Lobar intracerebral haemorrhage (LICH) is a rare cause of stroke which accounts for about 20% of primary intracerebral haemorrhages. The most common causes are cerebral amyloid angiopathy (CAA), high blood pressure and others, such as using antiplatelet or anticoagulation agents.
We analysed a series of patients with LICH and compared it with subgroups of patients with LICH who were previously receiving antiplatelet or anticoagulation agents. We determined the volume of the bleeding and its predictive value for mortality.
Patients and methodsWe consecutively and retrospectively included 162 patients diagnosed with LICH and cared for in the Neurology Department of Hospital Meixoeiro in Vigo between 1991 and 2009. We collected demographic characteristics, risk factors, aetiologies and symptoms, and conducted a comparative analysis between the general series and the subgroups of patients receiving anticoagulation and antiplatelet agents.
ResultsIn the general series, the most common cause was possible or probable CAA followed by hypertension. In the subgroup of patients receiving antiplatelet or anticoagulation agents there were no differences in the variables studied, except for the frequency of heart disease. Nonetheless, there were differences with respect to age, heart disease and bleeding volume between the general series (patients not treated with antiplatelet or anticoagulation agents) when compared with the subgroups of patients receiving antiplatelet and anticoagulation agents.
ConclusionsWe provide new information regarding the clinical behaviour of LICH and its differences in patients receiving antiplatelet or anticoagulation agents. Mortality is higher in cases of LICH on anticoagulants. Female sex and the volume of bleeding are predictors of mortality.
La hemorragia intracerebral lobular (HIL) es una causa poco frecuente de ictus y representan cerca del 20% de las hemorragias intracerebrales primarias. La causa más frecuente son la angiopatía amiloidea cerebral (AAC), la hipertensión arterial (HTA) y otras como el tratamiento antiagregante o anticoagulante.
Analizar una serie de pacientes con HIL y compararla con subgrupos de pacientes con HIL antiagregados o anticoagulados previamente. Determinar el volumen de la hemorragia y su valor predictivo de mortalidad.
Pacientes y métodosSe incluyó de forma consecutiva y retrospectiva a 162 pacientes diagnosticados de HIL y atendidos en el servicio de neurología del Hospital Meixoeiro de Vigo entre los años 1991 y 2009. Se recogieron características demográficas, factores de riesgo, etiologías y clínica, y se realizó un análisis comparativo entre la serie general y los subgrupos de paciente antiagregados y anticoagulados.
ResultadosEn la serie general la causa más frecuente fue la AAC posible o probable seguida de la HTA. En los subgrupos de pacientes antiagregados o anticoagulados no había diferencias en las variables estudiadas excepto en la frecuencia de cardiopatía. Sí existían diferencias en cuanto a la edad, la cardiopatía y la volumen de la hemorragia entre la serie general (sin los pacientes antiagregados o anticoagulados) cuando se compararon con los subgrupos de antiagregados y anticoagulados.
ConclusionesAportamos algunas novedades respecto al comportamiento clínico de la HIL y sus diferencias en los pacientes antiagregados o anticoagulados. La mortalidad es superior en las HIL anticoaguladas. Son variables predictivas de defunción el sexo femenino y el volumen de la hemorragia.
Due to their specific location, aetiology, and prognosis, lobar intracerebral haemorrhages (LICH) are different from other types of intracerebral haemorrhages (ICH). According to different series, they account for 14%–39% of all ICH.1–5 LICH occurs in the cortical–subcortical junction of the cerebral lobes and usually extends along a plane parallel to the adjacent cerebral cortex. It may affect any of the cerebral lobes, but it most commonly presents in one or more lobes of the parietal-occipital-temporal association area.2–5 The various causes of LICH coincide with the aetiologies of other types of ICH; in LICH, however, high blood pressure plays a less important role and cerebral amyloid angiopathy (CAA) appears more frequently. Although high blood pressure causes 80% of basal ganglia haemorrhages, it only occurs in 30%–50% of LICH cases.4–8 Other aetiological factors, such as arteriovenous malformations, occur in 7%–20% of LICH cases; tumours are present in 7%–9%, and blood or clotting disorders are found with 5%–20% cases of haemorrhages.2–5,9
The purpose of this study is to evaluate a consecutive series of 162 patients with LICH and analyse LICH patient subgroups with a prior history of antiplatelet or anticoagulation treatment. We recorded demographic, aetiological, and clinical differences, outcome, and the bleeding volume plus its predictive value for mortality.
Patients and methodsWe conducted a retrospective study of a series of 162 consecutive LICH patients drawn from a database of stroke patients admitted to the neurology department at Hospital Meixoeiro in Vigo (CHUVI) between January 1991 and December 2009. Stroke subtypes were classified according to the guidelines drawn up by the Spanish Society of Neurology's Cerebrovascular Diseases Study Group.1 All patients in the series were admitted to the neurology department in the first 24 h after symptom onset. They were diagnosed and monitored by one of the department's neurologists according to the Spanish Society of Neurology's stroke care protocols.1 Patients cared for by other departments were not included in the study.
Patients were diagnosed with LICH if they had non-traumatic intraparenchymal haemorrhages and met the following criteria: (a) medical history, examination and brain CT compatible with intracerebral haemorrhage; (b) primary location of the haemorrhage in the cortical or cortical–subcortical region, in one or more cerebral lobes (in some cases, it was difficult to distinguish between lobar and deep cerebral haemorrhages. Those located within 1cm of the lateral ventricular wall were considered deep or basal ganglia haemorrhages; when the haemorrhage location was unclear, the CT scan was reviewed by a group of neurologists and radiologists in order to reach a consensus). Some included cases also featured (c) cortical vessel rupture with bleeding into the subarachnoid space or ventricular invasion.2
The following exclusion criteria were applied: traumatic LICH, LICH secondary to an arteriovenous malformation bleed, cavernous haemangioma, primary or metastatic brain tumours, and cerebral infarctions with haemorrhagic conversion. Cases of LICH associated with subarachnoid haemorrhage were included when the latter was found to be secondary to the lobar haemorrhage and not associated with aneurysm or arteriovenous malformation. In all patients, we ruled out the presence of an underlying pathology behind the haemorrhage with the aid of subsequent neuroimaging studies (CT scan or brain MRI).
The data collection protocol included the following: age, sex, and intracerebral haemorrhage risk factors according to international criteria.3,10,11 Diagnosis of any risk factor or factors and their treatment at the time of the haemorrhage had to be recorded in the patient's medical history. We also recorded clinical manifestations upon admission, possible aetiology or aetiologies of LICH indicated by the neurologist on the patient's discharge report, and outcome as either death or survival. LICH cases associated with possible, probable, or definite diagnoses of CAA were classified according to the Boston CAA Group guidelines.12 We specifically recorded prior treatment with oral antiplatelet and anticoagulation agents.
All patients had a brain CT scan taken during the first 6 h after admission. Bleed volume was measured using the standard formula for volume (cc)=A·B·C/2 (where A is the largest cross-sectional diameter of the haemorrhage in cm, B is the largest diameter perpendicular to A, and C is the number of 1-cm tomographic slices intersecting the haemorrhage).13 The CT scanners used during these years were ProSpeed, HiSpeed Spiral and Lightspeed VCT models built by General Electric.
Certain patients had a brain MRI taken between the first and the sixth month after the haemorrhage so as to rule out LICH secondary to an underlying lesion.
Within this series, we analysed two subgroups: LICH patients previously treated with the anticoagulation agent Sintrom® and patients previously treated with antiplatelet agents (aspirin, triflusal, or clopidogrel). We compared patients’ different demographic, clinical and aetiological variables, as well as the bleed volume in the 2 subgroups, to patients with LICH who had not received antiplatelet or anticoagulation agents.
Statistical analysis was performed with the Statistical Package for Social Sciences programme version 15.0 (SPSS, Chicago, United States).
We performed a descriptive analysis of the variables included in the study. For qualitative variables, we used frequencies and percentages, and for quantitative variables, we used standard deviation, range, median, and interquartile range.
We used the t test to compare ages between the 2 patient subgroups receiving anticoagulation and antiplatelet agents. In the overall series, the t test was also used to compare patients with no anticoagulation and antiplatelet agents with those receiving either anticoagulation or antiplatelet agents. We used the median test to measure the haemorrhage volume shown by the CT scan in each of the different patient subgroups.
We compared risk factors, clinical data, and aetiology among different patient subgroups using univariate analysis (χ2 test or Fisher's exact test). The Kolmogorov–Smirnov test was used to study normality. P<.05 was considered statistically significant.
The variables best predicting risk of death were determined by means of a backward stepwise logistic regression model based on the maximum likelihood method. Death was considered the dependent variable (coded as 1=yes, 0=no). Covariates were those shown to be clinically relevant or significant in the univariate analysis. The odds ratio (OR) and 95% confidence interval were estimated based on the coefficients and standard deviations. Sensitivity and specificity of the bleed volume with respect to death were determined by using receiver operating characteristic (ROC) curves to determine the best cut-off point.
ResultsA total of 5248 stroke patients were treated by the Hospital Meixoeiro neurology department between January 1991 and December 2009. Of these patients, 994 (18.9%) were diagnosed with ICH. The ICH group included 162 patients with LICH. That subgroup comprised 16.4% of the patients with ICH and 3% of the total stroke patients treated in this department.
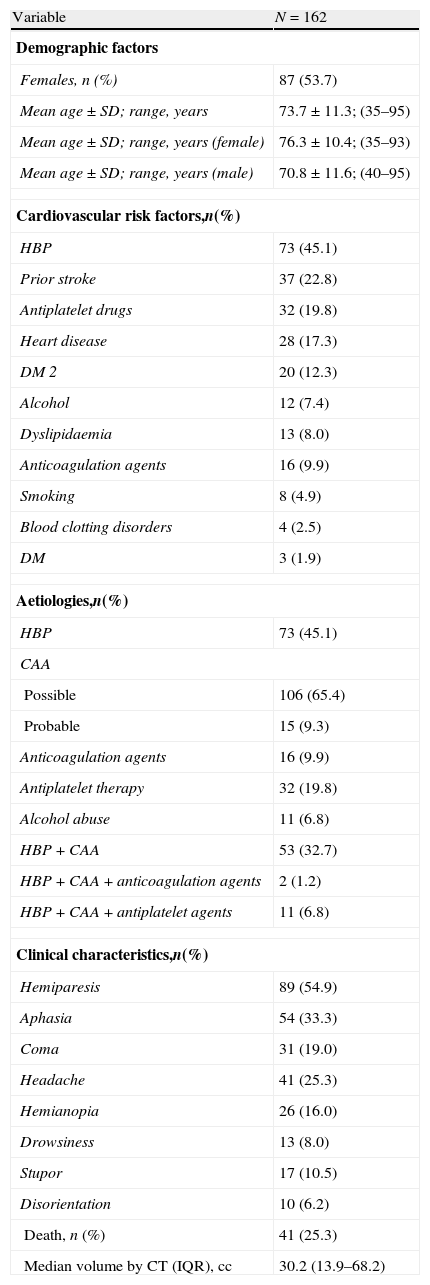
Results of the retrospective analysis of the general series of 162 LICH patients are shown in Table 1. Of the LICH patient total, 54% were women and the mean age of both sexes was 73.7 years, with a range of 35–95 years. Women had a mean age of 76.3 with a range of 35–93 years, while men had a mean age of 70.8 with a range of 40–95 years. Vascular risk factors, aetiology, symptoms, outcome (death/survival), and haemorrhage volume measured by CT are shown in Table 1. Since LICH may have multiple causes, we determined the number of patients with both high blood pressure and CAA, and the combination of HBP, CAA, and antiplatelet or anticoagulation therapy.
Descriptive results from the general series.
| Variable | N=162 |
| Demographic factors | |
| Females, n (%) | 87 (53.7) |
| Mean age±SD; range, years | 73.7±11.3; (35–95) |
| Mean age±SD; range, years (female) | 76.3±10.4; (35–93) |
| Mean age±SD; range, years (male) | 70.8±11.6; (40–95) |
| Cardiovascular risk factors,n(%) | |
| HBP | 73 (45.1) |
| Prior stroke | 37 (22.8) |
| Antiplatelet drugs | 32 (19.8) |
| Heart disease | 28 (17.3) |
| DM 2 | 20 (12.3) |
| Alcohol | 12 (7.4) |
| Dyslipidaemia | 13 (8.0) |
| Anticoagulation agents | 16 (9.9) |
| Smoking | 8 (4.9) |
| Blood clotting disorders | 4 (2.5) |
| DM | 3 (1.9) |
| Aetiologies,n(%) | |
| HBP | 73 (45.1) |
| CAA | |
| Possible | 106 (65.4) |
| Probable | 15 (9.3) |
| Anticoagulation agents | 16 (9.9) |
| Antiplatelet therapy | 32 (19.8) |
| Alcohol abuse | 11 (6.8) |
| HBP+CAA | 53 (32.7) |
| HBP+CAA+anticoagulation agents | 2 (1.2) |
| HBP+CAA+antiplatelet agents | 11 (6.8) |
| Clinical characteristics,n(%) | |
| Hemiparesis | 89 (54.9) |
| Aphasia | 54 (33.3) |
| Coma | 31 (19.0) |
| Headache | 41 (25.3) |
| Hemianopia | 26 (16.0) |
| Drowsiness | 13 (8.0) |
| Stupor | 17 (10.5) |
| Disorientation | 10 (6.2) |
| Death, n (%) | 41 (25.3) |
| Median volume by CT (IQR), cc | 30.2 (13.9–68.2) |
CAA: cerebral amyloid angiopathy; SD: standard deviation; DM 2: type 2 diabetes mellitus; HBP: high blood pressure; IQR: interquartile range; CT scan: computed tomography (brain).
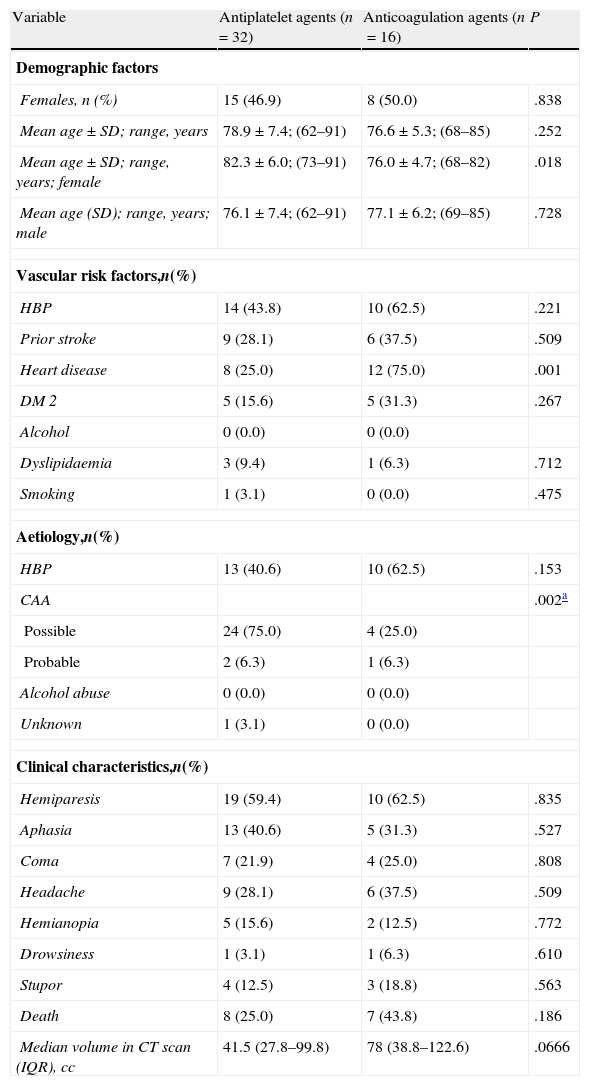
The results of the subgroups of patients who received antiplatelet agents (n=32) or anticoagulation agents (n=16) prior to LICH are shown in Table 2. The table also displays age and sex distribution, vascular risk factors, aetiology, clinical characteristics, outcome, and bleeding volume.
Results of the comparative univariate analysis of patient subgroups treated with antiplatelet and anticoagulation agents.
| Variable | Antiplatelet agents (n=32) | Anticoagulation agents (n=16) | P |
| Demographic factors | |||
| Females, n (%) | 15 (46.9) | 8 (50.0) | .838 |
| Mean age±SD; range, years | 78.9±7.4; (62–91) | 76.6±5.3; (68–85) | .252 |
| Mean age±SD; range, years; female | 82.3±6.0; (73–91) | 76.0±4.7; (68–82) | .018 |
| Mean age (SD); range, years; male | 76.1±7.4; (62–91) | 77.1±6.2; (69–85) | .728 |
| Vascular risk factors,n(%) | |||
| HBP | 14 (43.8) | 10 (62.5) | .221 |
| Prior stroke | 9 (28.1) | 6 (37.5) | .509 |
| Heart disease | 8 (25.0) | 12 (75.0) | .001 |
| DM 2 | 5 (15.6) | 5 (31.3) | .267 |
| Alcohol | 0 (0.0) | 0 (0.0) | |
| Dyslipidaemia | 3 (9.4) | 1 (6.3) | .712 |
| Smoking | 1 (3.1) | 0 (0.0) | .475 |
| Aetiology,n(%) | |||
| HBP | 13 (40.6) | 10 (62.5) | .153 |
| CAA | .002a | ||
| Possible | 24 (75.0) | 4 (25.0) | |
| Probable | 2 (6.3) | 1 (6.3) | |
| Alcohol abuse | 0 (0.0) | 0 (0.0) | |
| Unknown | 1 (3.1) | 0 (0.0) | |
| Clinical characteristics,n(%) | |||
| Hemiparesis | 19 (59.4) | 10 (62.5) | .835 |
| Aphasia | 13 (40.6) | 5 (31.3) | .527 |
| Coma | 7 (21.9) | 4 (25.0) | .808 |
| Headache | 9 (28.1) | 6 (37.5) | .509 |
| Hemianopia | 5 (15.6) | 2 (12.5) | .772 |
| Drowsiness | 1 (3.1) | 1 (6.3) | .610 |
| Stupor | 4 (12.5) | 3 (18.8) | .563 |
| Death | 8 (25.0) | 7 (43.8) | .186 |
| Median volume in CT scan (IQR), cc | 41.5 (27.8–99.8) | 78 (38.8–122.6) | .0666 |
SD: standard deviation; IQR: interquartile range.
Patients treated with Sintrom® upon arrival at the emergency department had an anticoagulation level of 3.26 (measured by the International Normalised Ratio, INR) with a range of 1.35–11.28 and a typical deviation of 2.4.
Comparison of both subgroups revealed significant age-related differences between women receiving antiplatelet agents and those treated with anticoagulation agents. As we might expect, heart disease is significantly more common among patients receiving anticoagulation agents. The presence of high blood pressure, diabetes mellitus type 2 (DM 2), and death was also significant. Deaths were more frequent in the group of patients receiving anticoagulation agents. Haemorrhage volume was larger in the patients receiving anticoagulation agents, but the difference was not significant.
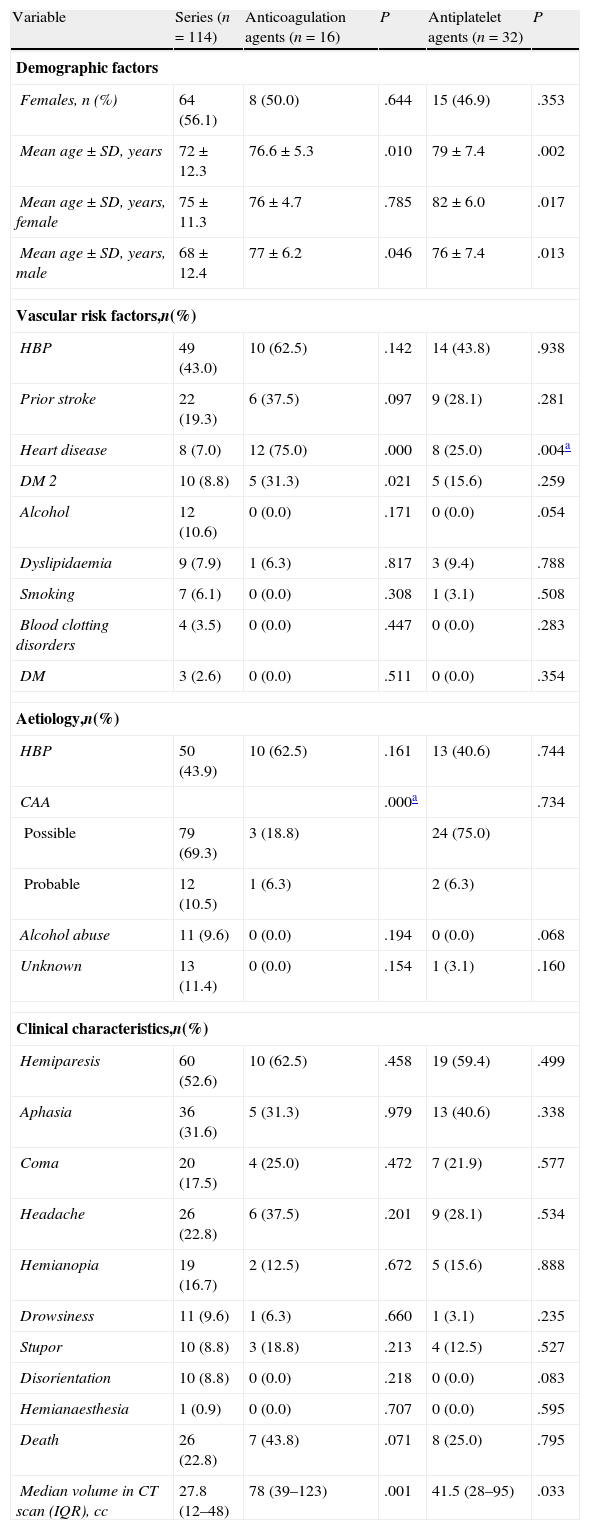
The comparative analysis between the general LICH series (excluding patients treated with antiplatelet or anticoagulation agents) and the group of patients receiving anticoagulation agents (Table 3) revealed significant differences related to age, but not to sex. Heart disease was also significantly more frequent in the group of patients receiving anticoagulation agents, as was to be expected.
Results of the comparative univariate analysis of the general series (excluding patients treated with antiplatelet or anticoagulation agents) and the patient subgroups treated with antiplatelet or anticoagulation agents.
| Variable | Series (n=114) | Anticoagulation agents (n=16) | P | Antiplatelet agents (n=32) | P |
| Demographic factors | |||||
| Females, n (%) | 64 (56.1) | 8 (50.0) | .644 | 15 (46.9) | .353 |
| Mean age±SD, years | 72±12.3 | 76.6±5.3 | .010 | 79±7.4 | .002 |
| Mean age±SD, years, female | 75±11.3 | 76±4.7 | .785 | 82±6.0 | .017 |
| Mean age±SD, years, male | 68±12.4 | 77±6.2 | .046 | 76±7.4 | .013 |
| Vascular risk factors,n(%) | |||||
| HBP | 49 (43.0) | 10 (62.5) | .142 | 14 (43.8) | .938 |
| Prior stroke | 22 (19.3) | 6 (37.5) | .097 | 9 (28.1) | .281 |
| Heart disease | 8 (7.0) | 12 (75.0) | .000 | 8 (25.0) | .004a |
| DM 2 | 10 (8.8) | 5 (31.3) | .021 | 5 (15.6) | .259 |
| Alcohol | 12 (10.6) | 0 (0.0) | .171 | 0 (0.0) | .054 |
| Dyslipidaemia | 9 (7.9) | 1 (6.3) | .817 | 3 (9.4) | .788 |
| Smoking | 7 (6.1) | 0 (0.0) | .308 | 1 (3.1) | .508 |
| Blood clotting disorders | 4 (3.5) | 0 (0.0) | .447 | 0 (0.0) | .283 |
| DM | 3 (2.6) | 0 (0.0) | .511 | 0 (0.0) | .354 |
| Aetiology,n(%) | |||||
| HBP | 50 (43.9) | 10 (62.5) | .161 | 13 (40.6) | .744 |
| CAA | .000a | .734 | |||
| Possible | 79 (69.3) | 3 (18.8) | 24 (75.0) | ||
| Probable | 12 (10.5) | 1 (6.3) | 2 (6.3) | ||
| Alcohol abuse | 11 (9.6) | 0 (0.0) | .194 | 0 (0.0) | .068 |
| Unknown | 13 (11.4) | 0 (0.0) | .154 | 1 (3.1) | .160 |
| Clinical characteristics,n(%) | |||||
| Hemiparesis | 60 (52.6) | 10 (62.5) | .458 | 19 (59.4) | .499 |
| Aphasia | 36 (31.6) | 5 (31.3) | .979 | 13 (40.6) | .338 |
| Coma | 20 (17.5) | 4 (25.0) | .472 | 7 (21.9) | .577 |
| Headache | 26 (22.8) | 6 (37.5) | .201 | 9 (28.1) | .534 |
| Hemianopia | 19 (16.7) | 2 (12.5) | .672 | 5 (15.6) | .888 |
| Drowsiness | 11 (9.6) | 1 (6.3) | .660 | 1 (3.1) | .235 |
| Stupor | 10 (8.8) | 3 (18.8) | .213 | 4 (12.5) | .527 |
| Disorientation | 10 (8.8) | 0 (0.0) | .218 | 0 (0.0) | .083 |
| Hemianaesthesia | 1 (0.9) | 0 (0.0) | .707 | 0 (0.0) | .595 |
| Death | 26 (22.8) | 7 (43.8) | .071 | 8 (25.0) | .795 |
| Median volume in CT scan (IQR), cc | 27.8 (12–48) | 78 (39–123) | .001 | 41.5 (28–95) | .033 |
SD: standard deviation; IQR=interquartile range.
There were no significant differences for the other vascular risk factors except for DM 2. There were also no significant differences between the 2 subgroups with regard to aetiology and symptoms. Deaths were more frequent in the group of patients treated with anticoagulation agents. However, this result was not significant compared to the group of LICH patients without antiplatelet or anticoagulation treatment. Nevertheless, the median haemorrhage volume was 3 times higher in the patient subgroup treated with anticoagulation agents, and the difference was clearly significant (Table 3).
In our series, the median haemorrhage volume was 30.2cc. In our patients, the volume of the CT scan had a high predictive value for death (area under the ROC curve, 0.88; P<.001). The cut-off point was 37.8cc (sensitivity 78% and specificity 72%).
In the group of patients receiving antiplatelet agents, the median volume was 41.5cc. In LICH patients who did not receive antiplatelet or anticoagulation agents, median volume was 27.8cc. This difference was significant (Table 3). In this subgroup, the cut-off point for death was a bleeding volume of 58cc, with a sensitivity of 100% and a specificity of 76% (area under the ROC curve, 1; P<.001).
There was no relationship between INR (mean, 3.2) upon arrival at the hospital and haemorrhage volume in the anticoagulation group. One patient had an INR of 11.28 and a haemorrhage volume of 84cc, and another patient had an INR of 2.7 and a haemorrhage volume of 283cc. Haemorrhage volume also had a low predictive value for death in this subgroup since the area under the ROC curve was only 0.68.
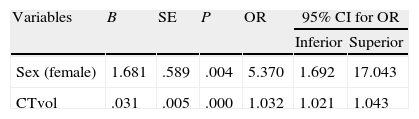
According to the multivariate logistic regression analysis of the general series, there was a significant association between death and the variables sex and haemorrhage volume quantified by CT. OR for haemorrhage volume was 1.032, and OR for female sex was 5.37 (Table 4). In the anticoagulation group, there was no significant link between bleeding volume and death due to the dispersion of the data and the low number of patients.
DiscussionBetween 1991 and 2009, LICH accounted for 3% of the total stroke patients treated in the neurology department at Hospital Meixoeiro, Vigo, Spain; this figure was similar to that reported in another study.4 LICH also accounted for 16.4% of the total ICH patients attended, a lower percentage than that reported by other published studies.2,14 In our study, females are slightly predominant and mean age is similar to that in other studies.4,15
Vascular risk factors recorded in our series are similar to those in the literature,2–4 with the exception of one series with an increased incidence of high blood pressure.8 There were no significant differences between the patient subgroup treated with antiplatelet agents and the subgroup on anticoagulation agents except that heart disease was more frequent in the second group, which was to be expected.
In our general series, the frequency of clinical manifestations (hemiparesis, aphasia, and changes in alertness) was similar to those listed in other series of LICH patients.2,4,8,9 Headache frequency, however, was lower among our patients.2–4 Our study found no significant differences in clinical presentation between the general series and the LICH patient subgroups treated with antiplatelet and anticoagulation agents, or between the last two subgroups.
We found a high mortality rate for LICH (25% in our series) which was very similar to rates given in other studies.3–5,7 This study found no significant differences for percentage of deaths between the general series without antiplatelet and anticoagulation agents (114 patients) and the patient subgroup treated with antiplatelet agents (Table 3). Comparing the general series with the patient group on anticoagulation agents shows that the mortality rate is almost twice as high in the anticoagulation group. This rate approaches statistical significance but does not reach it, probably due to the low number of patients in the anticoagulation group (Table 3). There are more deaths in the anticoagulation group than in the antiplatelet group (Table 2), but the difference is not significant.
The literature does not report the incidence of death in LICH patients previously treated with antiplatelet or anticoagulation agents, which is why our study provides a novel contribution. However, we found one study of a series of ICH patients previously treated with antiplatelet agents in which the in-hospital mortality rate was nearly identical to the one in our antiplatelet subgroup (about 25% in both studies).16 There are also series of ICH cases associated with anticoagulation in which in-hospital mortality rates are quite similar to the one in our anticoagulation subgroup (between 35% and 45%).17–19 The aetiologies (or combinations of aetiologies) underlying LICH were similar to those in other published series: high blood pressure, diabetes, heart disease, prior stroke, and dyslipidaemia.2–5 According to the Boston CAA Group guidelines, the presence of possible or probable CAA (close to 75%) was similar to that reported by a study including an anatomical pathology assessment.12 This highlights the important role that disease plays in lobar haemorrhages. Incidence of CAA was similar to the above study in the antiplatelet patient group, but lower in the anticoagulation group.
In our series, the median bleeding volume was 30.2cc, slightly lower than the volume in an earlier series published by our group,5 and also lower than the volumes reported in other studies.3,15,20 As we stated in another previous study, the haemorrhage volume quantified by CT upon admission showed a high predictive value for death in the overall series.5
We also found that haemorrhage volume had a high predictive value for death, with a cut-off point of 58cc, in the patient group treated with antiplatelet agents. This is a new finding which is relevant for managing LICH and for potential approaches to treatment. However, the same result could not be demonstrated in the anticoagulation group since this patient subgroup was quite small and bleeding volumes varied greatly.
The considerable difference between mean haemorrhage volume in the patient subgroup without antiplatelet or anticoagulation agents and the antiplatelet patient subgroup (27.8cc versus 41.5cc) may be explained by the action of the antiplatelet agents, and also in part by the significant age difference between groups. It was recently determined that LICH volume is related to age. The older the patient is, the larger the bleed volume.15
CAA is the most frequent cause of LICH5,12 and it is associated with ApoE alleles ¿2 and ¿4.14 It is often present in patients with Alzheimer disease.21 Brain microbleeds, especially those located in the cortex, can be detected by using gradient echo MR imaging. They are indicative of CAA.22,23 It is also known that these microbleeds are predictors of a greater tendency towards LICH in patients treated with anticoagulation agents. Elderly patients should therefore be checked for microbleeds prior to anticoagulation treatment, and presence of microbleeds must be factored into the risk-benefit ratio for anticoagulation.17,24 It was recently shown that antiplatelet treatment with aspirin in LICH patients may increase the risk of bleed recurrence.25
Our study has a number of limitations since it is a retrospective series spanning 19 years. Patients were not monitored after discharge, which is why we have no information regarding long-term outcomes, bleed recurrences, or other potentially interesting variables. The small size of the LICH patient subgroup treated with anticoagulation agents does not permit us to gather significant data. However, death rates in this group are much higher than in the rest of the series.
Our study reveals interesting information on LICH aetiology, clinical manifestations, and the differences in death rate associated with prior use of antiplatelet or anticoagulation agents. We also feel that it would be interesting to define cut-off points for haemorrhage volume that would enable us to predict patient mortality in the different subgroups under study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Romero López J, et al. Hemorragia intracerebral lobular: análisis de una serie y características en pacientes antiagregados y anticoagulados. Neurología. 2012;27:387–93.