nasogastric tubes, and arterial lines as much as possible. |
| – In cases of overdose, fibrinogen and other coagulation factors are often depleted. Waiting for normal physiological regeneration of these factors is usually sufficient. If haemorrhage occurs, follow the recommendations specific to the case. |
| – If there is an anaphylactic reaction, suspend the infusion and treat as appropriate. |
| – Blood pressure is to be checked every 15minutes during the infusion and the first hour thereafter; every 30minutes in the following 6 hours; and every hour until reaching the 24-hour mark. Blood pressure should be checked more frequently if it exceeds 180/105. |
| Blood pressure monitoring |
| – Blood pressure should be below 185/105 before the infusion is started. |
| – If blood pressure exceeds 185/105 in 2 measurements taken 5–10minutes apart, treat according to current recommendations (see text). If these measures do not decrease the reading, the patient should not undergo thrombolysis. If blood pressure rises and cannot be lowered after treatment has been started, the infusion should be discontinued. |
| Managing haemorrhage after thrombolysis |
| – Cerebral haemorrhage should be suspected when the patient experiences neurological impairment, intense headache, vomiting, or a blood pressure spike. |
| – Systemic haemorrhage may be visible or occult (haemodynamic changes). |
| – Discontinue rtPA infusion |
| – Perform emergency cranial CT (for cerebral haemorrhage). |
| – Measure coagulation times, fibrinogen, and platelets; run cross-match. |
| – Administer Haemocomplementan P® to replace fibrinogen: (1–2 vials, 1g). The maximum dose is 2–3g daily. |
| – Cryoprecipitates with a high platelet and factor VIII content, fresh plasma, and fresh blood are not recommended, since only fibrinogen will be depleted rather than the other factors listed above. |
| – Antifibrinolytic agents (tranexamic acid: amchafibrin®) may elicit thrombotic phenomena. |
LMWH: low molecular weight heparin; SAH: subarachnoid haemorrhage; INR: international normalised ratio; NIHSS: National Institute of Health Stroke Scale; rtPA: recombinant tissue plasminogen activator; BP: blood pressure; CT: computed tomography; aPTT: activated partial thromboplastin time.
Telemedicine systems that enable live communication between stroke centres of reference and hospitals lacking on-call neurologists may be useful as links to specialised resources where geographical barriers prevent or delay direct access. These systems help increase the number of patients evaluated by neurology specialists and the number of specific treatments administered, thereby reducing treatment delays (level of evidence 2a).13
Most stroke patients require hospitalisation. Possible exceptions include patients with a prior diagnosis of dementia or terminal illness and those not wishing to be hospitalised, provided that these cases will receive appropriate care outside the hospital.14
Stroke patients should be monitored in stroke units; studies with a 1A level of evidence show that SU care is linked to better outcomes due to reduced morbidity and mortality and a decreased probability of complications and/or dependence with an acceptable cost-efficiency ratio.15–19 These benefits are the result of non-invasive neurological monitoring and the use of general care protocols intended to maintain homeostasis, in addition to proper application of specific treatments.20–22 Care in SUs is linked to better care quality indicators (mean hospital stay, readmission, mortality, and need for institutionalisation) and it also significantly reduces monetary costs in stroke care.19,23 SUs are structurally delimited divisions that are functionally dependent on neurology departments and provide specialised care to stroke patients. They are coordinated and staffed by neurology specialists with support from other related medical specialties (cardiology, vascular surgery, neuroradiology, neurosurgery, rehabilitation, emergency medicine, etc.), from physiotherapists who provide rapid treatment, and from social workers. They are equipped to provide non-invasive continuous monitoring and have trained nursing staff with a recommended nurse-to-patient ratio of at least 1:6. Their personnel and diagnostic services are available 24hours a day, and their protocols and clinical channels for patient management are based on scientific evidence. The number of beds in the SU must be planned according to the needs and the size of the population served by the hospital. Stroke care plans recommend one monitored SU bed per 100000 inhabitants.24,25
Admission criteria are as follows: patients with acute-phase stroke (<48hours from onset), mild to moderate neurological deficit, and transient ischaemic attack. No age limits were established. Exclusion criteria are irreversible brain damage, prior dependent status or diagnosis of dementia, concurrent severe or terminal illness, and acute head trauma.
- 1.
Recommendations call for admission to an acute SU with the necessary equipment (level of evidence 1a; grade A recommendation).
- 2.
Emergency, in-hospital care is recommended for all patients with acute stroke (level of evidence 3a; grade B recommendation).
- 3.
Reducing time to provide neurological care to a minimum and establishing specific pre-hospital care coordination systems are also recommended (level of evidence 2a; grade B recommendation).
- 4.
Telemedicine systems with live, remote supervision by an expert neurologist may be useful to evaluate the patient and the specific treatment decision if in situ care is not available (level of evidence 1b; grade A recommendation).
Non-pharmacological brain protection refers to maintaining vital signs within normal limits (blood pressure, blood sugar, blood gas values, and body temperature), and preventing and detecting complications in their early stages. These measures significantly improve mortality and morbidity rates over the medium term, which is why a patient's vital signs and neurological status must be monitored in the first 48hours after stroke, or until the patient is in stable condition.21
Maintaining proper respiratory function is a priority for the general management of these patients. In most cases, placing patients in a semi-seated position is sufficient. However, when respiratory function is compromised, patients will need orotracheal intubation and mechanical ventilation. Hypoxia due to partial airway obstruction, pneumonia, or hypoventilation may increase the area of the lesion and indicate a poorer prognosis.26 Data indicate that administering supplementary low-flow oxygen to stroke patients reduces the rate of nocturnal desaturation (a common occurrence in these patients), and could therefore lead to better outcomes.27 If readings show hypoxia (<95% oxygen saturation [SaO2], the patient will require oxygen therapy (evidence level 2b).10
Blood pressure managementArterial hypertension is very frequent during the acute phase of stroke. In many cases, it decreases spontaneously within a few days of stroke onset. During the acute phase of stroke, antihypertensive drugs should be used with caution. Since the mechanisms regulating cerebral circulation in the ischaemic area will be damaged, a decrease in perfusion pressure could compromise regional blood flow in the penumbra, thereby exacerbating ischaemia and worsening the patient's neurological state.28 Numerous studies indicate that the relationship between mortality/functional prognosis after stroke and systolic/diastolic blood pressure follows a U-shaped curve. As such, the probability of death or dependency is higher for either low or high blood pressure values during the acute phase; the most favourable figures are 110–180/70–105 (evidence level 2a).29–32 For this reason, treating high blood pressure is only recommended if systolic pressure is over 185 or diastolic pressure exceeds 105.
Data show that providing monitored treatment for hypertension during the acute phase of stroke is safe.33–36 However, only one study shows that it is beneficial,35 while others show no decrease in vascular events or any effects of treatment that would improve outcomes.36 One study suggests treatment may be detrimental.37
When possible, doctors should administer oral drugs with little effect on regional cerebral blood flow, such as angiotensin receptor blockers, angiotensin convertase enzyme inhibitors, or beta-blockers.35 Drugs eliciting rapid, intense decreases in arterial pressure, such as calcium channel blockers or diazoxide, are to be avoided. If drugs must be administered intravenously, their effect should be predictable and easily reversible, as in the cases of labetalol, urapidil, or nitroprusside. Drugs must be administered under strict surveillance to prevent sudden drop or any decreases of more than 20%.1,32
Certain exceptional situations that alter blood pressure levels call for treatment, such as co-presence of myocardial ischaemia, heart failure, aortic dissection, or hypertensive encephalopathy.
Once the acute phase is over, antihypertensive treatment should be started as a secondary prevention strategy, in accordance with specific guidelines.
Hypotension is uncommon following a stroke. It may be caused by a decrease in volume or failure to pump blood, and if the condition is present, doctors should rule out such complications as myocardial infarction, aortic dissection, pulmonary embolism, or digestive tract haemorrhage. In addition to treating the cause, hypotension should be corrected using volume expander drugs, and occasionally, vasodepressors (dopamine).
Hyperthermia is associated with poorer prognosis in cerebral infarct such that at temperatures exceeding 37.5°C, there is an increased probability of disease progression and death (level of evidence 2a).38,39 Some studies show that treatment with antipyretic drugs improves outcomes in patients with high temperatures, but that these drugs are futile when used routinely in patients with normal temperatures (level of evidence 1b).40 When a patient presents fever, doctors should investigate and treat the cause. Antipyretics (paracetamol or metamizole, as well as physical cooling means if necessary) are recommended if axillary temperature is higher than 37.5°C. Experimental data show that hypothermia reduces the size of the infarct. Clinical studies show that it is possible to induce hypothermia with physical or pharmacological means, but at this time, no data point to the utility of hypothermia as a technique for improving functional outcomes or reducing mortality. In fact, hypothermia is associated with a greater risk of developing infectious complications, especially pneumonia.41 However, there are initiatives promoting further studies of the utility of hypothermia under optimal clinical conditions for this treatment.42,43
Glycaemic controlHyperglycaemia in the acute phase of stroke, as well as persistent hyperglycaemia>155 in the first 48hours after stroke onset, are linked to poorer functional outcomes and increased mortality (level of evidence 2b).44–47 Hyperglycaemia is associated with infarct progression,45 decreases the effectiveness of thrombolysis, and increases risk of haemorrhage following thrombolysis (level of evidence 2b).48,49 Insulin treatment corrects hyperglycaemia in acute stroke, but this intervention has not been shown to provide better outcomes (level of evidence 1b).50,51 The available data recommend maintaining glycaemia below 155mg/dL and avoiding glucose solutions in the first 24 to 48hours, except in diabetic patients who are more likely to experience hypoglycaemia, especially those who had previously been treated with oral antidiabetic agents.46,47 However, hypoglycaemia should be treated by administering glucose. This condition may elicit focal conditions that resemble stroke or exacerbate existing symptoms; on the other hand, some stroke patients will not show symptoms of hypoglycaemia. For these reasons, we recommend monitoring glycaemia in all patients over at least 6hours during the acute phase of stroke, or more frequently in cases in which glycaemia does not remain within the normal limits.
Hydration and nutritional balanceUndernutrition after a stroke promotes complications.52–54 Patients may experience serious nutritional difficulties due to dysphagia or low level of consciousness. Enteral nutrition through a nasogastric tube is required if these conditions persist more than 48–72hours.54 Patients’ ability to swallow should be examined daily to limit the risk of aspiration. Since dysphagia for liquids is more common, liquids should be avoided in early stages until after verifying that the patient can swallow normally. If the patient experiences dysphagia for liquids, dehydration can be prevented by providing liquids with thickeners or gelatine. Avoiding prolonged fasting reduces mortality and complication rates, but the effect on functional outcomes is unclear (level of evidence 1b).54
Physiotherapy in the acute phaseEarly mobilisation reduces the incidence of other complications: shoulder pain, pressure ulcers, muscle spasms, pressure paralysis, etc. Published studies and meta-analyses indicate that physiotherapy and rehabilitation are effective for functional recovery over the medium term, and that therapy is more effective when started in early stages and focusing specifically on rehabilitation for concrete tasks (level of evidence 1a).55–57 Although passive physiotherapy should be initiated early on, active rehabilitation should be postponed until the patient is in stable condition and not at risk for haemodynamic destabilisation.
Certain substances may delay recovery after a stroke due to their potential depressant effect on the central nervous system, especially substances with GABA agonists. As a result, they should be avoided as much as possible during the acute phase of stroke. These substances include neuroleptics, benzodiazepines and other anxiolytics, barbiturates, phenytoin and other anticonvulsants, and antispasmodic drugs. If these drugs are necessary, they should be administered with caution (level of evidence 3a).58 On the other hand, early use of serotonin reuptake inhibitor-type antidepressants (fluoxetine, citalopram) in indicated cases has been observed to improve mood disorders and promote functional recovery (level of evidence 1a).59–61
- 1.
Administering oxygen to patients with hypoxia (SaO2<95%) is recommended, as is providing intubation and ventilation support to patients with compromised airway (level of evidence 2b; grade B recommendation).
- 2.
Antihypertensive drugs should be used cautiously during the acute phase of stroke. Hypertension should be treated if systolic arterial pressure readings exceed 185mmHg, or if diastolic readings exceed 105mmHg (level of evidence 2a, grade B recommendation).
- 3.
Hyperthermia exceeding 37.5°C is to be avoided. Among the drugs that have been studied, paracetamol has been shown to safely and effectively reduce body temperature (level of evidence 1b; grade A recommendation).
- 4.
Avoid administration of glucose except to treat hypoglycaemia.
- 5.
Glycaemia levels>155mg/dL should be prevented (level of evidence 2a; grade B recommendation).
- 6.
Patients should be checked for dysphagia in order to prevent pulmonary aspiration. Doctors must prevent undernutrition and determine whether enteral nutrition with a nasogastric tube will be necessary during the first few days (level of evidence 1a; grade A recommendation).
- 7.
Early use of passive mobilisation exercises is recommended (evidence level 1a; grade A recommendation).
- 8.
Drugs that may have a detrimental effect on functional recovery should be avoided (level of evidence 3a; grade B recommendation).
The most frequent neurological complications are oedema and intracranial hypertension, epileptic seizures, and haemorrhagic conversion of a cerebral infarct.
Cerebral oedema after ischaemic stroke, with intracranial hypertension, may lead to cerebral herniation. This tends to be the cause of death within the first week after a large hemispheric infarct, especially in young patients or cases of cerebellar infarct occurring with compression of the fourth ventricle and aqueduct of Sylvius and potential secondary hydrocephalus.62 The term ‘malignant middle cerebral artery infarction’ (MMCAI) was coined to designate slowly evolving infarcts in this territory that are caused by either occlusion of the main trunk of the middle cerebral artery (MCA) or of the distal portion of the intracranial internal carotid artery (ICA).63 The entity is characterised by clinical signs of total anterior circulation infarct with a decreased level of consciousness and radiological findings of ischaemia affecting more than 50% of the MCA territory. Following that, frank oedema appears with a more or less pronounced mass effect and midline shift; this is generally related to the decrease in consciousness. The mortality rate of MMCAI is 80%, even with aggressive medical treatment (intubation and anti-inflammatory drugs).64 Clinical and radiological data let us predict this poor prognosis, which is helpful for selecting patients who may benefit from aggressive treatment. Careful monitoring is therefore the key to detecting decreased consciousness in early stages and providing treatment before damage becomes irreversible.
Treatment is initially preventive, and it consists of such general strategies as restricting fluids moderately and avoiding hypo-osmolar solutions (such as 5% glucose), and treating associated illnesses that could worsen oedema (hypoxia, hypercapnia, hyperthermia, arterial hypertension, urinary retention, etc. Other strategies include elevating the head of the bed 30° to improve venous return and decrease intracranial pressure (ICP) (level of evidence 3b).65
High doses of steroids should not be used as they neither lessen mortality nor decrease sequelae, but rather promote infection and complicate glycaemic control (level of evidence 1a).66–68
Osmotic agents (mannitol 20% or glycerol 10%) may decrease ICP, but their effect is temporary and they have not been proved effective for reducing mortality or sequelae. Routine use of osmotic agents is not recommended in cases of cerebral oedema in acute stroke (level of evidence 1a).69–71 In the same way, hyperventilating an intubated patient to decrease the partial pressure of arterial carbon dioxide (PaCO2) elicits a drop in intracranial pressure. However, since its effect is brief, it is used as an adjuvant technique prior to decompressive craniectomy.
Decompressive craniectomy in MMCAI has been shown to decrease mortality and, in some cases, sequelae as well. This is true when the technique is performed early (within 48hours of symptom onset), in younger patients (≤60 years) and when clinical data do not indicate herniation or concomitant conditions that could increase the probability of complications such as haemodynamic instability, risk of haemorrhage, severe comorbidity, etc. (evidence level 1a) (Table 3).72–76 Craniectomy must be extensive (at least 12cm) with a dural aperture. Placing an ICP sensor has not been shown to be useful (level of evidence 2b).77
Decompressive craniectomy in malignant infarct of the medial cerebral artery.
| Indications |
| – Age≤60 years. |
| – Symptom onset≤48hours. |
| – Clinical, radiological, and neurosonological signs of extensive MCA infarct or carotid infarct (TACI) |
| • NIHSS>15 at time of admission. |
| • After admission, decline in neurological condition (≥4 points on NIHSS) and or level of consciousness (≥1 point on NIHSS item 1a) when other non-neurological causes have been ruled out. |
| • Infarct volume≥145cm3 determined by diffusion MRI, or: |
| • CT showing impairment of ≥50% of the MCA, especially if mass effect is present. |
| • Increase in mass effect compared to baseline CT scan. |
| • Neurosonology or angiography indicative of occluded carotid or M1 segment of the MCA. |
| – Stable haemodynamic situation. |
| – Informed consent signed by family member or representative. |
| Contraindications or exclusion criteria |
| – Age>60 years. |
| – Poor baseline condition prior to stroke; Rankin score>2. |
| – Neurological impairment due to other treatable causes. |
| – Concomitant illnesses that are severe and/or have a poor prognosis of survival. |
| – Clotting disorders or elevated risk of haemorrhage. |
| – Contraindication for anaesthesia. |
| – Clinical or radiological data for cerebral herniation or brain death. |
| – Consent not given by family member or representative. |
| – Patient left express instructions in a living will or other instrument to refuse care that would permit survival with loss of autonomy. |
MCA: middle cerebral artery; NIHSS: National Institute of Health Stroke Scale; MRI: magnetic resonance imaging; TACI: total anterior circulation infarct; CT: computed tomography.
In cases of large cerebellar infarcts producing obstructive hydrocephalus or affecting the brainstem, suboccipital craniectomy78 is an effective treatment for both hydrocephalus and brainstem compression (level of evidence 3a). This treatment is preferred to ventricular catheter placement, which may elicit upward transtentorial herniation of the oedematous cerebellum. Isolated ventricular drainage is therefore not recommended in these cases.10 Time and age criteria are not as restrictive as in cases of hemicraniectomy in MMCAI.
Epileptic seizures may complicate the acute phase of a stroke. Seizures are usually partial, with or without secondary generalisation. The recurrence rate is low for seizures occurring shortly after the stroke, but higher for those with a later onset (3%–5% of all cases); 54% to 55% of patients who experience late-onset seizures will develop epilepsy.79,80 Antiepileptic agents should only be used for recurring seizures, and prophylactic use of these drugs is not indicated in patients who have never had seizures. First-generation antiepileptic drugs, especially phenytoin, are not the most appropriate during the acute phase of stroke because they may interfere with the patient's recovery. Lamotrigine and gabapentin have been evaluated as treatment for post-stroke seizures, and their efficacy/safety profiles are better than those of carbamazepine, oxcarbazepine, or topiramate due to having fewer drug interactions (level of evidence 1a).80,81 Levetiracetam seems to be useful in patients with late-onset post-stroke seizures, and it may also be effective in the acute phase (level of evidence 3b).82,83
- 1.
Patients with extensive anterior territory infarcts or cerebellar infarcts should be monitored closely, and doctors are recommended to use general techniques for preventing oedema (level of evidence 3b; grade B recommendation).
- 2.
Corticosteroids should not be used in cerebral oedema caused by ischaemia (level of evidence 1a; grade A recommendation).
- 3.
If clinical and imaging signs of MMCAI are present, doctors should consider decompressive hemicraniectomy within 48hours of stroke onset in patients younger than 60 and where signs of transtentorial herniation are absent. Osmotherapy and hyperventilation are to be carried out in preparation for this procedure (level of evidence 1a; grade A recommendation).
- 4.
Suboccipital decompressive craniectomy is recommended in cases of cerebellar infarct in which the brainstem is affected due to compression or obstructive hydrocephalus (level of evidence 3b; grade B recommendation).
- 5.
Prophylactic use of antiepileptic drugs is not recommended in patients with no previous history of seizures (level of evidence 1a; grade A recommendation). Antiepileptics should be administered to patients with recurring seizures (level of evidence 1a; grade A recommendation). Data are insufficient to indicate any single antiepileptic drug as a first-choice treatment.
The most frequent infections are pneumonia and urinary tract infection. Pneumonia develops in patients with an altered level of consciousness, decreased cough reflex, or dysphagia. This disease is a significant cause of death in stroke patients. Doctors must identify patients at risk for pneumonia and employ preventative measures such as lung isolation where necessary, respiratory physiotherapy, aspiration of secretions, and preventing vomiting.
Urinary tract infections may cause sepsis in up to 5% of all stroke patients. These infections are more frequent in women and patients with more severe strokes. Doctors recommend avoiding situations, such as urinary catheterisation, that can favour these infections, except where strictly necessary.84
Presence of fever indicates that the patient must be checked for pneumonia or UTI; if either is suspected, antibiotic treatment should be started shortly thereafter (level of evidence 2b).84 Empirical antibiotic treatment is recommended, using amoxicillin/clavulanate at high doses (1–2g IV/8h) since this antibiotic covers most microbes that could be responsible. Patients allergic to that treatment should use quinolones (ciprofloxacin 200–400mg/12h). Treatment should be adjusted to fit the culture findings or symptoms if there is no response.
Deep vein thrombosis is another common complication. It sometimes provokes pulmonary thromboembolism, which in turn causes death in 25% of stroke cases. Administering low molecular weight heparin is an effective means of preventing venous thrombosis (level of evidence 1a).85 Aspirin has also proved its efficacy for preventing pulmonary thromboembolism (level of evidence 1a).86,87 There is no evidence that physical devices, such as compression stockings or intermittent pneumatic compression systems, significantly reduce the incidence of venous thrombosis (level of evidence 1a).88
- 1.
Early delivery of antibiotic treatment is recommended for infectious complications (level of evidence 2b; grade B recommendation).
- 2.
Low molecular weight heparin or heparinoids are recommended to prevent deep vein thrombosis and pulmonary embolism in immobilised patients. If these treatments are contraindicated or an alternative is required, aspirin may be used (level of evidence 1a; grade A recommendation).
Depending on the type of cerebral ischaemia, there are 2 theoretical approaches to limiting brain damage. These approaches are improving or re-establishing cerebral blood flow (CBF) in the ischaemic area, and employing drugs intended to inhibit the cellular and molecular alterations that lead to ischaemia-reperfusion injury in the potentially salvageable area or ischaemic penumbra (pharmacological brain protection).89 Both therapeutic strategies must be implemented in early stages to prevent the irreversible progression of different lesion mechanisms. Doctors recently advanced the concept of damage repair based on the potential existence of plasticity phenomena that may be activated or reinforced through therapeutic interventions.90,91 Treatments employed for this purpose may have a wider window of opportunity.
Measures intended to improve or re-establish CBFEnsuring the proper perfusion pressure to maintain a stable haemodynamic situation in the ischaemic area is a crucial target. Antithrombotic and thrombolytic drugs and mechanical thrombectomy have all been used to recanalise and reperfuse ischaemic tissue.
Antithrombotic agentsAnticoagulantsHeparin is used in the acute phase of cerebral ischaemia because of its potential effects, which are stopping thrombus progression or promoting thrombus resolution, and preventing early stroke recurrence in cases of ischaemic stroke caused by embolic mechanisms.
Unfractionated heparinResults from the International Stroke Trial (IST) show that administering subcutaneous heparin calcium does not improve patient outcomes. Although this treatment does prevent early recurrence, that effect is counteracted by an associated increased risk of haemorrhage, which is especially pronounced if aspirin is also used (level of evidence 1b).86 Similarly, a subanalysis within this study was unable to show any benefits of heparin treatment among patients with atrial fibrillation.92 Other studies evaluating heparin sodium (partial thromboplastin time 1.5–2 times the control) show efficacy for preventing early recurrences in patients with cerebral infarcts of cardioembolic origin,93,94 but this efficacy comes at the expense of an increase in haemorrhages.
Intravenous heparin has not demonstrated any benefits in cases of progressive cerebral infarct.95
A meta-analysis of studies of unfractionated heparin as acute-phase treatment for strokes of all aetiologies revealed no overall benefits.96 While the meta-analysis found a decreased incidence of deep vein thrombosis, pulmonary thromboembolism, and early stroke recurrence, any benefits were cancelled out by the increased risk of potentially severe haemorrhage (level of evidence 1a). A meta-analysis of studies specifically evaluating heparin use in patients with acute-phase cardioembolic infarct97 concludes that heparin, compared to aspirin or placebo, does not significantly decrease mortality, dependency, or stroke recurrence in the first 14 days. In addition, it causes a nearly threefold increase in the frequency of symptomatic cerebral haemorrhages. Furthermore, it produces no significant reduction in cases of deep vein thrombosis or pulmonary embolism compared to aspirin (level of evidence 1a).
Low molecular weight heparin and heparinoidsNumerous trials have studied the effect of LMW heparin and heparinoids on acute ischaemic stroke, and overall results have been negative. In a study with nadroparin (FISS), researchers observed a lower 6-month mortality rate among patients receiving treatment.98 However, these results were not confirmed by a later European clinical trial (FISS bis) which also showed a higher rate of haemorrhagic complications at higher doses.99 The TOAST clinical trial (Trial of Org 10172 in Acute Stroke Treatment), employing intravenous danaparoid treatment within 24hours of a cerebral infarct, showed a lower number of haemorrhages among treated patients, but no benefits with regard to preventing stroke progression and early recurrence, or a more favourable outcome at 3 months.100 Other studies comparing dalteparin101 or tinzaparin102 to aspirin have delivered similar results. Meta-analyses of studies of heparinoids and low-molecular weight heparins show that although these treatments lower the incidence of deep vein thrombosis and pulmonary thromboembolism, they do not improve outcomes or decrease early stroke recurrence. They may also cause an increase in intracranial haemorrhages (level of evidence 1a).85,103
Studies of the defibrinogenating drug ancrod have revealed no benefits and an increased risk of cerebral haemorrhage.104,105
Antiplatelet drugsThe only antiplatelet drug to have been studied in the acute phase of cerebral stroke is aspirin. The International Stroke Trial (IST)86 and the Chinese Acute Stroke Trial (CAST)87 show that administering aspirin dosed at 300mg/day in the first 48hours and in the first 2 weeks was beneficial for patient outcomes at 6 months. The treatment also reduced early recurrence and mortality rates. Meta-analysis of both studies shows an absolute decrease of 0.7% in the recurrence rate and of 0.4% in the mortality rate. The increase in haemorrhages was 0.2%. The overall benefit was an absolute decrease of 0.9% in the risk of death or recurrence (level of evidence 1a).106
Other intravenously administered antiplatelet drugs, such as abciximab or tirofiban, have been studied both in monotherapy and combined with thrombolysis as means of achieving arterial recanalisation in acute cerebral infarct.107,108 Despite some promising early results, clinical trials with abciximab in the first 6hours after stroke onset109 show an increase in the rate of haemorrhages among treated patients (evidence level 1b).110
In all clinical trials employing thrombolysis, no antithrombotic agents were administered until 24hours after delivering the thrombolytic drug, as is currently recommended.
- 1.
Aspirin is recommended in the first 48hours following a cerebral infarct except where contraindicated (evidence level 1a; grade A recommendation). Early treatment with anticoagulants is not recommended for patients with acute cerebral infarct (evidence level 1a; grade A recommendation).
- 2.
For patients undergoing thrombolysis, no antithrombotic drug should be used until 24hours after the procedure (evidence level 1a; grade B recommendation).
At present, we have sufficient evidence, based on randomised studies (NINDS, ECASS, ATLANTIS),111–115 meta-analysis of these clinical trials,116–118 and post-marketing clinical practice studies,119,120 to recommend thrombolytic treatment with intravenous recombinant tissue plasminogen activator (rtPA) dosed at 0.9mg/kg in acute stroke patients at less than 4.5hours after onset of a cerebral infarct. This treatment is linked to better clinical and functional outcomes at the 3-month mark (level of evidence 1a).1,10,111,120 Haemorrhagic complications, particularly symptomatic cerebral haemorrhage, are the main risk in rtPA treatment. The rate of symptomatic cerebral haemorrhage in the SITS_ISTR registry, containing nearly 24000 patients, was 2%.120 In general, the rate of haemorrhagic complications decreases and treatment has an appropriate safety margin if dosing recommendations and patient selection criteria are followed strictly (Table 2).
At present, age>80 years is not considered an exclusion factor for thrombolytic treatment. The frequency of favourable functional outcomes among SITS patients older than 80 and treated with IV rtPA was significantly higher than in patients from trials employing neuroprotection and no thrombolysis (VISTA registry). The effect was similar to that in younger patient groups (level of evidence 2a).121
Doctors have also questioned whether prior stroke with diabetes mellitus should be an exclusion factor.122
Epileptic seizures at the time of stroke onset increase the likelihood of a diagnostic error, but it is understood that seizures do not constitute a reason for denying thrombolytic treatment if the infarct is confirmed by neuroimaging techniques.123,124
The sooner thrombolysis is performed, the greater its benefits,117 which is why all unnecessary delays are to be avoided.
We do not recommend administering other thrombolytic agents systemically, since this practice is associated with a high rate of haemorrhagic complications (evidence level 1a).1,10
Up to a third of the patients treated with intravenous thrombolysis present artery reocclusion. This is more frequent in cases in which recanalisation is incomplete or when an extracranial/intracranial tandem lesion is present.125 Various strategies have been studied to improve the recanalisation rate after intravenous thrombosis and decrease the frequency of reocclusion. The CLOTBUST study shows that applying ultrasound to the occluded artery while simultaneously administering rtPA improves the recanalisation rate and patient outcomes (level of evidence 1b).126 Researchers have also observed that simultaneous administration of echo-enhancing agents can improve the recanalisation rate, but doubts remain as to the safety of the procedure (level of evidence 1b).127 Other treatments designed to prevent reocclusion, such as associating rtPA with anticoagulants (tirofiban, argatroban) or fast-acting antiplatelet drugs (abciximab, eptifibatide), did not return favourable results.107,108,128
Clinical trials are currently examining other synthetic thrombolytic agents (desmoteplase, reteplase, tenecteplase) which have been modified for better thrombolytic capacity and increased affinity and selectivity for thrombin bound to the thrombus in order to reduce associated haemorrhagic complications. Results are promising but not yet confirmed.129–133
Results from studies evaluating the utility of intra-arterial thrombolysis, whether in monotherapy or in combination with intravenous thrombolysis, are promising, and studies continue. Nevertheless, endovascular techniques are becoming increasingly common in daily clinical practice. Available evidence is based on only a very few randomised controlled trials in addition to some case series and prospective registry studies. Based on existing data from the randomised, placebo-controlled PROACT II trial, intra-arterial thrombolysis with recombinant pro-urokinase is an effective means of recanalising arteries occluded by thrombus, and applying that treatment is linked to a higher percentage of patients being independent at 3 months. It increases the risk of cerebral haemorrhaging, but not of mortality (level of evidence 1b).134,135 Despite the above, it was not approved by regulatory authorities. The procedure necessarily involves a longer delay in administering treatment. At present, no data support the premise that intra-arterial thrombolysis offers better outcomes than intravenous thrombolysis, although the technique has been used in patients with large vessel occlusions, patients with salvageable tissue at more than 4.5hours after stroke onset, and where intravenous thrombolysis was contraindicated (evidence level 2b). The therapeutic window would be 6hours from stroke onset for the anterior territory (level of evidence 1b), 12hours for the posterior territory, and up to 24hours in cases with progressive or fluctuating onset. However, some case series include patients with posterior territory strokes treated as much as 48hours after onset (level of evidence 4).136–139
The sparse data on combining intravenous and intra-arterial thrombolysis suggest that this treatment may be safe.140,141 The IMS I study found that a partial dose of intravenous rtPA followed by intra-arterial thrombolysis yielded functional outcomes that were better than those in historical controls in the NINDS trial, but no better than outcomes in NINDS trial patients who had received thrombolytic agents (level of evidence 1b).142 The IMS III study, which is in progress and has recruited more than 300 patients, evaluates whether intravenous thrombolysis plus endovascular intervention (intra-arterial thrombolysis or mechanical device) is more beneficial than intravenous treatment alone.143
It is also possible to perform mechanical thrombectomy using intra-arterial devices that break up and extract the blood clot. The MERCI study reported recanalisation in 45% of the patient total. This rate was 64% in the group receiving combined treatment with intra-arterial rtPA. Fifty per cent of the recanalised patients improved significantly. Nevertheless, clinical outcomes were no better than those reported by the PROACT study.144 The Multi-MERCI study reported recanalisation in 55% of the cases; 3-month outcome was good in 36% of the patients. There was also a significant increase in haemorrhages and a 34% mortality rate, which could be linked to the fact that these patients had suffered severe strokes.145 In a combined analysis of the MERCI and Multi MERCI studies compared to PROACT II, researchers concluded that results from embolectomy were similar to those from the PROACT II treatment group, and the mortality rate also resembled that in the PROACT II control group.146 The single-arm study of the PENUMBRA device yielded better results than the MERCI study, with a recanalisation rate of 81% and no significant increases in mortality. Clinical outcomes, however, were poor. The percentage of patients with favourable clinical outcomes resembled the rate in the PROACT study control group.147 Multiple randomised and prospective registry studies are underway to evaluate the efficacy and safety of numerous mechanical thrombectomy devices for use in both the anterior and posterior territories. Today, mechanical intra-arterial recanalisation procedures may be considered an option in patients in whom intravenous thrombolysis is contraindicated, provided that salvageable tissue remains. The therapeutic window is 8hours for the anterior territory (level of evidence 1b). Although the posterior territory is less commonly affected, the therapeutic window in these cases may be the same as in intra-arterial pharmacological thrombolysis (level of evidence 4). These procedures should only be carried out in centres with the proper equipment and experience.138
- 1.
Thrombolytic treatment with IV rtPA dosed at 0.9mg/kg is recommended as treatment for acute cerebral infarct up to 4.5hours after stroke onset. Treatment should be performed as early as possible. Patient selection should follow established criteria strictly. Guidelines for administering treatment, and for treating arterial hypertension or haemorrhagic complications, are shown in Table 3 (level of evidence 1a; grade A recommendation).
- 2.
Treatment must be indicated by neurologists with expertise in stroke management and performed in centres equipped to provide specialist care, preferably in an SU. These centres must also be able to treat potential complications (extrapolation from Level 1 studies; grade B recommendation).
- 3.
Antithrombotic drugs (heparin, aspirin) are not recommended in the 24hours following IV thrombolysis (extrapolation from Level 1 studies; grade B recommendation).
- 4.
Intra-arterial thrombolysis may be useful in patients with large-vessel occlusion stroke, and who are not candidates for intravenous thrombolysis, until 6hours post-infarct (level of evidence 1b; grade B recommendation).
- 5.
The utility of combined intra-arterial and intravenous treatment has not yet been established, but it may be an option for patients presenting large-vessel occlusion who do not respond to intravenous treatment (evidence level 2b; grade B recommendation).
- 6.
Mechanical thrombolysis may be useful until 8hours post-stroke in patients who are not candidates for intravenous thrombolysis or who have experienced treatment failure (level of evidence 1b; grade B recommendation).
- 7.
At present, endovascular treatment is only recommended when performed in centres with SUs and experience in neurointervention. Ideally, this procedure is performed according to a case registry or clinical study protocol (level of evidence 5; grade D recommendation).
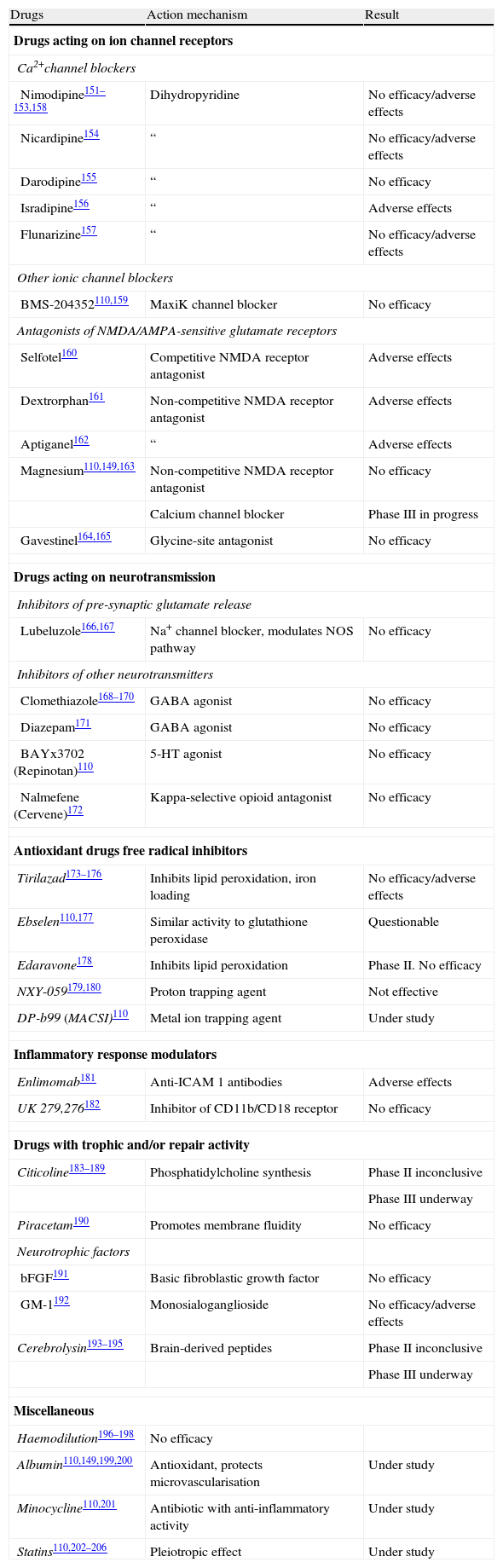
Progress in our understanding of the cellular and molecular changes underlying the pathophysiology of cerebral ischaemia has sparked investigations into multiple drugs that may be able to prevent these changes, and therefore inhibit the mechanisms responsible for damage due to cerebral ischaemia and reperfusion. This capacity is known as ‘neuroprotection’ or ‘pharmacological cerebral protection’.1,110,148,149 Researchers have studied numerous pharmacological agents known as neuroprotectors; at least in theory, these agents may lessen the damage caused by interruption of the CBF by inhibiting one or more of the biochemical mediators of ischaemia-reperfusion damage. Many of the more than 1000 completed experimental studies have shown positive results, although these results were not confirmed by most of the 400 clinical studies that have since been performed (Table 4). The lack of clinical confirmation for results from experimental studies is partially due to poor adaptation between basic translational research strategies and clinical trial design.150 We will not provide detailed descriptions of all clinical trials for each of these different drugs because this exceeds the scope of these guidelines. Instead, we will focus on those drugs for which trials are still underway and on drugs that may be clinically useful based on their promising results.
Pharmacological cerebral protection in cerebral ischaemia: clinical trials.
| Drugs | Action mechanism | Result |
| Drugs acting on ion channel receptors | ||
| Ca2+channel blockers | ||
| Nimodipine151–153,158 | Dihydropyridine | No efficacy/adverse effects |
| Nicardipine154 | “ | No efficacy/adverse effects |
| Darodipine155 | “ | No efficacy |
| Isradipine156 | “ | Adverse effects |
| Flunarizine157 | “ | No efficacy/adverse effects |
| Other ionic channel blockers | ||
| BMS-204352110,159 | MaxiK channel blocker | No efficacy |
| Antagonists of NMDA/AMPA-sensitive glutamate receptors | ||
| Selfotel160 | Competitive NMDA receptor antagonist | Adverse effects |
| Dextrorphan161 | Non-competitive NMDA receptor antagonist | Adverse effects |
| Aptiganel162 | “ | Adverse effects |
| Magnesium110,149,163 | Non-competitive NMDA receptor antagonist | No efficacy |
| Calcium channel blocker | Phase III in progress | |
| Gavestinel164,165 | Glycine-site antagonist | No efficacy |
| Drugs acting on neurotransmission | ||
| Inhibitors of pre-synaptic glutamate release | ||
| Lubeluzole166,167 | Na+ channel blocker, modulates NOS pathway | No efficacy |
| Inhibitors of other neurotransmitters | ||
| Clomethiazole168–170 | GABA agonist | No efficacy |
| Diazepam171 | GABA agonist | No efficacy |
| BAYx3702 (Repinotan)110 | 5-HT agonist | No efficacy |
| Nalmefene (Cervene)172 | Kappa-selective opioid antagonist | No efficacy |
| Antioxidant drugs free radical inhibitors | ||
| Tirilazad173–176 | Inhibits lipid peroxidation, iron loading | No efficacy/adverse effects |
| Ebselen110,177 | Similar activity to glutathione peroxidase | Questionable |
| Edaravone178 | Inhibits lipid peroxidation | Phase II. No efficacy |
| NXY-059179,180 | Proton trapping agent | Not effective |
| DP-b99 (MACSI)110 | Metal ion trapping agent | Under study |
| Inflammatory response modulators | ||
| Enlimomab181 | Anti-ICAM 1 antibodies | Adverse effects |
| UK 279,276182 | Inhibitor of CD11b/CD18 receptor | No efficacy |
| Drugs with trophic and/or repair activity | ||
| Citicoline183–189 | Phosphatidylcholine synthesis | Phase II inconclusive |
| Phase III underway | ||
| Piracetam190 | Promotes membrane fluidity | No efficacy |
| Neurotrophic factors | ||
| bFGF191 | Basic fibroblastic growth factor | No efficacy |
| GM-1192 | Monosialoganglioside | No efficacy/adverse effects |
| Cerebrolysin193–195 | Brain-derived peptides | Phase II inconclusive |
| Phase III underway | ||
| Miscellaneous | ||
| Haemodilution196–198 | No efficacy | |
| Albumin110,149,199,200 | Antioxidant, protects microvascularisation | Under study |
| Minocycline110,201 | Antibiotic with anti-inflammatory activity | Under study |
| Statins110,202–206 | Pleiotropic effect | Under study |
AMPA: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA: gamma-aminobutyric acid; NMDA: N-methyl-d-aspartic acid.
Citicoline is an intermediary of phosphatidylcholine synthesis. It facilitates acetylcholine synthesis in the brain, reduces accumulation of free fatty acids in ischaemic tissue, and displays antioxidant activity.183 Numerous clinical trials have shown that citicoline administered in the first 24hours after stroke onset can deliver favourable outcomes. The best dosage seems to be 2000mg/day during 6 weeks.184–187 These data suggest that citicoline has a therapeutic effect188 (level of evidence 2b), and they are being investigated in a new phase-III trial, the ICTUS study.110,189
High-dose human serum albuminMany of albumin's activities may exert a protective effect,149 and albumin has shown its efficacy in experimental studies. The ALIAS I clinical study was a small pilot study of escalating doses in which human albumin was administered to patients in the first 16hours after stroke. An analysis comparing outcomes between treated patients and historical controls from the NINDS study showed that patients receiving higher doses of albumin in addition to thrombolytic treatment were 3 times more likely to have a favourable outcome (level of evidence 2b).199 To investigate these data, another phase-III study is being carried out at present.110,200
MinocyclineMinocycline is a tetracycline-derived antibiotic. Experimental studies of this drug point to a protective effect mediated by its anti-inflammatory and anti-apoptotic activity. A small clinical trial found that patients treated with minocycline had better 3-month outcomes than those on a placebo, as measured by the NIHSS score, Barthel index, and modified Rankin scale (level of evidence 2b).201
StatinsIn addition to their lipid-lowering activity, statin drugs exert a protective effect in cerebral ischaemia. Several studies have shown that statin treatment prior to a stroke is associated with better prognosis, and that discontinuing statins is an independent factor for poor prognosis (level of evidence 2b). Some studies indicate that early treatment with statins improves outcomes in acute stroke patients.202–206 Further studies are currently underway.110
Brain repairBrain repair is an endogenous, natural process beginning after injury occurs. It includes processes of cellular proliferation, neurogenesis, angiogenesis, and synaptogenesis, which may be promoted through rehabilitation and the delivery of trophic factors, drugs with trophic effect, and stem cells.207 The different rehabilitation techniques favour brain plasticity processes, thereby promoting better functional recovery.57–59 Different trophic factors such as erythropoietin (EPO), granulocyte colony-stimulating factor (GCSF), insulin-like growth factor-1 (IGF-1), or basic fibroblast growth factor (bFGF) have been tested in clinical trials after animal models delivered promising results. Some have had to be interrupted due to adverse effects, while others showed the treatment to be safe, and still others showed a negative effect for these treatments (Table 4).191,192,208 Drugs exerting a trophic effect, such as citicoline183,189 or cerebrolysin,193,195 have also demonstrated activity that favours repair mechanisms.
Regarding cell therapy, different cell lines have been used in animal models of cerebral infarct, and they have yielded good results in the areas of brain repair and functional recovery.209 In addition, there have been numerous clinical trials in which stem cell transplants in stroke patients have demonstrated safety and tolerability.91 Many different trials are currently underway to investigate distinct cell lines, which include neural stem cells (CTX0E03) in the PISCES study and the mesenchymal stem cells in the ISIS study.110,210,211
- 1.
For ischaemic stroke patients previously treated with statins, those drugs should not be discontinued (level of evidence 2a; grade B recommendation).
- 2.
Patients should begin appropriate rehabilitation programmes as soon as they are in stable condition (level of evidence 1a; grade A recommendation).
- 3.
At present, data are not sufficient to recommend generalised use of any drugs with potential protective or repair activity as treatment for cerebral infarct.
- 4.
Stem cell therapy is currently under investigation and cannot be recommended for clinical practice.
Cerebral venous thrombosis has different clinical manifestations that may include headache (the most frequent symptom), visual disorders, papilloedema and altered level of consciousness (if intracranial pressure increases significantly), and venous infarct with focal deficits or convulsions.212–214
Radiological tests are fundamental for diagnosis. Compatible signs can usually be observed in a simple CT scan, which is the imaging technique of choice in emergency departments due to its availability. If the cerebral venous thrombosis is suspected, CT is performed with and without contrast. New helical CT scanners perform non-invasive venography; this technique can confirm the presence of thrombosis in venous sinuses and the extent of thrombosis by showing lack of flow in the affected sinus. CT is indicated in suspected cases of cerebral venous thrombosis so that the emergency department can confirm the diagnosis. Magnetic resonance imaging is the most sensitive technique for confirming this type of thrombosis.213,214 Performing conventional invasive arteriography is rarely necessary.
Doctors will subsequently complete all specific studies needed to determine aetiology (prothrombotic states, clotting disorders, other haematological diseases, drug abuse, autoimmune or connective tissue diseases, cancer, infections, etc.).214 Any such disorders will be treated specifically.
Some randomised clinical trials have been carried out to test treatments. A small double-blind study compared a placebo to dose-adjusted unfractionated heparin (activated partial thromboplastin time at least twice that of the control). This study, containing only 20 patients, was terminated prematurely after showing better outcomes among patients treated with heparin.215
Another randomised study compared nadroparin (90 anti-Xa U/kg/12h) to a placebo over a 3-week period. After 12 weeks, 13% of the patients in the anticoagulated group (3 of 30) and 21% of the patients in the placebo group (6 of 29) showed poor outcomes; for the nadroparin group, absolute benefit was 7% and reduction in relative risk was 38%. There were no new symptomatic cerebral haemorrhages. Furthermore, the placebo group contained twice as many patients with intracranial hypertension as the nadroparin group (28% vs 13%).216 Meta-analysis of these 2 studies found that anticoagulation was associated with a lower risk of death or dependency, but the difference was not significant.217 The studies suggest that the risk of cerebral haemorrhage associated with heparin treatment is low in these patients. They also indicate a low risk of growth when haemorrhage does occur, which indicates that heparin treatment has a favourable risk-to-benefit ratio (level of evidence 1a). A recent non-randomised study including 624 patients suggests that low molecular weight heparin is safer and more effective than unfractionated heparin in patients with cerebral venous thrombosis, especially in patients experiencing haemorrhagic lesions at onset (evidence level 2b).218
Regarding other techniques for recanalising thrombosed sinuses, one systematic review suggests that local pharmacological thrombolysis may be beneficial in more severe cases and may even reduce mortality rates (level of evidence 3a).219 Mechanical thrombectomy is technically feasible, but experience with the technique is only anecdotal at present.214
In patients with large parenchymatous lesions and significant mass effects leading to herniation, decompressive craniectomy decreases mortality and is generally associated with good functional outcomes (level of evidence 2b).220
- 1.
Anticoagulant treatment with low molecular weight heparin or IV heparin is recommended for cerebral venous thrombosis even in cases with haemorrhagic lesions (level of evidence 1a; grade A recommendation). Low molecular weight heparin is preferable to unfractionated heparin in cases with haemorrhagic lesions (level of evidence 2b; grade B recommendation).
- 2.
Local pharmacological thrombolysis may be an option in severe cases (level of evidence 3a; grade B recommendation.
It is often said that “time is brain”, and we should not forget that effective treatments are available, such as hospitalisation in an SU, intravenous thrombolysis, and other promising therapies such as neurovascular interventions. Additional treatments are currently being studied.
As we learn from this review, many aspects in the treatment of cerebral infarcts have been thoroughly verified, and their grades of recommendation are therefore high. Nevertheless, numerous potential treatments have yet to be tested. We should adhere closely to guidelines when treating our patients, while also encouraging researchers to complete clinical trials that may yield sufficiently reliable new treatment options. Treating cerebral infarcts according to evidence-based recommendations will ensure safer and more effective management of these processes, and also help unify criteria and reduce care-related costs for these patients.
FundingThis study has received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
Coordinator: Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid.
A.1. Drafting committee
Exuperio Díez-Tejedor (Coordinator), Hospital Universitario La Paz, Madrid; Blanca Fuentes (Secretary), Hospital Universitario La Paz, Madrid; María Alonso de Leciñana, Hospital Universitario Ramón y Cajal, Madrid; José Álvarez-Sabin, Hospital Universitari Vall d’Hebron, Barcelona; Juan Arenillas, Hospital Universitario Clínico, Valladolid; Sergio Calleja, Hospital Universitario Central de Asturias, Oviedo; Ignacio Casado, Hospital San Pedro, Cáceres; Mar Castellanos, Hospital Josep Trueta, Girona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Antonio Dávalos, Hospital Universitari Germans Trias i Pujol, Badalona; Fernando Díaz-Otero, Hospital Universitario Gregorio Marañón, Madrid; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; José Antonio Egido, Hospital Clínico Universitario San Carlos, Madrid; Juan Carlos López Fernández, Hospital Universitario Dr. Negrín, Las Palmas; Mar Freijo, Hospital Universitario de Basurto, Bilbao; Blanca Fuentes, Hospital Universitario La Paz, Madrid; Jaime Gállego, Hospital General de Navarra, Pamplona; Andrés García Pastor, Hospital Universitario Gregorio Marañón, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; Francisco Gilo, Hospital Universitario La Princesa, Madrid; Pablo Irimia, Clínica Universitaria de Navarra, Pamplona; Aida Lago, Hospital Universitari La Fe, Valencia; José Maestre, Hospital Universitario Virgen de las Nieves, Granada; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Joan Martí-Fábregas, Hospital de la Santa Creu i Sant Pau, Barcelona; Patricia Martínez-Sánchez, Hospital Universitario La Paz, Madrid; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Carlos Molina, Hospital Universitari Vall d’Hebron, Barcelona; Ana Morales, Hospital Universitario Virgen de la Arrixaca, Murcia; Florentino Nombela, Hospital Universitario La Princesa, Madrid; Francisco Purroy, Hospital Universitari Arnau de Vilanova, Lleida; Marc Ribó, Hospital Universitari Vall d’Hebron, Barcelona; Manuel Rodríguez-Yañez, Hospital Clínico Universitario, Santiago de Compostela; Jaime Roquer, Hospital del Mar, Barcelona; Francisco Rubio, Hospital Universitari de Bellvitge, Barcelona; Tomás Segura, Hospital Universitario de Albacete, Albacete; Joaquín Serena, Hospital Josep Trueta, Girona; Patricia Simal, Hospital Clínico Universitario San Carlos, Madrid; Javier Tejada, Hospital Universitario de León, León; José Vivancos, Hospital Universitario La Princesa, Madrid.
A.2. Review or institutional committee
José Álvarez-Sabín, Hospital Universitari Vall d’Hebron, Barcelona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; José Larracoechea, Hospital de Cruces, Bilbao; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Jorge Matías-Guiu, Hospital Clínico Universitario San Carlos, Madrid; Francisco Rubio, Hospital de Bellvitge, Barcelona.