Recent studies on uric acid as a biomarker for the prognosis of acute stroke have found conflicting results.
MethodsWe collected blood samples from 600 consecutively admitted patients at our tertiary hospital and analysed the relationship between uric acid levels and functional prognosis (measured using the modified Rankin Scale [mRS]). Patients who had received reperfusion therapy were excluded since this may have influenced uric acid levels.
ResultsA total of 73% of patients had mRS scores ≤2; the mean uric acid level was 5.22mg/dL. We found a nonlinear relationship between functional prognosis at discharge and serum uric acid levels at admission when the National Institutes of Health Stroke Scale score was excluded from the analysis.
ConclusionsSerum uric acid levels in patients with acute ischaemic stroke are significantly associated with functional prognosis at discharge, although this relationship is nonlinear. In fact, poorer prognosis is associated both with very low and with very high concentrations of uric acid. This suggests a dual role of uric acid in relation to stroke: on the one hand, as an associated risk factor, and on the other, as a possible neuroprotective factor due to its antioxidant effect.
En el proceso de búsqueda de biomarcadores para el pronóstico del ictus agudo, en los últimos años los estudios realizados en torno al ácido úrico (AU) han mostrado resultados contradictorios.
MétodosSe recogieron muestras analíticas de 600 pacientes ingresados de manera consecutiva en un hospital de tercer nivel y se analizó la relación entre los niveles de ácido úrico y el pronóstico funcional de los pacientes medido mediante la escala de Rankin modificada (mRS). Se excluyeron los pacientes que habían recibido terapias de reperfusión ya que podría existir un efecto diferencial en los mismos respecto a los no tratados.
ResultadosEl 73% de los pacientes tuvieron una mRS ≤2 y los niveles medios de ácido úrico fueron de 5,22mg/dl. Se encontró una relación no lineal entre el pronóstico funcional al alta y los niveles de ácido úrico sérico en el momento del ingreso al excluir del análisis la medida de la National Institutes of Health Stroke Scale (NIHSS).
ConclusionesLos valores séricos de AU en pacientes afectos de un ictus isquémico agudo se asocian significativamente con el pronóstico funcional en el momento de su alta, pero esta relación es no lineal. Se asocia un peor pronóstico a las concentraciones extremas, muy bajas o muy elevadas de AU. Esto podría revelar un doble papel del AU en su relación con el ictus, como factor de riesgo asociado y/o como posible neuroprotector dado su papel antioxidante.
Numerous biomarkers have been explored in the context of ischaemic stroke; however, it is yet to be ascertained whether any adds prognostic value to clinical variables.1 Among these, uric acid has shown divergent results, with high levels of uric acid being associated with both positive and negative outcomes.2–4
Hyperuricaemia is associated with multiple traditional risk factors for stroke, such as arterial hypertension.5 Oxidative stress and free radical formation play an essential role in the pathogenesis of acute stroke.6 Uric acid, the main endogenous antioxidant in plasma, is responsible for two-thirds of all free radical–scavenging capacity7; it may therefore play a protective role against some vascular diseases,8 such as stroke.
In animal models, uric acid concentration increases significantly during ischaemic stroke, remaining elevated for up to 48hours.9 Clinical outcomes in humans seem inconsistent, however. Some studies have found a strong correlation between hyperuricaemia and good functional prognosis in patients admitted due to stroke,2,10 whereas others report poor prognosis in patients with ischaemic stroke.4,11 Although few studies have addressed this issue in patients receiving thrombolysis, their results suggest that high uric acid levels positively influence treatment outcomes.12
These results suggest a synergistic effect between uric acid and recombinant tissue plasminogen activator (rTPA, Alteplase). In a phase III study published in 2014, adding uric acid to thrombolytic therapy was not found to significantly increase the percentage of successful outcomes (scores 0–1 on the modified Rankin Scale [mRS]). However, as it was not associated with any safety risk, the researchers suggest conducting further studies in search of positive results.13
Research into the activity of uric acid in patients with ischaemic stroke has delivered diverging results. Uric acid may be regarded as a potential therapeutic target due to its role as a marker of vascular risk and its prognostic and therapeutic value in acute stroke.
Our study aimed to determine whether serum uric acid levels are associated with better functional outcomes at discharge in patients with ischaemic stroke, and to analyse the potential association between uric acid and other clinical and analytical variables.
Patients and methodsWe prospectively gathered epidemiological and clinical data from 600 consecutive patients with stroke admitted to the neurology department at Hospital Universitario de Gran Canaria Dr. Negrín over a period of 18 months. We excluded patients with haemorrhagic stroke. Patients receiving thrombolytic therapy were also excluded due to the potential differential effect between patients receiving and not receiving thrombolysis. Hospital Universitario de Gran Canaria Dr. Negrín is a tertiary care hospital serving a population of approximately 335 000 in the northern part of the island of Gran Canaria and on the island of Lanzarote.
Patients were admitted to the neurology department within 72hours of symptom onset; data were gathered at admission. Fasting blood samples were obtained within 24hours of admission. Clinical prognosis was established at discharge using the mRS.14,15 The mRS is the most frequently used scale in stroke research, although it evaluates patient performance in activities of daily living, which cannot be properly evaluated during hospitalisation.
Data analysisWe analysed the relationship between blood uric acid level and each of the variables recorded in our sample to explore any significant associations that may affect outcomes.
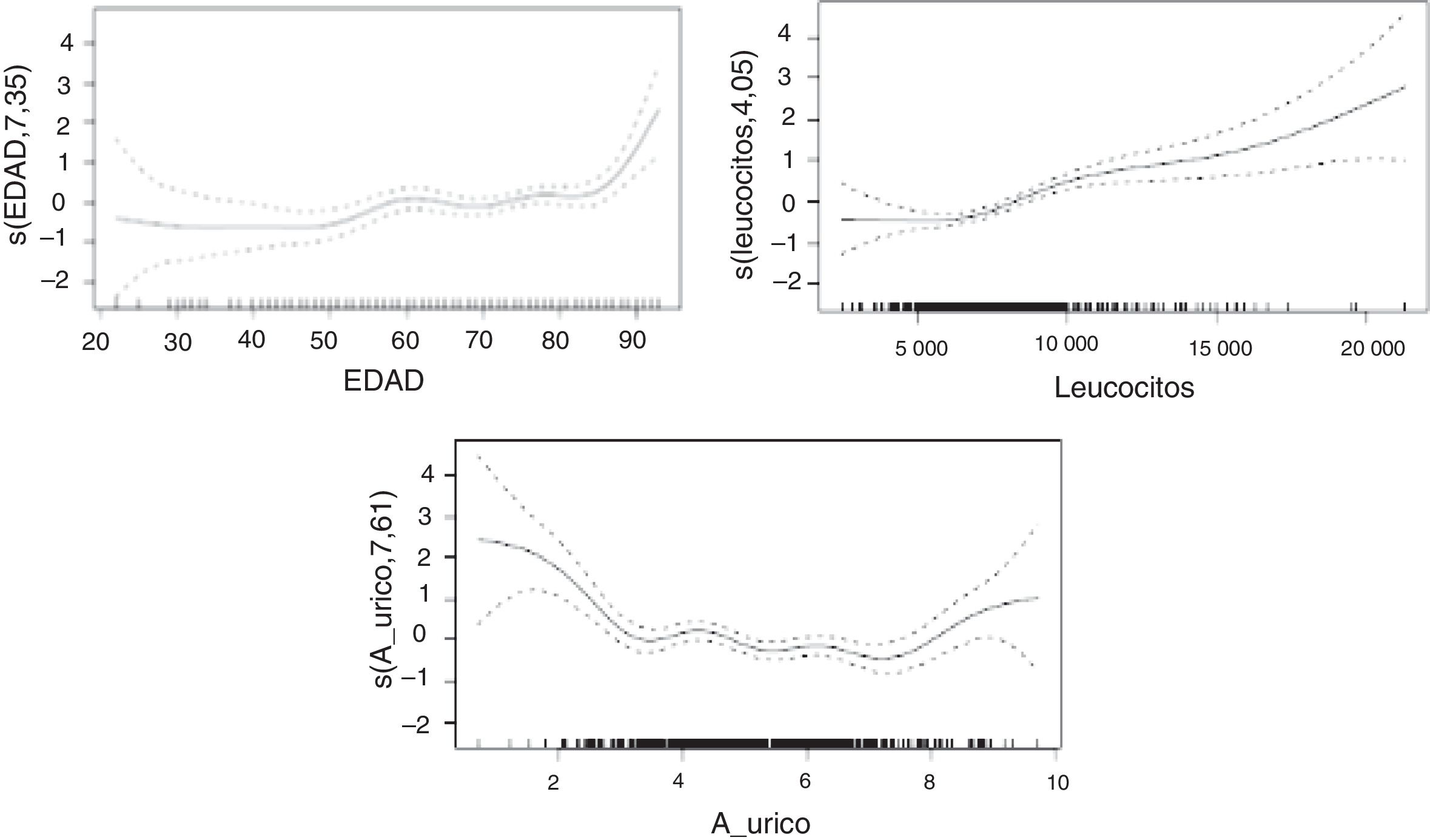
We performed 2 multivariate analyses to determine which variables were independently associated with mRS score; the latter was the dependent variable in both cases. In the first multivariate analysis, variables were selected based on a complete enumeration algorithm and the Bayesian information criterion. The second multivariate analysis was similar to the first, but did not include National Institutes of Health Stroke Scale (NIHSS) scores. We explored potential nonlinear effects of the numerical variables selected using additive models (Hastie and Tibshirani, 1986). These nonlinear effects were estimated with cubic splines.
Hypothesis contrast testing results were considered to be statistically significant for P values below .05. Data were analysed with the R statistical software, version 3.1.0.16
ResultsDescription of the samplePatients’ mean age (SD) at admission was 68 (12.9) years. Men accounted for 55.2% of the sample (n=331); 419 patients (69.8%) had hypertension. Around 37% of patients had diabetes mellitus or dyslipidaemia, 20% had atrial fibrillation, and 25.5% were active smokers. Mean NIHSS score was 3 (SD: 7.14; interquartile range: 1–5).
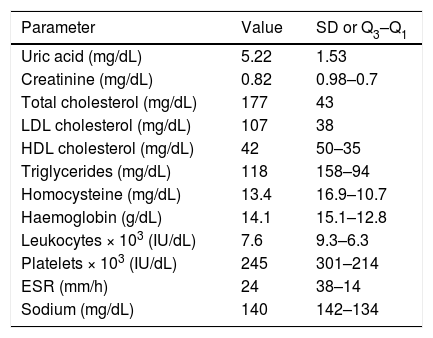
Table 1 shows the analytical parameters of our sample (complete blood count and biochemical analysis).
Distribution of analytical variables in our sample.
| Parameter | Value | SD or Q3–Q1 |
|---|---|---|
| Uric acid (mg/dL) | 5.22 | 1.53 |
| Creatinine (mg/dL) | 0.82 | 0.98–0.7 |
| Total cholesterol (mg/dL) | 177 | 43 |
| LDL cholesterol (mg/dL) | 107 | 38 |
| HDL cholesterol (mg/dL) | 42 | 50–35 |
| Triglycerides (mg/dL) | 118 | 158–94 |
| Homocysteine (mg/dL) | 13.4 | 16.9–10.7 |
| Haemoglobin (g/dL) | 14.1 | 15.1–12.8 |
| Leukocytes × 103 (IU/dL) | 7.6 | 9.3–6.3 |
| Platelets × 103 (IU/dL) | 245 | 301–214 |
| ESR (mm/h) | 24 | 38–14 |
| Sodium (mg/dL) | 140 | 142–134 |
ESR: erythrocyte sedimentation rate; Q1: first quartile; Q3: third quartile; SD: standard deviation.
Doppler ultrasound of the supra-aortic trunks detected carotid artery stenosis > 50% in 49 patients (8.2%) and <50% in 21 (3.5%); the remaining 530 patients (88.3%) did not present significant stenosis.
According to the TOAST classification,17 23% of strokes were small-vessel occlusions, 21% were due to large-artery atherosclerosis, 19% were cardioembolic, and 37% were of undetermined or other determined aetiology.
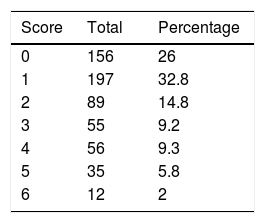
At discharge, patients were classified by degree of functional dependence using the mRS (Table 2).14,15
Following the models used in previous studies,10,18 functional prognosis was classified as either good (mRS scores ≤2) or poor (>2). At discharge, 442 patients (73.7%) had a good functional prognosis and 158 patients (26.3%) had a poor functional prognosis or died during hospitalisation.
Additive multivariate regression model for mRS scoresWe created an additive regression model including the most significant variables.
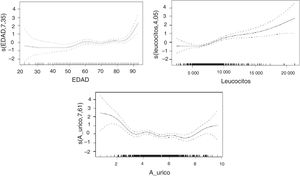
We first used the variables age (P<.001), white blood cell count (P<.001), and NIHSS score (P<.001). Additive models showed nonlinear effects for white blood cell count and NIHSS score.
Exploratory models showed a strong association between uric acid levels and functional prognosis at discharge when NIHSS scores were excluded. NIHSS scores reflect severity and, indirectly, infarct volume; this is why this variable may interfere with the analysis of the direct effects of such other markers as uric acid. We therefore performed a second multivariate analysis, excluding NIHSS scores; this allowed us to better analyse the effect of uric acid on functional prognosis at discharge.
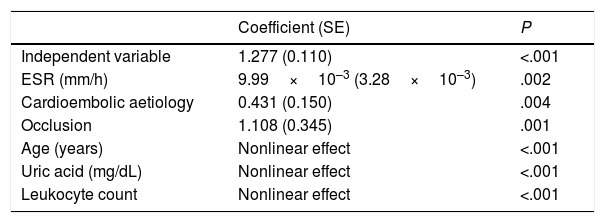
In the second model (Table 3), erythrocyte sedimentation rate, cardioembolic aetiology, and presence of carotid artery occlusion showed a linear relationship with mRS score at discharge, whereas age, uric acid level, and white blood cell count showed a nonlinear relationship. In the sample as a whole, no significant differences in uric acid levels were observed between men and women.
Additive multivariate regression model for modified Rankin Scale scores.a
| Coefficient (SE) | P | |
|---|---|---|
| Independent variable | 1.277 (0.110) | <.001 |
| ESR (mm/h) | 9.99×10–3 (3.28×10–3) | .002 |
| Cardioembolic aetiology | 0.431 (0.150) | .004 |
| Occlusion | 1.108 (0.345) | .001 |
| Age (years) | Nonlinear effect | <.001 |
| Uric acid (mg/dL) | Nonlinear effect | <.001 |
| Leukocyte count | Nonlinear effect | <.001 |
ESR: erythrocyte sedimentation rate; SE: standard error.
The nonlinear relationships between mRS score and uric acid level, age, and white blood cell count are shown in Fig. 1.
A strong association was found between age and mRS score, with older patients scoring considerably higher on the scale. Regarding uric acid levels, extreme values in our sample were associated with high mRS scores; the correlation was less marked in the middle range. Higher concentrations of white blood cells were associated with more marked changes in mRS scores.
DiscussionUnderstanding the pathophysiological processes of acute stroke is essential for the treatment and prognosis of these patients.
Successive studies have shed light on the stages leading to neuronal death, including necrosis and apoptosis; oxidative mechanisms play an essential role in both processes.6,19–21
Uric acid, the main endogenous antioxidant of the human body, has been proposed as a potential protective factor against some vascular diseases, including stroke.7,8 Several studies have analysed the association between serum uric acid levels and functional prognosis of patients with stroke, with diverging results.2,4,11,18,22–25
Our analysis revealed a significant correlation between uric acid levels at admission and functional prognosis at discharge. Higher uric acid levels were found to be correlated with better functional prognosis. These findings are consistent with those of some previous studies,2,10 including a recent meta-analysis by Wang et al.25 The benefits of uric acid are attributed to its antioxidant properties, helping reduce neuronal damage by preventing necrosis or apoptosis in the area of ischaemia.7,26,27
In the analysis including potential confounding factors associated with stroke and uric acid level, such as age, cholesterol level, white blood cell count, and haemoglobin concentration, the statistically significant association between uric acid level and functional prognosis was nonlinear (P<.001): patients with extreme uric acid levels showed poorer functional prognoses. These results are consistent with those reported in the 2010 study by Seet et al.,24 who observed a U-shaped relationship between uric acid level and functional prognosis, suggesting that patients with stroke and severe hypouricaemia may be more vulnerable to increased oxidative stress, and that patients with hyperuricaemia may have poorer functional prognosis due to unknown mechanisms. The researchers stress that these findings do not imply a causal relationship.
In our study, the relationship between uric acid level and patient functional prognosis describes a U- or J-shaped curve: both the high and the low extremes of uric acid level at the time of stroke are associated with poor functional outcomes at discharge.
These findings coincide with those of studies reporting an association between uric acid level and variables of neurological prognosis24 or vascular health.28 This suggests a dual role of uric acid in the pathogenesis of stroke.
Hypouricaemia may be deleterious, decreasing the body’s antioxidant defence against stroke, in which oxidative stress plays a major role.29 Several studies with in vitro and in vivo models have demonstrated the neuroprotective role of uric acid under conditions of acute ischaemia26,30; therefore, low uric acid levels usually predict poor prognosis of acute stroke. Based on the above, uric acid therapy has been approved as an adjuvant to thrombolysis for the treatment of acute stroke. Several studies report that uric acid therapy during the acute phase of stroke does not have deleterious effects and may even be beneficial, especially in women, who have lower baseline levels.13,31,32
Hyperuricaemia, on the other hand, is associated with poor functional prognosis during the acute phase of stroke. However, no study has yet analysed this statistically significant association. From a pathophysiological viewpoint, it is reasonable to link poor prognosis to poor “vascular health” in patients with high uric acid levels and multiple concomitant cardiovascular risk factors. Our study lacks detailed information on associated comorbidities that may confirm this hypothesis. Uric acid level is an independent predictor of arterial hypertension.33 Hyperuricaemia promotes lipid peroxidation, which increases the vulnerability of atherosclerotic plaques,34 inhibits nitric oxide production, and induces smooth muscle cell proliferation and endothelial dysfunction.35,36 High uric acid levels also alter platelet adhesiveness, which may have an impact on intravascular aggregation and coagulation.28 The antioxidant properties of uric acid may be altered in certain situations, for example in cases of low ascorbic acid levels, promoting oxidation.37 Therefore, in the event of cerebral blood flow alterations and subsequent necrosis and apoptosis, a patient with hyperuricaemia may have fewer resources for improving perfusion in the infarcted area, probably due to reduced collateral circulation.
Our study is not without limitations. The differences between patients from different studies may affect results. We decided to exclude patients with haemorrhagic stroke or receiving thrombolysis, as their clinical and pathophysiological characteristics may have different effects: for example, uric acid may be especially protective in patients receiving thrombolytics.12 Furthermore, we did not adjust uric acid levels for drug use, nor did we measure oxidative stress, which may have helped to control confounding factors. No data were gathered on infarct size, and NIHSS scores were excluded from the final analysis due to the possibility of uric acid consumption occurring during the acute phase of stroke, increasing in parallel with stroke size; our results should therefore be interpreted with caution.
However, the greatest limitation of our study is probably the lack of a precise record of the timing of blood sample collection. In our setting, blood is usually extracted within one day of hospital admission; depending on the time of admission, blood may be extracted in less than 6hours or up to 24hours.
Blood uric acid levels change over the first days after stroke onset. Amaro et al.38 observed a maximal decrease in uric acid levels at 6hours after stroke in 8 patients receiving thrombolytics. Brouns et al.39 observed a biphasic pattern in uric acid concentrations after stroke, with the lowest values recorded 7 days after the event.
Our study showed a significant association between serum uric acid levels and functional prognosis at discharge in patients with acute ischaemic stroke. The relationship is not linear, however: extreme uric acid concentrations were associated with poorer prognoses.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cabrera Naranjo FH et al. Uricemia como factor pronóstico del ictus isquémico agudo. Neurología. 2021;36:279–284.
This study was partially presented at the 2016 Annual Meeting of the European Academy of Neurology (Copenhagen).