Headache is the most common complication associated with exposure to high altitude, and can appear as an isolated high-altitude headache (HAH) or in conjunction with acute mountain sickness (AMS). The purpose of this article is to review several aspects related to diagnosis and treatment of HAH.
DevelopmentHAH occurs in 80% of all individuals at altitudes higher than 3000m. The second edition of ICHD-II includes HAH in the chapter entitled “Headaches attributed to disorder of homeostasis”. Hypoxia elicits a neurohumoral and haemodynamic response that may provoke increased capillary pressure and oedema. Hypoxia-induced cerebral vasodilation is a probable cause of HAH. The main symptom of AMS is headache, frequently accompanied by sleep disorders, fatigue, dizziness and instability, nausea and anorexia. Some degree of individual susceptibility and considerable inter-individual variability seem to be present in AMS. High-altitude cerebral oedema is the most severe form of AMS, and may occur above 2500m. Brain MRI studies have found variable degrees of oedema in subcortical white matter and the splenium of the corpus callosum. HAH can be treated with paracetamol or ibuprofen. Pharmacological treatment of AMS is intended to increase ventilatory drive with drugs such as acetazolamide, and reduce inflammation and cytokine release by means of steroids.
ConclusionsSymptom escalation seems to be present along the continuum containing HAH, AMS, and high-altitude cerebral oedema.
La cefalea es la complicación más frecuente de la exposición a la altitud y puede aparecer de forma aislada o bien asociada al mal de altura (MA). El objetivo de este artículo es revisar los aspectos relacionados con el diagnóstico y tratamiento de la cefalea de elevada altitud (CEA).
DesarrolloEl 80% de las personas presentan CEA por encima de los 3.000m de altitud. La segunda versión de la Internacional Classification of Headache Disorders (ICHD-II) incluye la CEA en el capítulo «Cefalea atribuida a trastornos de la homeostasia». La hipoxia desencadena una respuesta neurohumoral y hemodinámica que provoca un aumento de la presión capilar y edema. La vasodilatación cerebral inducida por hipoxia es una causa probable de CEA. El síntoma cardinal del MA es la cefalea, que se suele asociar con trastornos del sueño, fatiga, mareo e inestabilidad, náuseas y anorexia. Parece existir una cierta susceptibilidad así como una gran variación interindividual. La forma más grave es el edema cerebral de altitud y puede suceder por encima de los 2.500m. Estudios de resonancia de encéfalo han mostrado la presencia de edema en sustancia blanca y esplenio del cuerpo calloso. La CEA puede tratarse con paracetamol e ibuprofeno. El tratamiento farmacológico del MA tiene la finalidad de incrementar la respuesta ventilatoria, mediante fármacos como la acetazolamida, y reducir los procesos inflamatorios y de liberación de citocinas, mediante el empleo de esteroides.
ConclusionesParece haber una progresión en la expresión de los síntomas entre la CEA, el MA y el edema cerebral de altitud.
The first written record about high-altitude headache dates back to 30 BCE and is owed to Too Kin, an officer of the Imperial Chinese Army. Too Kin, along with his troops, experienced an episode of altitude sickness in a mountain range in Afghanistan which he named the Great Headache Mountain and the Little Headache Mountain.1 Two millennia went by before Paul Bert, the chair of physiology at the Sorbonne after Claude Bernard, developed the modern discipline of altitude physiology.2 In 1913, Thomas Ravenhill provided the first clinical descriptions of signs and symptoms associated with rapid ascent to high altitude in the north of Chile, and reported on both high-altitude cerebral oedema and high-altitude pulmonary oedema.3
Headache is the most common complication of exposure to high altitude, and it can appear as isolated high-altitude headache (HAH) or in conjunction with acute mountain sickness (AMS). HAH is a global health problem whose incidence has increased over the past decades due to different factors. These include more opportunities for travel, exercise, and tourism which therefore expose thousands of tourists, travellers and sports enthusiasts to a rapid increase in altitude, frequently with no previous acclimation.4 Exposure to altitude is considered high for subjects at elevations of 1500 to 3700m above sea level, very high at 3700 to 5500m, and extreme above 5500m.5
However, a person with high altitude exposure can experience different types of headaches in addition to headache associated with altitude. This being the case, episodic migraine crises precipitated by hypoxia and altitude, as well as headache linked to acute mountain sickness, must be considered. The purpose of this article is to review several aspects related to diagnosis and treatment of HAH and AMS. To this end, we searched the Medline database for all articles published in English or Spanish up to February 2012, using the keywords ‘headache altitude’ and ‘acute mountain sickness’.
High-altitude headacheEpidemiologyIt is estimated that at least 25% of non-acclimated individuals exposed to altitudes of 1859 to 2750m experience high-altitude headache. At altitudes above 3000m, 80% of individuals will have HAH and almost 100% will experience headache at 4500m or higher.4
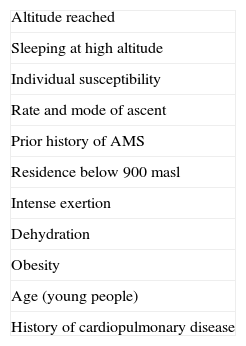
Definition, clinical presentation and risk factorsThe second edition of the International Classification of Headache Disorders (ICHD-II)6 includes high-altitude headache (subsection 10.1.1) in chapter 10 (Headache attributed to disorder of homoeostasis), section 10.1 (Headache attributed to hypoxia and/or hypercapnia). Diagnostic criteria are listed in Table 1.
Diagnostic criteria for HAH
| A. Headache must present with at least 2 of the following characteristics and fulfil criteria C and D: |
| 1. Bilateral |
| 2. Frontal or frontotemporal |
| 3. Dull or pressing pain |
| 4. Mild to moderate intensity |
| 5. Aggravated by exertion, movement, straining, coughing, or bending down |
| B. Ascent to altitudes above 2500m |
| C. Headache develops within 24hours after ascent |
| D. Headache resolves within 8hours after descent |
HAH can appear as an isolated symptom within the first 24hours of exposure to altitudes above 2500m without previous acclimation, or it may present along with more varied signs and symptoms which constitute AMS, as we will discuss in a later section.7
HAH has a mild to moderate intensity and can be described as bilateral dull or pressing pain in the frontal, frontoparietal, or holocranial regions.8,9 Clinically, this headache is usually aggravated by exertion and head or body movement. It may also present with throbbing pain which, according to different studies, affects 30%8 to 75%9 of individuals. Headaches either begin upon awakening, or wake subjects at night, in at least 25% of the cases. This type of headache seems to be more intense in women and in individuals with a history of other types of headache in daily life.8
The connection between HAH and episodic migraine is yet to be determined. As pain in HAH seems to be more intense in patients with a previous history of migraine, some authors hypothesise that it may be associated with migraine.8 Migraine without aura should be differentiated from HAH, especially in subjects with a prior history of migraine. It is believed that some cases of migraine with aura could be related to the presence of right-to-left shunt blood vessels that are increasingly active with physical exertion and altitude.10 Doctors have also described isolated cases of primary cluster headache triggered by exposure to high altitude and responding to oxygen therapy.11
Rapid ascent to a high altitude is the main risk factor.7,12–14 Known risk factors for HAH are listed in Table 2. According to a study comprising 506 mountaineers who experienced headache at altitudes of 2200 to 3817m, risk factors for HAH were previous history of migraine, intense physical exertion, and low arterial oxygen saturation.14 Adults and the elderly have a lower incidence of headache than the younger population, which may be due to the effect of cerebral atrophy. A good level of physical fitness (‘being fit’) does not prevent HAH.
PathogenesisThere is an inverse relationship between altitude/atmospheric pressure and PaO2. Chemoreceptors in the carotid body of a person gaining altitude detect a reduction in PaO2. Hypoxia promotes a neurohumoral and haemodynamic response that gives rise to increased capillary pressure and vascular and cerebral oedema. Hypoxia-induced cerebral vasodilation is a probable cause of headache. Cerebral blood flow is elevated in the hypoxic state and returns to pre-ascent values with acclimation.7
Cerebral autoregulation, a process aimed at regulating cerebral perfusion according to variations in blood pressure, is altered by hypoxia and in individuals suffering from HAH and AMS.15 Studies performed with transcranial Doppler, which measures velocity in the middle cerebral artery (MCA) as an indicator of cerebral blood flow, show considerable variations in flow velocity.16 Sea-level assessment of dynamic cerebral autoregulation, which uses transcranial Doppler to calculate velocity in the MCA, can act as a predictor for susceptibility to AMS.17 A lower baseline autoregulation index may be considered a potential risk factor for AMS.
However, researchers have also proposed other factors apart from hypobaric hypoxia that favour HAH, for example, alterations in the blood-brain barrier promoted by different mediators, such as vascular endothelial growth factor, nitric oxide, or bradykinin.4 Some also suggest that the trigeminovascular system may be activated at high altitude by either chemical stimuli (nitric oxide) or mechanical stimuli (vasodilation).18
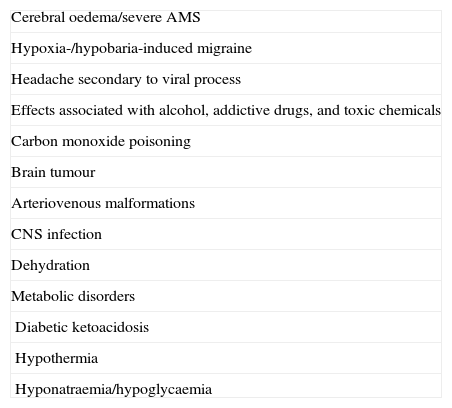
DiagnosisFor HAH to be diagnosed, the condition must appear at altitudes above 2500m and it may not be attributable to other causes. For this reason, differential diagnosis for HAH must consider an array of toxic and metabolic causes and space-occupying cerebral lesions listed in Table 3. The differential diagnosis commonly includes headache due to viral infections and dehydration.19
Differential diagnosis for HAH
| Cerebral oedema/severe AMS |
| Hypoxia-/hypobaria-induced migraine |
| Headache secondary to viral process |
| Effects associated with alcohol, addictive drugs, and toxic chemicals |
| Carbon monoxide poisoning |
| Brain tumour |
| Arteriovenous malformations |
| CNS infection |
| Dehydration |
| Metabolic disorders |
| Diabetic ketoacidosis |
| Hypothermia |
| Hyponatraemia/hypoglycaemia |
Incidence of AMS is approximately 45% to 95%, depending on the series. It is estimated that almost 50% of trekkers ascending above 5000m experience acute mountain sickness.20
AcclimationImmediate physiological changes induced by hypoxia and altitude include increases in heart and respiratory rates, increased diuresis, alterations in taste perception, nasal congestion, and sometimes syncope. Acclimation can be considered the final stage of a process in which individuals adapt to altitude hypoxia. While acclimation takes several days to weeks, symptoms of AMS present at the onset of this process.7
SymptomsThe main symptom of AMS is headache, frequently accompanied by sleep disorders, fatigue, dizziness and instability, nausea, and anorexia. Insomnia is the second most frequent symptom and presents in at least 60% of all individuals reaching 3500m. Non-restorative sleep may be secondary to periodic breathing that interrupts sleep architecture with a pattern of hypoxia-hyperventilation-hypocapnia; other factors such as headache and fatigue may also promote insomnia.21 High-altitude syncope seems to be a vasovagal phenomenon related to hypoxia, although at times the presence of arrhythmias may trigger it.22
The frequency and intensity of symptoms associated with AMS seem to point to some degree of individual susceptibility and considerable inter-individual variability. For this reason it is believed that there may be a certain genetic predisposition to AMS. In any case, human response to hypobaric hypoxia seems to be polygenic23 and determined by different expression of genes encoding different proteins, such as serum erythropoietin, hypoxia-inducible factor 1alpha (HIF-1alpha), angiotensin-converting enzyme, aldosterone, and nitric oxide synthase activity, among others.4
PathophysiologyFrom a pathophysiological point of view, the main pathogenetic factor in acute mountain sickness is hypobaric hypoxia, which can be exacerbated by hypoventilation, a periodic breathing pattern, and relatively intense physical exertion. The combination of these factors leads to an increase in capillary permeability, sodium retention, vasodilation, and an increase in cerebral blood flow and pulmonary hypertension.24
Apart from O2 and CO2 concentrations, several factors can affect blood vessel tonicity in hypoxia, mainly cerebral adenosine, potassium ion, and nitric oxide synthase levels. Vascular permeability may be influenced not only by hypoxia but also by different chemical mediators, such as hypoxia-inducible factor 1 (HIF-1)25 and vascular endothelial growth factor (VEGF).26
DiagnosisThe Lake Louise AMS scoring system was developed for establishing an early diagnosis and monitoring severity of AMS symptoms in individuals exposed to high altitudes.27 It consists of 2 sections, a self-reported questionnaire and a clinical assessment form. A diagnosis of AMS requires the presence of the main symptom, headache (even when it presents with mild intensity), plus at least 1 additional symptom.
Diagnostic criteria for AMS by Lake Louise Consensus Group are listed in Table 4. Scores of 3 to 5 on the questionnaire indicate mild AMS and scores of 6 or more indicate severe AMS.28 An adapted Spanish version of the Lake Louise AMS questionnaire was validated a few years ago.29 The Environmental Symptoms Questionnaire is a more detailed and time-consuming questionnaire that may be useful for assessing severity of AMS symptoms in applied research.30
Lake Louise diagnostic criteria for AMS
| Headache in non-acclimated individuals who ascend rapidly to an altitude above 2500m, and at least 1 of the following symptoms: |
| - Gastrointestinal: anorexia, nausea and vomiting |
| - Insomnia |
| - Dizziness/vertigo |
| - Fatigue |
| - Onset within 6-24hours after ascent, sometimes within the first hour |
masl: metres above sea level.
High-altitude cerebral oedema (HACE) is the most severe form of AMS. It may occur above 2500m and should therefore be considered in differential diagnosis of HAH. HACE is a potentially highly severe encephalopathy that may affect 0.5% to 1% of all individuals suffering from AMS.31 In addition, it is estimated that 5% of non-acclimated individuals may present cerebral oedema above 4500m. The incidence of high-altitude pulmonary oedema is approximately 2%.32
SymptomsThe mildest forms of cerebral oedema present with headache, dizziness, vertigo, and reaction time retardation. The most severe forms can present with ataxia, altered level of consciousness, hallucinations, seizures, stupor, and coma. Above 7500m, 32% of climbers experience hallucinations. An increase in intracranial pressure may lead to papilloedema, retinal haemorrhage and paralysis of cranial nerves, especially cranial nerve VI. The most severe forms may progress to cerebral herniation, and intracranial hypertension resulting in death.33 Retinal haemorrhages, which are frequent during high-altitude ascent, occur in 30% and 50% of individuals suffering from AMS and HACE, respectively.34
Neuroimaging findingsNeuroimaging studies (cerebral computed tomography and brain magnetic resonance imaging) showed small ventricles and cerebral sulci effacement in climbers suffering HACE. It is not yet known whether cerebral oedema is of vasogenic or cytotoxic origin, or whether one precedes the other or if both present simultaneously.7 Hypoxia may produce a generalised vasogenic oedema subsequent to an increase in blood-brain barrier permeability. However, both cytotoxic35 and vasogenic36 cellular oedema have been detected. Cytotoxic oedema may derive from disorders in ATP-dependent sodium-potassium pump functioning.
Individuals suffering from high-altitude cerebral and pulmonary oedema showed hyperintense areas in the splenium of the corpus callosum and in the centrum semiovale, but no abnormalities in cortical grey matter.24 On this basis, many authors suggest that high-altitude cerebral oedema is predominantly a reversible vasogenic cerebral oedema in the white matter.36 Brain MRI has also shown hyperintensities in subcortical white matter in individuals above 7000m.36
Additional brain MRI studies in HACE patients revealed haemosiderin depositions, mostly in the corpus callosum.37
PathogenesisHACE pathogenesis has traditionally been based on the theory of intracranial hypertension secondary to hypobaric hypoxia. However, some authors have stated that correlation between headache in AMS and cerebral oedema is unclear.4 It has recently been suggested that hypoxia may affect not only cerebral blood flow, but also cause a certain degree of cerebral venous insufficiency. Thus, a slight increase in central venous pressure (as occurs with hypoxia-induced pulmonary vasoconstriction, for example) may compromise cerebral venous outflow at high altitudes and favour oedema.38 Cerebral oedema is considered the main factor leading to increased brain volume and intracranial hypertension. However, there are other mechanisms, such as increases in cerebral blood flow or venous outflow obstruction, which may also increase intracranial pressure.
Other neurological and neuropsychological complications associated with high altitudeCerebrovascular complications described in association with high altitude include transient ischaemic attack and ischaemic stroke. Their origin may be related to vasospasm and vasoconstriction phenomena favoured by the hypocapnia, severe dehydration, and thrombophilia associated with hypoxia.39 Some cases of patients who died at high altitude due to cerebral venous thrombosis can be found in medical literature.40 Histopathological and postmortem examinations have confirmed the presence of ring-shaped microhaemorrhages associated with cerebral venous thrombosis.24,40,41
Exposure to hypobaric hypoxia leads to neuropsychological disturbances in perception, memory, language, reaction time, learning, and psychomotor skills. Learning capacity, short-term memory and spatial memory are affected at altitudes above 4500m, and more profoundly at more than 6000m. Symptom intensity varies according to ascent rate and altitude.42,43
TreatmentHigh-altitude headachePharmacological treatment in the acute phase must distinguish isolated headache in AMS from cerebral oedema. As HAH is frequently self-limited and remits in 2 or 3 days, climbers are recommended to stop climbing, rest, and rehydrate when the condition presents. If symptoms persist or worsen, climbers should descend 500 to 1000m.5 Adequate hydration, analgesics and anti-inflammatory agents may improve symptoms.19
HAH can be treated with paracetamol (500-1000mg) and anti-inflammatory agents such as ibuprofen (400-600mg).19,44 Triptans are not effective, although they can be useful for migraine associated with hypoxia. At extreme altitudes, supplementary oxygen (2-4L/minute) may be necessary during the night.7 Once oxygen therapy has been administered, HAH should improve within about 15 minutes; this event may be an indicator for ruling out migraine.
Acute mountain sickness and cerebral oedemaPharmacological treatment of AMS is intended to increase ventilatory drive with drugs such as acetazolamide, and reduce inflammation and cytokine release by means of steroids.
Acetazolamide is a carbonic anhydrase inhibitor that acts on the brain in addition to the blood cells, renal tubules, chemoreceptors, and systemic and pulmonary vessels. It provokes metabolic acidosis, thus increasing respiratory minute volume. Cerebral oedema can be treated with acetazolamide (125-250mg every 8-12h),45 dexamethasone (4mg/6h, orally),46 and/or portable hyperbaric chamber (193mbar during 1h),47 depending on intensity and severity of symptoms.48 Dexamethasone seems to block VEGF expression and reverts hypoxia-induced cerebral oedema.49
PreventionHigh-altitude headacheA slow ascent which promotes acclimation is recommended. At 2500m and above, the maximum advisable ascent rate would be approximately 600m per day. This may reduce the incidence of AMS by 40%. Diet is also important and should be based on carbohydrates. Alcohol should be avoided.
Results from the HEAT trial (Headache Evaluation at Altitude Trial) were published recently; this clinical trial compared the effects of 600mg ibuprofen or 85mg acetazolamide or placebo for preventing HAH in 343 healthy subjects at 4300m.50 Headache incidence rates were similar in participants treated with acetazolamide (27.1%) or ibuprofen (27.5%), and both were lower than that among participants receiving the placebo (45.3%). This suggests that both drugs in such doses are equally efficacious against HAH.50
Acute mountain sicknessIndividuals ascending above 3000m or with a previous history of AMS-associated headache are recommended to take acetazolamide as preventive treatment. The recommended preventive dosage is 125 to 250mg every 12hours, at least 24hours before ascent and for 2 days while the subject is at high altitude.48,51 Higher doses (375mg every 12hours) do not seem to be more efficacious than a dose of 125mg every 12hours.52 However, results from the SPACE trial (spironolactone and acetazolamide trial in the prevention of acute mountain sickness) showed that spironolactone does not seem to prevent AMS compared to results from acetazolamide.53
Dexamethasone (8mg per day, administered in several doses) is effective for preventing AMS because it reduces cytokine-release and capillary permeability.54 Prednisolone at a dose of 20mg every 24hours for 2 days before ascent and 3 days while at high altitude is also beneficial.55
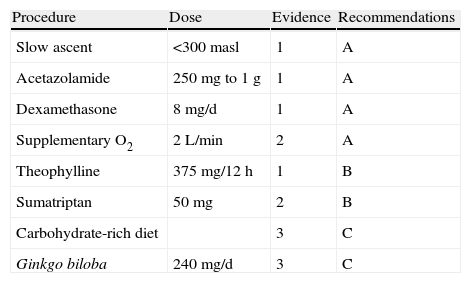
Ginkgo biloba extracts have been used to promote free radical elimination and prevent AMS. However, the PHAIT trial (prevention of high altitude illness trial) showed that it does not seem to be efficacious for preventing AMS compared to results from acetazolamide.56 Aspirin, at a dose of 325mg three times daily, seems to reduce the incidence of headache associated with physical exertion at high altitude.57 Different recommendations on preventive measures for HAH, are listed in Table 5 according to level of evidence.
Recommended preventive measures for HAH according to the level of evidence
| Procedure | Dose | Evidence | Recommendations |
| Slow ascent | <300masl | 1 | A |
| Acetazolamide | 250mg to 1g | 1 | A |
| Dexamethasone | 8mg/d | 1 | A |
| Supplementary O2 | 2L/min | 2 | A |
| Theophylline | 375mg/12h | 1 | B |
| Sumatriptan | 50mg | 2 | B |
| Carbohydrate-rich diet | 3 | C | |
| Ginkgo biloba | 240mg/d | 3 | C |
masl: metres above sea level.
We need new clinical trials to confirm whether sumatriptan, dosed at 50mg before ascent, is efficacious for preventing AMS.58 Additional trials will have to confirm the results of a small study that found that low doses of theophylline (300mg per day for 5 days) were efficacious for preventing AMS.59
ConclusionHAH can occur in isolation or as the main symptom of AMS. In any case, clinical symptoms seem to display a progressive pattern along the continuum of HAH, AMS, and HACE. At present, the recent discoveries of different chemical mediators provide us with a better understanding of the pathogenesis of this continuum and of the therapeutic targets acted upon by acetazolamide and steroids.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Carod-Artal FJ. Cefalea de elevada altitud y mal de altura. Neurología. 2014;29:533–540.
This study was presented as a lecture to the Headache Study Group at the 63rd Annual Meeting of the Spanish Society of Neurology, November 2011, Barcelona.