Chronic immune-mediated ataxic neuropathies may be associated with anti-disialosyl antibodies, and sometimes with ophthalmoplegia and high levels of IgM paraprotein, a component that acts as a cold agglutinin. The disease is generally known as chronic ataxic neuropathy, ophthalmoplegia, IgM paraprotein, cold agglutinins, and disialosyl antibodies (CANOMAD), or chronic ataxic neuropathy with disialosyl antibodies (CANDA) in patients not presenting oculomotor alterations.1,2
The disease often presents a relapsing-remitting course characterised by the appearance or worsening of ophthalmoplegia, sensory symptoms, and bulbar/limb weakness.
Relapses generally improve and resolve with monthly (5 days per month) or weekly treatment with intravenous immunoglobulins (IVIG) and/or corticosteroids.3–5 However, up to 30% of patients with CANDA/CANOMAD do not respond to maintenance therapy with IVIG. Some authors report their experience with rituximab (RTX), which has been used successfully to treat other immune-mediated neuromuscular diseases.6–12
We present a case with chronic ataxic neuropathy associated with anti-disialosyl antibodies in which we opted to start RTX treatment after the occurrence of several relapses despite maintenance treatment with IVIG; the patient remained asymptomatic for 19 months.
Clinical caseThe patient was a 77-year-old man, a smoker, with no family history of neuromuscular diseases and with personal history of arterial hypertension and chronic obstructive pulmonary disease, who attended our centre due to difficulty performing fine manual tasks, gait instability, and paraesthesia in all 4 limbs. He reported no history of gastrointestinal or respiratory infection. Physical examination revealed hypoaesthesia in both hands and feet, reduced vibration sensitivity in both feet, generalised areflexia, ataxic gait (the patient walked with a cane), and positive Romberg sign. No ophthalmoplegia was detected, and the patient did not report diplopia. To quantify disease progression, we used the Inflammatory Neuropathy Cause and Treatment (INCAT) score, a clinical scale that evaluates disability of the upper and lower limbs. The scale is scored 0-10, with 0 indicating no functional impact and 10 indicating inability to perform purposeful movements of the arms or legs; at the baseline visit, the patient scored 4.
A neurophysiological study identified axonal sensorimotor neuropathy in all 4 limbs, with some signs of demyelination. The sensory neurography revealed a lack of response in the median, ulnar, and sural nerves bilaterally, and reduced amplitude in the right radial nerve. In the motor neurography, the bilateral median, ulnar, and anterior tibial nerves and the right peroneal nerve showed increased latency, reduced amplitude, and decreased conduction velocity disproportionate to the decreased amplitude.
Cerebrospinal fluid analysis detected 0 cells per mm3 (normal range: 0-5), 5 erythrocytes per mm3 (0-5), 59 mg/dL glucose (50-80 mg/dL), and 54.8 mg/dL proteins (15-60 mg/dL). ELISA detected anti-ganglioside IgM antibodies in the serum (GM1: 1/1035, GM2: 1/15 996, GD1a: 1/5016, GD1b: 1/17 326, GT1b: 1/16 796, GQ1b: 1/10 361, GD3: 1/14 966; normal range: 1/500). We also detected IgM paraproteinaemia (IgM titre: 325 mg/dL; normal range: 40-230) and cold agglutinins.
In the light of these results, we decided in February 2015 to administer IVIG (0.4 mg/kg/day for 5 days), which improved the sensory symptoms and ataxic gait (INCAT score of 1 due to mild gait instability). He presented no relapses in 2015 or 2016, although he did continue to present mild hypoaesthesia in the hands and feet, as well as decreased vibration sensitivity in the feet (INCAT score 0-1).
In September 2017, the patient presented a respiratory infection and developed quadriparesis, ataxia, and bulbar weakness, requiring admission to the intensive care unit and respiratory support. INCAT score was 9, as the patient could not stand unassisted and was unable to perform any purposeful movement with his hands. We administered IVIG, and the patient presented a progressive improvement. After 18 days, he recovered his baseline status and was discharged (INCAT score of 2).
In December 2017 and April 2018, he presented 2 episodes of severe exacerbation of ataxia (INCAT score of 5), which were treated with IVIG. We subsequently prescribed monthly IVIG treatment.
In July 2018, the patient presented a further exacerbation, in the form of gait ataxia (INCAT score of 7); we opted to start RTX treatment, with 2 doses of 1000 mg at an interval of 14 days.
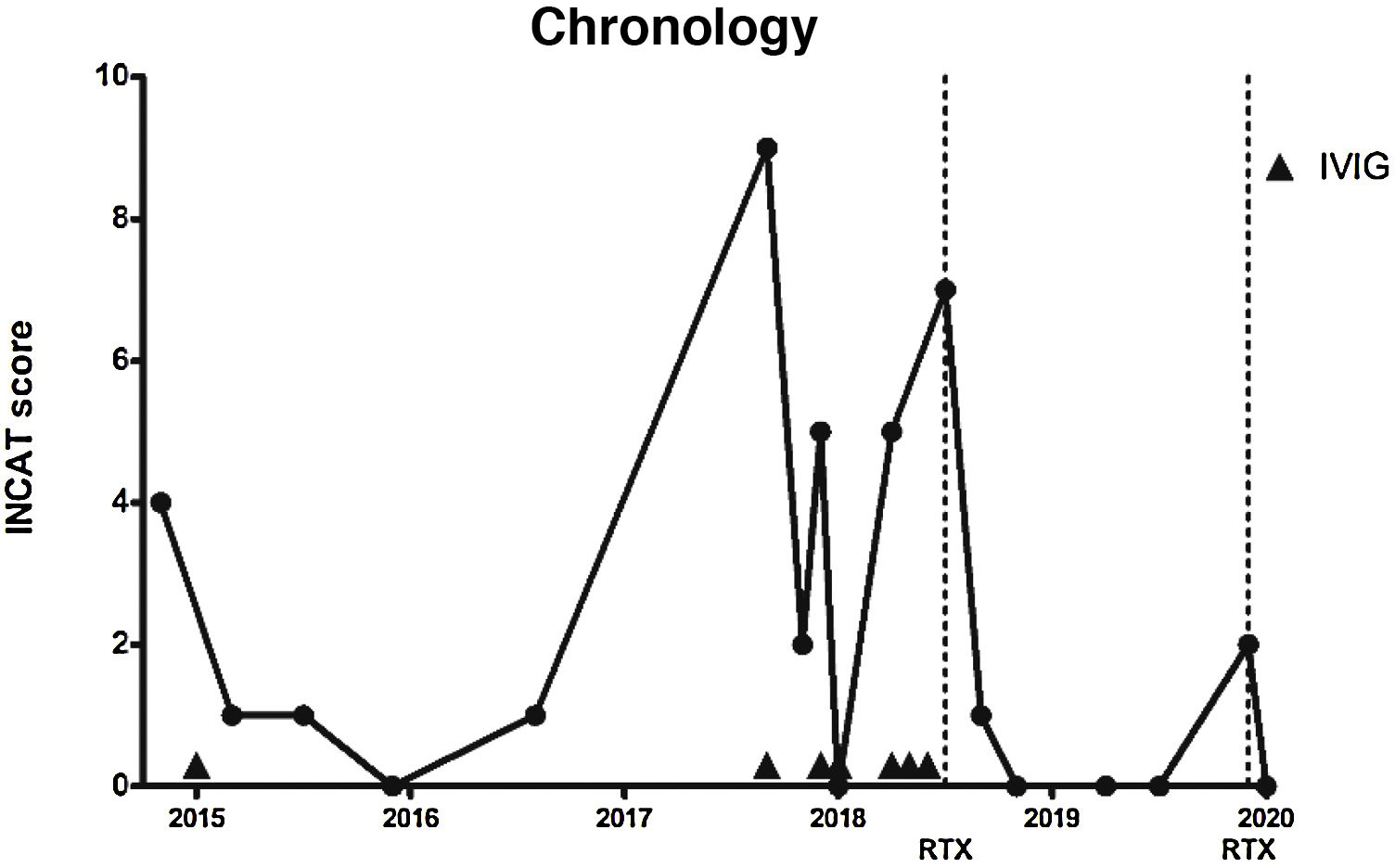
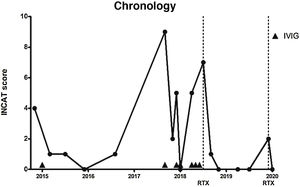
In the 19 months after onset of RTX treatment, only on one occasion did the patient present mild worsening of ataxia (INCAT score of 2), which improved with administration of a single dose of RTX (1000 mg). The patient currently presents no gait ataxia or sensory alterations (INCAT score of 0) (Fig. 1).
Timeline of disease activity, according to Inflammatory Neuropathy Cause and Treatment score and the treatments prescribed. Dotted lines indicate rituximab administration. Arrowheads indicate intravenous immunoglobulin administration.
INCAT: Inflammatory Neuropathy Cause and Treatment; IVIG: intravenous immunoglobulins; RTX: rituximab.
The objective of this letter is to highlight the effectiveness of RTX as a treatment for ataxic neuropathy with anti-ganglioside antibodies.
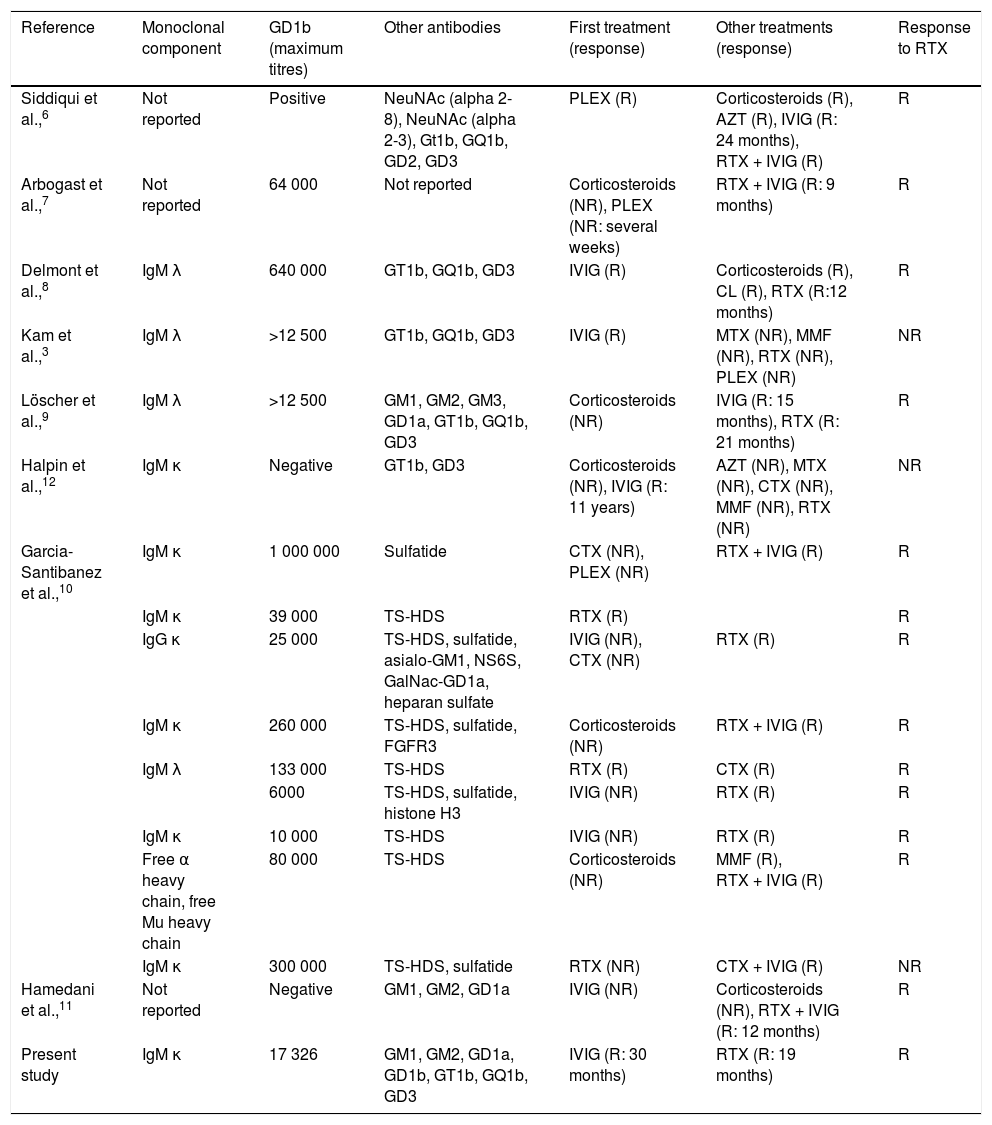
The literature currently includes 17 reported cases of CANOMAD/CANDA treated with RTX (Table 1).6–12 In line with the criteria used in studies of chronic inflammatory demyelinating polyradiculoneuropathy, we reviewed cases treated with RTX and classified patients as responders if they showed some improvement in motor or sensory symptoms, lasting at least 6 months, without a need for additional treatment. Those who required alternative treatment are classified as non-responders.13
Reported cases of CANOMAD/CANDA treated with rituximab.
| Reference | Monoclonal component | GD1b (maximum titres) | Other antibodies | First treatment (response) | Other treatments (response) | Response to RTX |
|---|---|---|---|---|---|---|
| Siddiqui et al.,6 | Not reported | Positive | NeuNAc (alpha 2-8), NeuNAc (alpha 2-3), Gt1b, GQ1b, GD2, GD3 | PLEX (R) | Corticosteroids (R), AZT (R), IVIG (R: 24 months), RTX + IVIG (R) | R |
| Arbogast et al.,7 | Not reported | 64 000 | Not reported | Corticosteroids (NR), PLEX (NR: several weeks) | RTX + IVIG (R: 9 months) | R |
| Delmont et al.,8 | IgM λ | 640 000 | GT1b, GQ1b, GD3 | IVIG (R) | Corticosteroids (R), CL (R), RTX (R:12 months) | R |
| Kam et al.,3 | IgM λ | >12 500 | GT1b, GQ1b, GD3 | IVIG (R) | MTX (NR), MMF (NR), RTX (NR), PLEX (NR) | NR |
| Löscher et al.,9 | IgM λ | >12 500 | GM1, GM2, GM3, GD1a, GT1b, GQ1b, GD3 | Corticosteroids (NR) | IVIG (R: 15 months), RTX (R: 21 months) | R |
| Halpin et al.,12 | IgM κ | Negative | GT1b, GD3 | Corticosteroids (NR), IVIG (R: 11 years) | AZT (NR), MTX (NR), CTX (NR), MMF (NR), RTX (NR) | NR |
| Garcia-Santibanez et al.,10 | IgM κ | 1 000 000 | Sulfatide | CTX (NR), PLEX (NR) | RTX + IVIG (R) | R |
| IgM κ | 39 000 | TS-HDS | RTX (R) | R | ||
| IgG κ | 25 000 | TS-HDS, sulfatide, asialo-GM1, NS6S, GalNac-GD1a, heparan sulfate | IVIG (NR), CTX (NR) | RTX (R) | R | |
| IgM κ | 260 000 | TS-HDS, sulfatide, FGFR3 | Corticosteroids (NR) | RTX + IVIG (R) | R | |
| IgM λ | 133 000 | TS-HDS | RTX (R) | CTX (R) | R | |
| 6000 | TS-HDS, sulfatide, histone H3 | IVIG (NR) | RTX (R) | R | ||
| IgM κ | 10 000 | TS-HDS | IVIG (NR) | RTX (R) | R | |
| Free α heavy chain, free Mu heavy chain | 80 000 | TS-HDS | Corticosteroids (NR) | MMF (R), RTX + IVIG (R) | R | |
| IgM κ | 300 000 | TS-HDS, sulfatide | RTX (NR) | CTX + IVIG (R) | NR | |
| Hamedani et al.,11 | Not reported | Negative | GM1, GM2, GD1a | IVIG (NR) | Corticosteroids (NR), RTX + IVIG (R: 12 months) | R |
| Present study | IgM κ | 17 326 | GM1, GM2, GD1a, GD1b, GT1b, GQ1b, GD3 | IVIG (R: 30 months) | RTX (R: 19 months) | R |
Responders (R) were defined as all patients showing some improvement in motor or sensory symptoms, lasting at least 6 months, with no need for additional treatment (except IVIG). Those who required alternative treatment are classified as non-responders (NR).
AZT: azathioprine; CL: chlorambucil; CTX: cyclophosphamide; FGFR3: fibroblast growth factor-3; IVIG: intravenous immunoglobulins; MMF: mycophenolate mofetil; MTX: methotrexate; PLEX: plasma exchange; RTX: rituximab; TS-HDS: trisulfated heparin disaccharide.
According to this classification, 14 patients (82.35%) responded to RTX, either alone or in combination with IVIG. The duration of the effect of RTX is not reported in the majority of cases.6–11 RTX was administered in combination with IVIG in 6 cases,6,7,10,11 and was mainly used as a second-line treatment.6–12
The protocol for further administration of RTX varies between cases: 6 months after the first dose,8 every 8 weeks,6 or when CD19+/CD20+ lymphocytes are detectable.9
In patients with neuromyelitis optica, CD19+/CD20+ lymphocyte levels have been used as a biomarker of response to RTX and a criterion for additional infusions of the drug.14 Evidence from patients receiving RTX treatment for neuropathy with anti-MAG antibodies (targeting MAG, a glycoprotein associated with myelin) and myasthenia gravis seems to suggest that levels of these cells are correlated with disease activity.14 This relationship has not been fully explored in the context of CANOMAD/CANDA.9
ConclusionsWe describe the case of a patient who presented chronic sensory ataxia affecting all 4 limbs, associated with IgM paraproteinaemia, anti-disialosyl antibodies, and cold agglutinins. The patient was diagnosed with CANDA syndrome and treated with IVIG, presenting an unsatisfactory response in terms of control of relapses. Since the onset of RTX treatment, 19 months ago, the patient has presented no further relapses, remaining asymptomatic.
Unlike the case of other chronic immune-mediated neuropathies, there is no general consensus on the best treatment for CANOMAD/CANDA. Given the great variability of clinical characteristics, the progression of the disease, and its low prevalence, it is difficult to conduct randomised trials.1 Therefore, in order to establish treatment protocols, there is a need to identify new markers of disease progression and to develop validated scales to assess clinical response.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bertran Recasens B, Figueras-Aguirre G, Royo de Mingo I, Rubio MA. Eficacia a largo plazo del rituximab en la neuropatía crónica atáxica con anticuerpos antigangliósidos. Neurología. 2021;36:739–742.