Decision-making is the process of selecting a course of action from among 2 or more alternatives by considering the potential outcomes of selecting each option and estimating its consequences in the short, medium and long term. The prefrontal cortex (PFC) has traditionally been considered the key neural structure in the decision-making process. However, new studies support the hypothesis that describes a complex neural network including both cortical and subcortical structures.
ObjectiveThe aim of this review is to summarise evidence on the anatomical structures underlying the decision-making process, considering new findings that support the existence of a complex neural network that gives rise to this complex neuropsychological process.
DevelopmentCurrent evidence shows that the cortical structures involved in decision-making include the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC). This process is assisted by subcortical structures including the amygdala, thalamus, and cerebellum.
ConclusionsFindings to date show that both cortical and subcortical brain regions contribute to the decision-making process. The neural basis of decision-making is a complex neural network of cortico-cortical and cortico-subcortical connections which includes subareas of the PFC, limbic structures, and the cerebellum.
La toma de decisiones (TD) puede definirse como la selección de una alternativa dentro de un rango de opciones existentes, considerando los posibles resultados de las selecciones realizadas y sus consecuencias en el comportamiento presente y futuro. Tradicionalmente, se ha afirmado que desde el punto de vista anatómico la base neural fundamental de este proceso lo constituye la corteza prefrontal (CPF); sin embargo, nuevos estudios validan la hipótesis de la existencia de una compleja red neural que incluyen estructuras tanto corticales como subcorticales.
ObjetivoLa presente revisión tiene como objetivo resumir la evidencia sobre las bases anatómicas relacionadas con el proceso de toma de decisiones tomando en consideración la información disponible hasta la actualidad, que valida la existencia de una compleja red neural que sirve de soporte a este complejo proceso neuropsicológico.
DesarrolloLa evidencia contemporánea indica que dentro de las bases neurales de la TD se encuentran regiones de la CPF como la corteza orbitofrontal, dorsolateral y el giro cingulado anterior. Además, el proceso es asistido por regiones subcorticales, como la amígdala, el hipocampo y el cerebelo.
ConclusionesLos resultados hasta el momento demuestran la importancia de las estructuras corticales y subcorticales en la toma de decisiones. Las bases neurales de la TD consisten en una compleja red neural con conexiones cortico-corticales y cortico-subcorticales, que incluyen tanto las subdivisiones de la CPF como las estructuras límbicas y el cerebelo.
Decision-making (DM) is defined as the process of selecting one alternative from a range of existing options, considering the potential outcomes of each selected option and estimating its consequences in present and future behaviour.1 In fact, each year 40% of all deaths globally are due to DM deficits affecting self-regulation.2 This complex process has been studied by several scientific disciplines (cognitive psychology, economics, computer science, and neuropsychology) using different theoretical models as a reference.3–6 Along these lines, the somatic-maker hypothesis proposed by Damasio is especially interesting for neurologists and neuropsychologists. This hypothesis represents a theoretical and methodological reference as it establishes the basis of the relationship between cortical and subcortical structures in decision-making, as well as between deficits and certain brain injuries.7,8
From an anatomical viewpoint, the prefrontal cortex (PFC) is considered the most important brain region when delimiting the neural substrates underlying this process. The PFC is composed of 3 brain subregions: the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), and the dorsolateral prefrontal cortex (DLPFC). These regions work in close relationship with such other regions as the thalamus, the amygdala, and the basal ganglia, to ensure an adequate DM process.9 These structures are organised in 3 fundamental circuits, which are related to DM: (1) the OFC and the limbic pathways, which are closely related with rewards and affective-based decisions; (2) the DLPFC, which specialises in integrating several sources of information, and (3) the ACC, which plays a relevant role in sorting among conflicting options and in outcome-processing (feedback).10 Furthermore, the prefrontal cortex is connected to other subcortical areas which are involved in the function of these cortical regions.11
The purpose of this study is to summarise the anatomical basis related to the process of DM, considering the information available to date, which demonstrates the existence of a complex neural network which supports this intricate psychological process.
DevelopmentOrbitofrontal cortex and decision makingThe PFC is a very important brain region in DM. The structures making up the PFC are the OFC, the ACC, and the DLPFC. The OFC is critical for decisions dependent on positive incentives (gain) as well as the emotional experience associated with outcomes (feedback).11 The OFC has connections with other structures related to DM (for example, the DLPFC and the ACC). Furthermore, it is characterised by strong bidirectional links with the temporal sensory association cortex, the amygdala, and the hippocampus; they all play a key role in emotional processing.12
The orbitofrontal cortex is functionally organised in such a way that the medial portion decodes rewards while the lateral portions evaluate punishment.13 The reward value for primary reinforcing factors (for example, taste), is encoded in posterior areas of the OFC, whereas the value of more complex, secondary reinforcing factors (such as money) are encoded in the anterior regions of the same structure.14 Patients with OFC lesions usually have 2 main types of deficits. First, these patients are unable to alter their decisions to a given stimulus despite a negative associated outcome. Second, patients are deficient in tasks requiring empathy or theory of mind.9
At a cellular level, studies with primates have shown that neuronal activity in the OFC during award-related tasks is high, particularly when the primate anticipates a large reward.15 In contrast, activation of this region decreases considerably when large penalties (punishments) are anticipated in association with decisions. This level of neuronal inhibition-excitation in the orbitofrontal region is also modulated by previous presentation of stimuli, and the immediacy of the reward associated with it.16
In studies performed on humans, evidence is based on analysing patients with frontal lobe lesions while they performed cognitive tasks. Studies using the Iowa Gambling Task (IGT)17 and the Cambridge Gambling Task (CGT)18 have confirmed the presence of difficulties in changing the initial DM pattern according to the contingencies that appear during the DM process. This impairment in making emotional decisions is fundamentally associated with strong links between the OCP, the insula, and the limbic system (hippocampus, amygdala, etc.).19
Dorsolateral prefrontal cortex and anterior cingulate cortexOptimal DM requires the participation of other highly specialised cortical structures, especially the DLPFC and the ACC.20 The DLPFC occupies the lateral and superior areas of the frontal lobes and its functional organisation lies along a dorsal–ventral axis. The dorsal DLPFC is responsible for monitoring information in working memory, whereas the ventral DLPFC regulates retrieval of information stored in the posterior cortical association regions.11,21 Like the OFC, the ventral-medial PFC has strong limbic and cortico-cortical connections, especially along the temporal, parietal, and occipital regions.21,22
The ACC occupies the medial portion of the prefrontal cortex. This region has cortico-cortical connections with the OFC and DLPFC, and subcortical projections to the nucleus accumbens. The anterior portion of the ACC has been demonstrated to be directly associated with depression, which is in turn related to emotion-based DM.23 The main role of the ACC in DM is related to the modulation of such other prefrontal regions as the PFC and DLPFC. This function is performed basically by analysing situations that are ambiguous or conflictive.24 The ACC also participates in optimising future decisions based on previous contingencies perceived during the option selection process.25 Functional magnetic resonance imaging (fMRI) has been used to confirm this feature. fMRI studies show increased ACC activation in periods of non-occurrence of rewards or when negative feedback related to nonoptimal responses increases.26
Participation of the thalamus and basal ganglia in decision-makingThe function of the prefrontal regions depends not only on such cortical structures as the PFC, DLPFC, and ACC, but also on white matter pathways and subcortical structures, including the thalamus, basal ganglia, and cerebellum, which are interconnected with the PFC. This is why damage to these regions can alter the DM process considerably.9 All prefrontal regions involved in DM send projections to the caudate nucleus/putamen through the subcallosal fasciculus of Muratoff or the external capsule.27,28 The basal ganglia, in turn, relay this information to the thalamus, which sends feedback projections to the original cortical region.29
In addition, the OFC and ACC project to the ventral striatum30 and, consequently, damage to this region may cause difficulties in adaptive DM, as it is associated with symptoms of disinhibition, loss of reward-contingencies, and labile behaviours.31 As previously explained, information is relayed from the basal ganglia to the thalamus, which projects back to the neocortex. The mediodorsal nucleus is reciprocally connected with the OFC and the DLPFC, the amygdala, and the olfactory cortex.32 Lesions to this thalamic nucleus affect DM since they cause apathy, aboulia, and disinhibition.33
Amygdala, cerebellum, and decision makingThe amygdala is another subcortical region involved in DM. This structure of the limbic system plays a key role in the emotional coding of environmental stimuli.34 The importance of the amygdala in DM basically resides in its participation in reward mechanisms: it interacts with the ventral striatum, promoting stimulus-reward associations.35–37
Metabolic studies using positron emission tomography (PET) have reported elevated amygdala activity in contexts of potential rewards.38 This is an important element in DM processes, in which continuous assessment of gains and losses in terms of the selection of one option over another is necessary. Other studies have also shown notable activation of this region in contexts of probable rewards.39 However, this activation is more likely to refer to the neural activation eliciting the stimulus (arousal) than to the value of the stimulus (valence, for example, pleasant/unpleasant). Several studies have reached this conclusion after analysing the activity levels of the amygdala in response to reward vs punishment stimuli and observing no differences in intensity with regard to valence.40,41
This finding, together with the well-known fact that the amygdala rapidly habituates to stimuli,42 helps us understand the role of this region in new decision situations which require fast evaluations that guarantee adaptive responses in brief periods. However, its function could be essentially subordinated to situations of strong emotional content which the subject making the decision is not familiar with. This hypothesis is supported by studies using fMRI that have shown a hypostimulation of this structure in MD tasks.43
The cerebellum is another region which has shown to play a key role in DM. The cerebellum has traditionally been thought to be involved in posture, motor control, and coordination. However, a considerable number of studies performed since 1990 have shown that functions of this structure are not limited to these processes.44 For example, posterior areas of the cerebellum participate in high level functions, such as working memory, language processing, spatial information analysis, and emotional regulation.45 The cerebellum also operates as an integral node in the cortical–subcortical circuits linked to the prefrontal cortex, modulating the function of these structures.28 Furthermore, it plays a key role in the development of attention processes.46
A recent study compared DM performance in patients with cerebellar damage with 2 other groups, one including patients with frontal damage, and the other a control group. According to the results of this study, patients with cerebellar damage showed poorer performance than the control group but better performance than the frontal-damaged group.44 These findings go in line with the data obtained by other authors who link DM with the cerebellum.47 According to these authors, the cerebellum belongs to the group of brain regions that activate in DM both under certainty and uncertainty, and it plays a key role in the internal representation of uncertain events, facilitating the prediction of future outcomes as well as inductive processes.
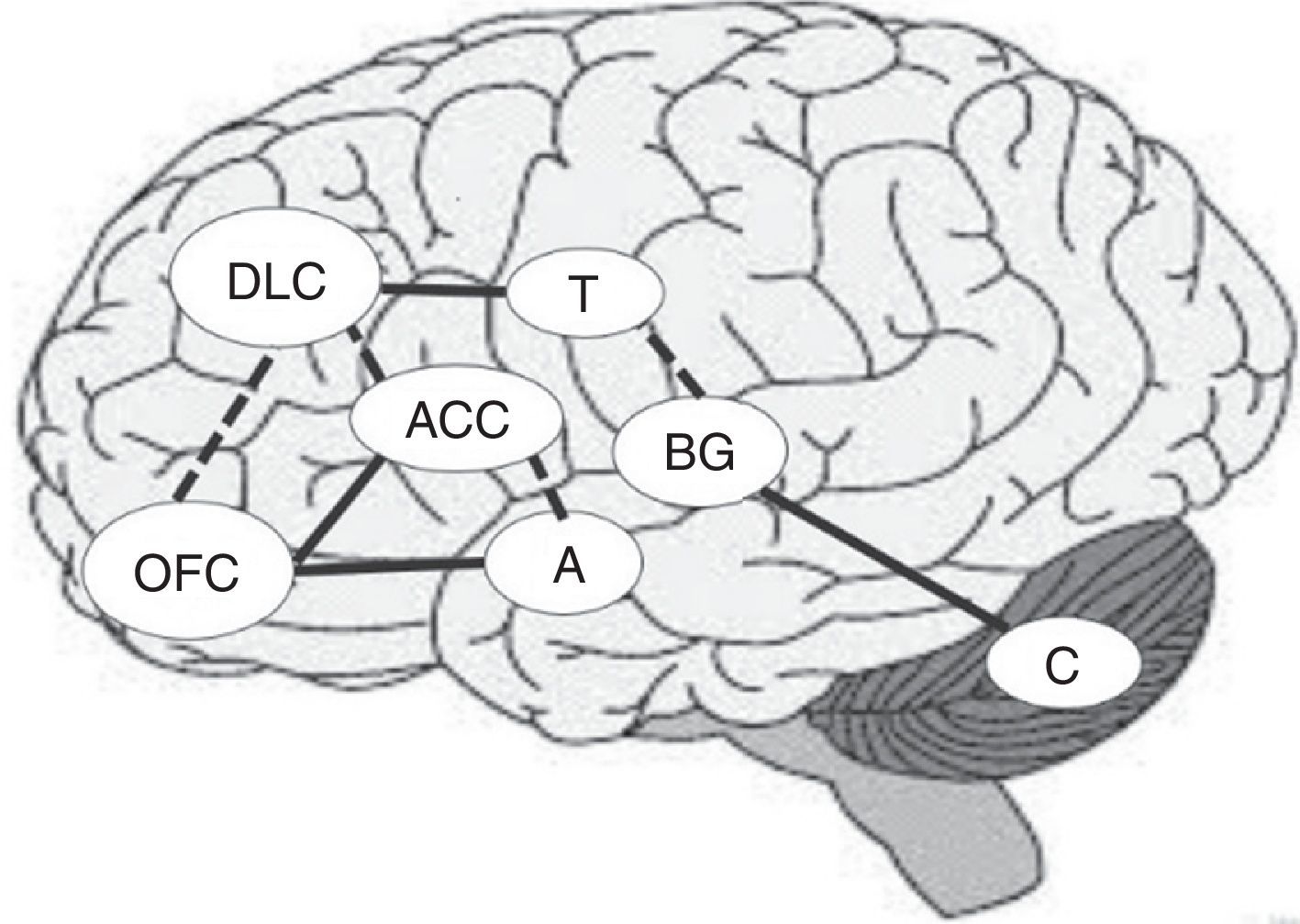
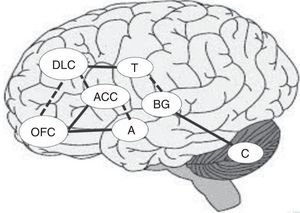
Together with the right prefrontal cortex and basal ganglia, the cerebellum is involved in the internal representation of geographical and temporal distances.48,49 For this reason, if the ability to establish temporal connections between actions and their consequences is impaired, the ability to learn from experience may also be impaired, making difficult to identify advantageous and disadvantageous decisions in the DM process.44Fig. 1 shows the main areas involved in the DM process and the interconnections between regions, including previously mentioned circuits. Solid lines indicate the strongest connections between 2 regions and dashed lines show the connections mainly involved in uncertainty situations (Fig. 1).
Brain damage and decision-making: dysfunctions in neurological diseasesThe systematic study of patients with neurological disorders has shown DM dysfunction in a significant number of diseases. In particular, DM dysfunctions have been found in: patients with traumatic brain injury (TBI),50,51 (behavioural variant) frontotemporal dementia,52 Parkinson's disease, substance dependent patients,53 and patients with frontal lobe and cerebellar damage caused by cerebrovascular accidents.44 They are also common in patients with autism or schizophrenia,54,55 subarachnoid haemorrhage,56 and bipolar disorder.57
In the specific case of frontotemporal dementia, patients have been found to show disturbed moral judgement in addition to behavioural disinhibition, perseveration, and hyperorality,21 since these patients have difficulty assessing the social consequences of their actions.52 This feature is mainly due to the fact that although DLPFC is preserved in this disease, the OFC is severely impaired. As previously stated, the OFC is responsible for the emotional processing of decision situations and behavioural inhibition.9
Furthermore, patients with surgical or traumatic brain injury also present pronounced DM deficits. Trauma to the OFC and temporal regions is frequent in these patients9 and usually damages the white matter, leading to diffuse axonal injury. From a behavioural point of view, in patients with TBI, DM deficits translate into marked impulsivity and difficulty in planning actions, which in turn manifest as aggressive behaviour.58
Clinical assessment of decision-makingRegarding diagnosis, several tests can be used to assess DM to detect neurological and neuropsychological deficits in both brain-damaged and apparently healthy patients. Some of the most widely used tests are the Balloon Gambling Task,59 risky decision making tasks,60 the IGT, and the Columbia Card Task.61–63 In addition to these, the Wisconsin Card Sorting Test (WCST)64–67 and the Risky Choice Task68 may also be used when appropriate.
We should highlight the IGT, a tool with a high sensitivity for detecting damage in prefrontal and subcortical areas involved in DM.17,43 This tool has detected DM difficulties in schizophrenia,69,70 attention deficit hyperactivity disorder,71 impulsive aggressive disorders,72 and Huntington disease,73 among others. Another advantage of the IGT is that it has a version specifically designed for children; this version, which is called the Hungry Donkey Task, is similar to the IGT but adjusted to the psychological and neuropsychological features of children.74 Finally, the IGT is known for being the DM evaluation tool which enjoys the most empirical support through the use of neuroimaging techniques, neurophysiological tools, and behaviour rating scales.75
ConclusionsData collected up to the present time has shown the importance of cortical and subcortical structures in DM. The essential neocortical structures in the adaptive functioning of this process are the OFC, the ACC, and the DLPFC. The subcortical regions most involved in DM are the ventral striatum, basal ganglia, amygdala, and cerebellum. From a functional viewpoint, the existence of a specific circuit in decision situations under uncertainty has been demonstrated. Detected interconnections involve the dorsolateral prefrontal cortex (related to working memory), the insula and the posterior cingulate cortex (associated with representation of mood states), the ventromedial prefrontal/medial orbitofrontal cortex (integration of the previously described processes), and the ventral striatum and the anterior cingulate cortex (involved in the implementation of decision behaviours).
Damage to any region of the neural network would affect decision-making, making it difficult for the body to adapt to its everyday context and preventing healthy functioning.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Broche-Pérez Y, Herrera Jiménez LF, Omar-Martínez E. Bases neurales de la toma de decisiones. Neurología. 2016;31:319–325.