The hemiplegic shoulder pain is common after a stroke. Its appearance brings pain and limits daily living activities as well as participation in specific Neuro-rehabilitation programs. All this leads to a worse functional outcome. Good management of patients can reduce both the frequency and intensity of shoulder pain, improving functional outcome.
DevelopmentWe conducted a literature search of various databases between 1980 and 2008. The articles were evaluated using the PEDro scoring system. Five evidence levels were established for the conclusions.
ConclusionsShoulder subluxation occurs at an early stage after stroke and is associated with subluxation of the shoulder joint and spasticity (mainly subscapularis and pectoralis). Slings prevent subluxation of the shoulder. It is preferable to move within a lower range of motion and without aggression to prevent the occurrence of shoulder pain. The injection of corticosteroids does not improve pain and range of motion in hemiplegic patients, while botulinum toxin combined with physical therapy appears to reduce hemiplegic shoulder pain.
El hombro doloroso hemipléjico es frecuente después de un ictus. Su aparición conlleva además del dolor, una limitación para las actividades de la vida diaria, así como para la participación en programas específicos de neurorrehabilitación. Todo este conjunto determina un peor resultado funcional. El buen manejo de los pacientes puede reducir tanto la frecuencia de aparición de hombro doloroso, como la intensidad del mismo, mejorando así el pronóstico funcional.
DesarrolloEntre los años 1980 y 2008 se llevó a cabo una búsqueda de la literatura en diferentes bases de datos. La evaluación de los artículos se realizó con el sistema de puntuación PEDro. Se establecieron 5 niveles de evidencia para obtener las conclusiones.
ConclusionesLa subluxación del hombro, ocurre de manera precoz tras el ictus y se asocia con subluxación de la articulación del hombro y con espasticidad (subescapular y pectoral mayor principalmente). Los cabestrillos previenen la subluxación del hombro. Es preferible realizar movimientos con un menor rango de movimiento y sin agresividad, para evitar la aparición del hombro doloroso. La inyección de corticoides no mejora el dolor ni el rango de movimiento de los pacientes hemipléjicos, mientras que la toxina botulínica combinada con fisioterapia parece reducir el dolor del hombro hemipléjico.
Cerebrovascular diseases are so prevalent that their impact, in economic, social and healthcare terms, is considerable. In the last few decades, there have been initiatives to promote treatment during the acute phase of a stroke1 and progress has been made toward understanding stroke physiopathology. These advances have improved treatment options, which in turn improves patient prognoses. Despite the considerable strides which have been made, stroke is still the leading cause of disability in developed countries. For this reason, measures designed to lessen the dependency of stroke patients should be under the control of the doctors involved in caring for those patients. Today, there can be no question that acute phase stroke is best managed by hospital stroke units coordinated by neurologists,2 and that professionals in these units should know how to prevent and manage the complications which may arise.
Painful hemiplegic shoulder (PHS) secondary to stroke is a common clinical entity; depending on the study cited, incidence rates range from 34%3 to 84%4 (53% in our geographical area5). Onset may in some cases be early, meaning within the first 2 weeks after the stroke; however, the condition typically presents after 2–3 months.6 PHS decreases a patient's ability to undertake functional and rehabilitation activities,7 and it is associated with a lower score on the Barthel score after discharge of the patient.8 It is a predictor of poor functional recovery of the arm and longer hospital stay, and the percentage of patients with PHS able to return to their own homes is lower.9
Factors that may contribute to the appearance of PHS can be categorised as those having to do with the shoulder joint itself (rotator cuff injury10 or subluxation of the humeral head11) and those related to a neurological disorder (lack of sensation, initial flaccid paralysis, hemispatial neglect and spasticity12,13).
Proper management of PHS in stroke patients will allow them to participate more fully in the neuro-rehabilitation process, and may therefore result in a better functional outcome. Neurologists, as the doctors responsible for stroke units, must be active members of the neuro-rehabilitation team,14,15 foreseeing any complications (PHS and others) that may delay recovery and treating them when they do appear.
Our objective is to undertake a review of medical literature on the subject of painful hemiplegic shoulder (excluding reflex sympathetic dystrophy) in order to present current research on the causes and treatment of PHS.
ProcedureUsing a variety of databases (CINAHL, EMBASE, MEDLINE and PSYCHINFO), we performed a literature search for the years 1980–2008. Published studies evaluating PHS aetiology and treatment were then selected. Studies cited in review articles, meta-analyses or systematic reviews and which were not detected by our original literature search were also included. Evidence for each intervention was evaluated on an individual basis.
Articles were evaluated using the PEDro scoring system (Physiotherapy Evidence Database, available at http://www.pedro.org.au) for randomised clinical trials (RCT). This tool is a system for evaluating a study's methodology which assigns a score between 1 and 10 (10 being the best); two different reviewers assessed each article, and resolved any discrepancies by reaching a consensus. Levels of evidence were determined according to the Eastern Ontario/Queen's Evidence Based Report, which is based on levels of evidence used by the United States Agency for Health Care Policy and Research (AHCPR) Guidelines for Stroke Rehabilitation. Five levels of evidence were established.
- •
Strong evidence (Level 1a): findings are supported by the results of 2 or more RCTs of at least fair quality (“fair quality” indicates a PEDro score ≥4).
- •
Moderate evidence (Level 1b): findings are supported by a single RCT of at least “fair” quality.
- •
Limited evidence (Level 2): findings are supported by at least 1 experimental study (prospective and retrospective controlled trials, single group interventions, etc.).
- •
Consensus (Level 3): in the absence of evidence, agreement of a group of experts on the appropriate treatment course. Consensus opinion is regarded as the lowest form of evidence.
- •
Conflicting (Level 4): disagreement between the findings of at least 2 RCTs, or when no RCT is available, or when 2 non-RCT studies are in disagreement. When there were more than 4 RCTs and results from only one were conflicting, the conclusion was based on the results of the majority of the studies, unless the study with conflicting results was of better quality.
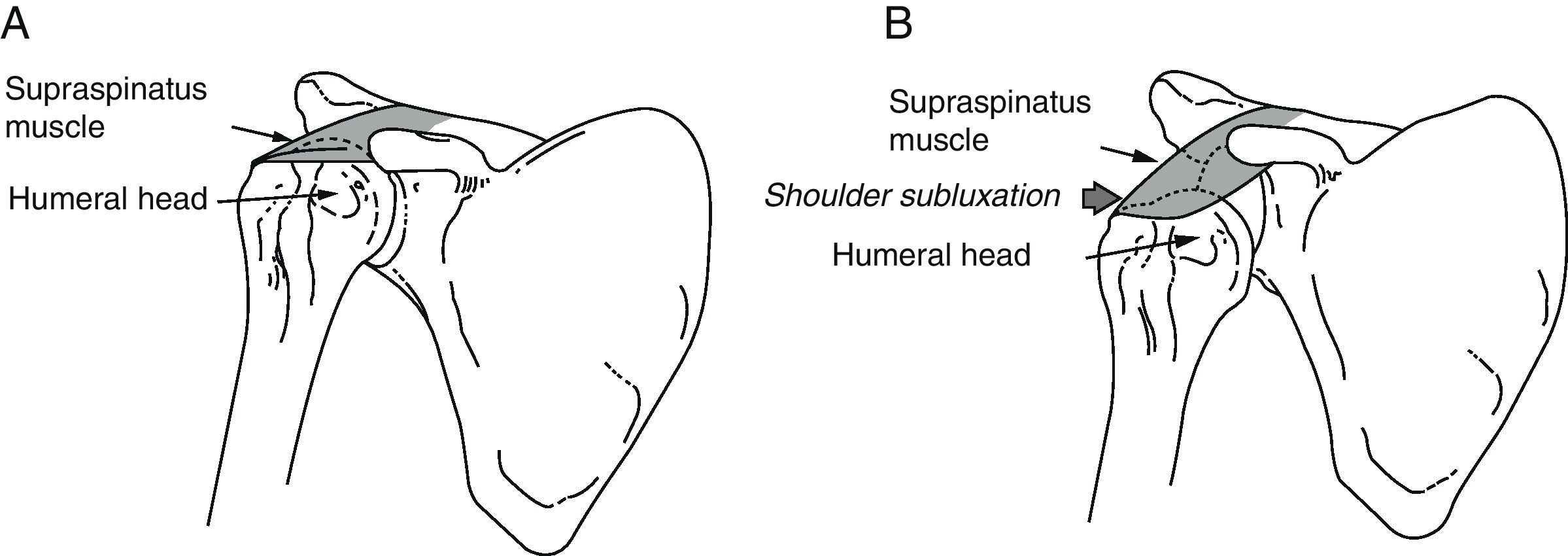
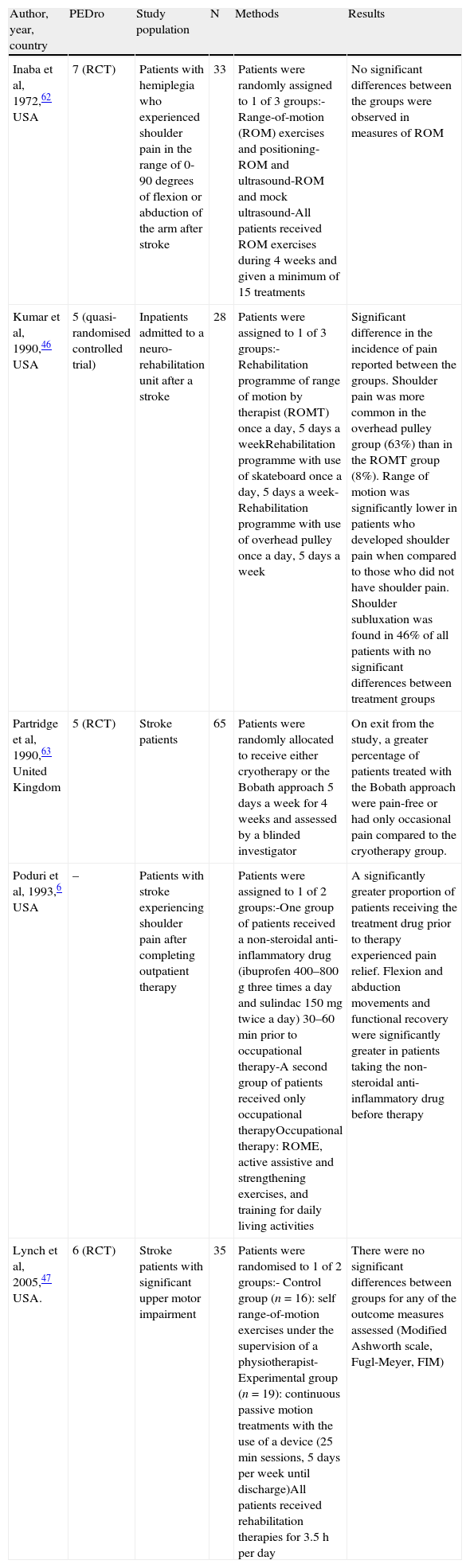
Shoulder subluxation occurs when the mechanical integrity of the glenohumeral joint is compromised, resulting in a palpable gap between the acromion and the humeral head (Fig. 1).
(A) Normal shoulder: the supraspinatus muscle keeps the humeral head inside the glenoid cavity. (B) Shoulder subluxation: during the initial phase of hemiplegia, the supraspinatus is flaccid. The weight of the arm can cause subluxation of the humeral head toward the inferior rim of the glenoid cavity.
The glenohumeral joint is multiaxial and has a wider range of motion than other joints. In order to achieve such a range of motion, the glenohumeral joint has to relinquish a more stable bone structure, and this lack is compensated by muscular stability. For this reason, a change in normal muscular function (as occurs after a stroke with motor sequelae) presents a potential risk for subluxation.
During the initial period following a stroke, the hemiplegic arm is flaccid or hypotonic. This is why the shoulder muscles are unable to anchor the humeral head within the glenoid cavity, resulting in a high risk of shoulder subluxation. During this period, the affected extremity should be properly supported; the weight of the arm itself may be enough to cause subluxation. Glenohumeral subluxation may also occur as a result of adopting incorrect sleeping postures, lack of support when the patient is in a vertical position, or tension on the hemiplegic arm when the patient is being moved from one place to another.
Results from clinical research have been largely contradictory up until now. Some studies point to a link between subluxation and PHS,9 while others refute it.16 Small sample sizes and differences in evaluation methodology have made this subject difficult to study.17
Shoulder subluxation is associated with pain.18,19 However, not all hemiplegic patients with subluxation suffer from shoulder pain, and the hypothesis that subluxation is the cause of pain in a hemiplegic shoulder remains controversial.17,18,20,21 Caillet21 states that during the flaccid stage, the scapula adopts an inferior, rotated position, since the serratus anterior muscle is paretic and the upper part of the trapezius muscle no longer supports the scapula. It is thought that the combination of flaccid supporting muscles and the inferior position of the scapula are factors that predispose subluxation of the humeral head toward the lower rim of the glenoid cavity. However, Prevost et al22 concluded that the position of the scapula is not an important factor in inferior subluxation in hemiplegic patients. Also, Price et al23 observed that subluxation in patients who have suffered cerebral infarctions is not related to the resting position of the scapula.
Spasticity and contracturesSpasticity is defined as a velocity-dependent increase in muscle tone, associated with a hyperactive stretch reflex. This symptom is part of upper motor neuron syndrome.24 Under normal circumstances, muscular balance is maintained between the different muscle pairs (agonists–antagonists). Following a stroke, muscular balance may be altered as muscle groups affected by spasticity become dominant. This produces the typical posture that reflects a spastic muscle pattern. Flexor tone is dominant in the upper limbs, resulting in retraction and depression of the scapula, in addition to internal rotation and adduction of the shoulder (subscapularis, pectoralis major, teres major and latissimus dorsi). The subscapularis and pectoralis muscles are the ones most involved in this process.25
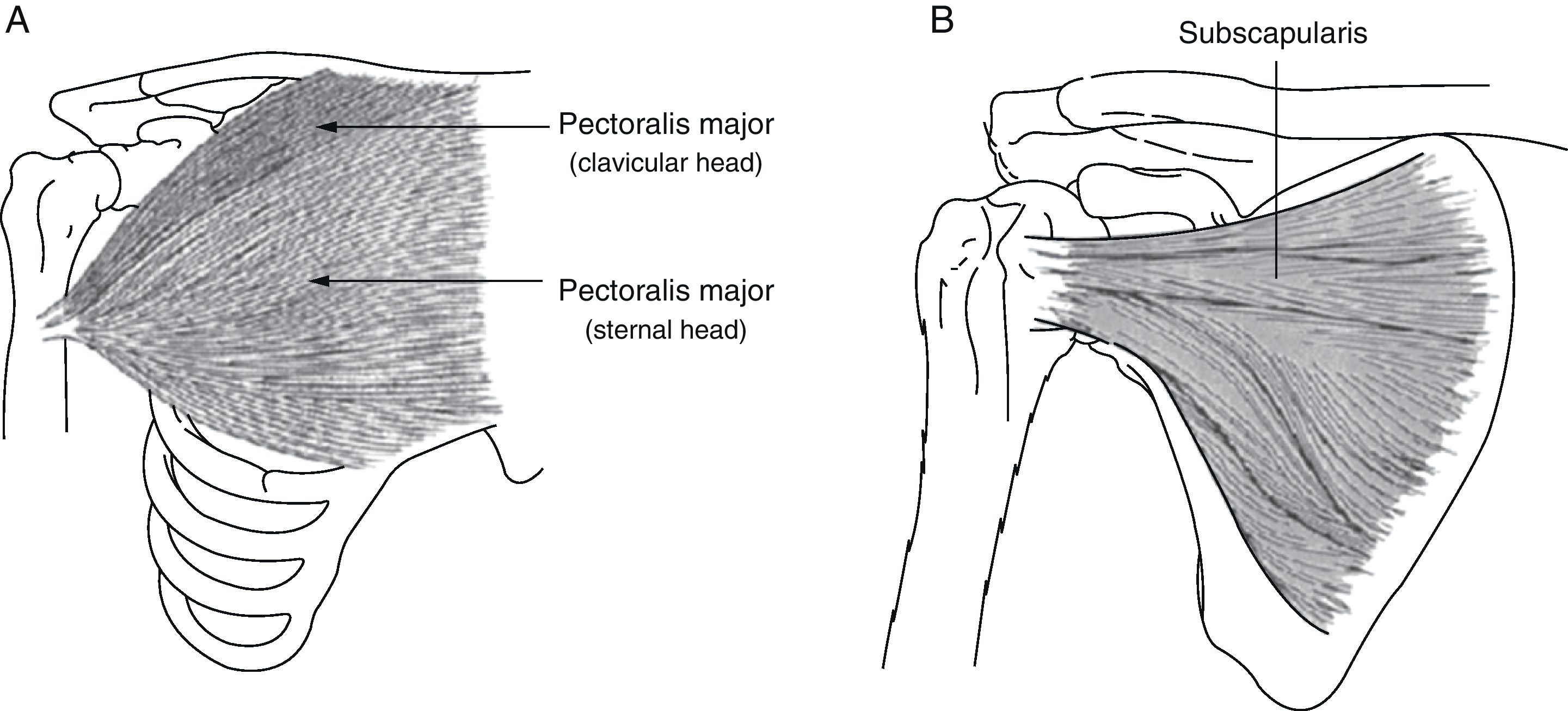
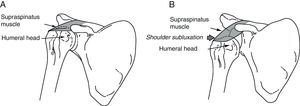
The subscapularis is one of the shoulder's internal rotators, and it also contributes to arm abduction and extension from a flexed position (Fig. 2). Subscapularis muscle spasticity limits abduction, flexion and external rotation. Zorowitz et al16 state that limitation of external rotation in the hemiplegic shoulder is the factor most closely linked to PHS, and Hecht26 attributes this problem specifically to the subscapularis.
(A) Pectoralis major: the function of the pectoralis major is to adduct, internally rotate and flex the arm. (B) Subscapularis: this muscle is one of the main internal rotators of the shoulder. As part of the synergistic flexor group in hemiplegic spasticity, the subscapularis is tonically active, which limits not only external rotation, but also shoulder abduction and flexion.
The pectoralis major performs flexion, adduction and internal rotation of the arm (Fig. 2); this muscle is important when abduction is more limited than external rotation.26
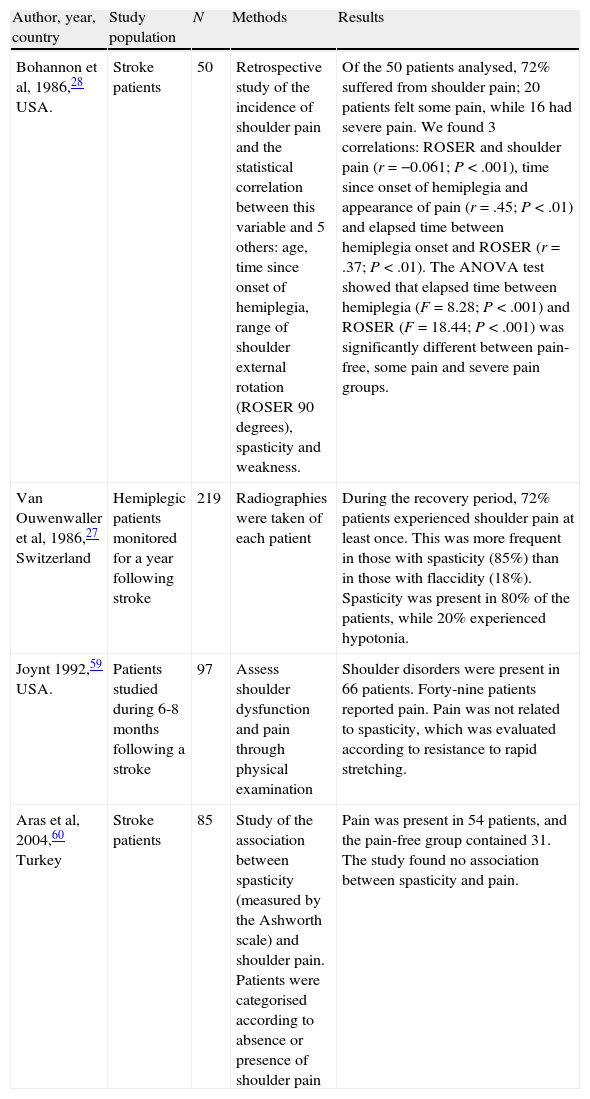
Van Ouwenaller et al27 identify spasticity as the main factor and the one most commonly involved in the onset of PHS. This argument is based on the possibility that musculoskeletal alterations occurring after the stroke are associated with PHS. In fact, PHS incidence is higher than normal among patients with spasticity, regardless of the intrinsic pathology affecting the shoulder.17 Poulin et al4 found that patients with PHS show significantly more spasticity in the affected limb than in limbs that are pain-free. Taking a dissenting view, Bohannon et al28 state that spasticity is not related to shoulder pain (Table 1).
Spastic and painful hemiplegic shoulder.
| Author, year, country | Study population | N | Methods | Results |
| Bohannon et al, 1986,28 USA. | Stroke patients | 50 | Retrospective study of the incidence of shoulder pain and the statistical correlation between this variable and 5 others: age, time since onset of hemiplegia, range of shoulder external rotation (ROSER 90 degrees), spasticity and weakness. | Of the 50 patients analysed, 72% suffered from shoulder pain; 20 patients felt some pain, while 16 had severe pain. We found 3 correlations: ROSER and shoulder pain (r=−0.061; P<.001), time since onset of hemiplegia and appearance of pain (r=.45; P<.01) and elapsed time between hemiplegia onset and ROSER (r=.37; P<.01). The ANOVA test showed that elapsed time between hemiplegia (F=8.28; P<.001) and ROSER (F=18.44; P<.001) was significantly different between pain-free, some pain and severe pain groups. |
| Van Ouwenwaller et al, 1986,27 Switzerland | Hemiplegic patients monitored for a year following stroke | 219 | Radiographies were taken of each patient | During the recovery period, 72% patients experienced shoulder pain at least once. This was more frequent in those with spasticity (85%) than in those with flaccidity (18%). Spasticity was present in 80% of the patients, while 20% experienced hypotonia. |
| Joynt 1992,59 USA. | Patients studied during 6-8 months following a stroke | 97 | Assess shoulder dysfunction and pain through physical examination | Shoulder disorders were present in 66 patients. Forty-nine patients reported pain. Pain was not related to spasticity, which was evaluated according to resistance to rapid stretching. |
| Aras et al, 2004,60 Turkey | Stroke patients | 85 | Study of the association between spasticity (measured by the Ashworth scale) and shoulder pain. Patients were categorised according to absence or presence of shoulder pain | Pain was present in 54 patients, and the pain-free group contained 31. The study found no association between spasticity and pain. |
Frozen shoulder or shoulder contracture (adhesive capsulitis) is characterised by presenting limited range of movement. It may arise as a result of immobilisation and atrophy due to disuse, and these conditions are present in stroke patients with residual hemiplegia. It is frequently associated with in cases of spastic hemiplegic shoulder.28–30
Rotator cuff abnormalitiesThe rotator cuff is the term for all of the tendons pertaining to a group of 4 muscles: the subscapularis, which rotates the arm inwardly; the supraspinatus, which raises the arm and separates it from the trunk; the infraspinatus, which aids in raising the arm during external rotation; and the teres minor, which also rotates the arm externally. This muscle group is frequently injured; typical injuries include strains, tendinitis, impingement, bursitis and sprains. The supraspinatus is the most commonly affected muscle. Its tendon runs below the bone and is susceptible to compression by the acromion. Degenerative changes are common in rotator cuff muscles, and they may contribute to PHS. The incidence of rotator cuff strain in hemiplegic patients is between 33%31 and 40%,32 while this percentage in the general population ranges from 20% to 40%.33
In the general population, shoulder pain is very often associated with abnormalities in this muscle group. It is therefore not surprising that a certain number of patients with PHS would also be affected.
In the early flaccid phase following the stroke, tissues in the area of the glenohumeral joint are especially susceptible to trauma due to traction in the joint, incorrect passive movements and the effect of gravity,34 all of which can contribute to muscle strain.17 That being said, Rizk et al35 were not able to document higher incidence of strain among PHS patients.
Managing painful shoulder in hemiplegic patientsAn optimal treatment approach has not yet been established, and this is due in part to lack of consensus regarding pain aetiology. As a result, the literature cites a wide range of treatments with varying degrees of success.36 Since PHS treatment is complex, preventive measures should be taken immediately after the stroke. This normally falls to the neurologist in the stroke unit who is responsible for the patient. Early passive movement and providing support and protection for the shoulder during its flaccid phase are considered important in order to minimise the risk of PHS.25
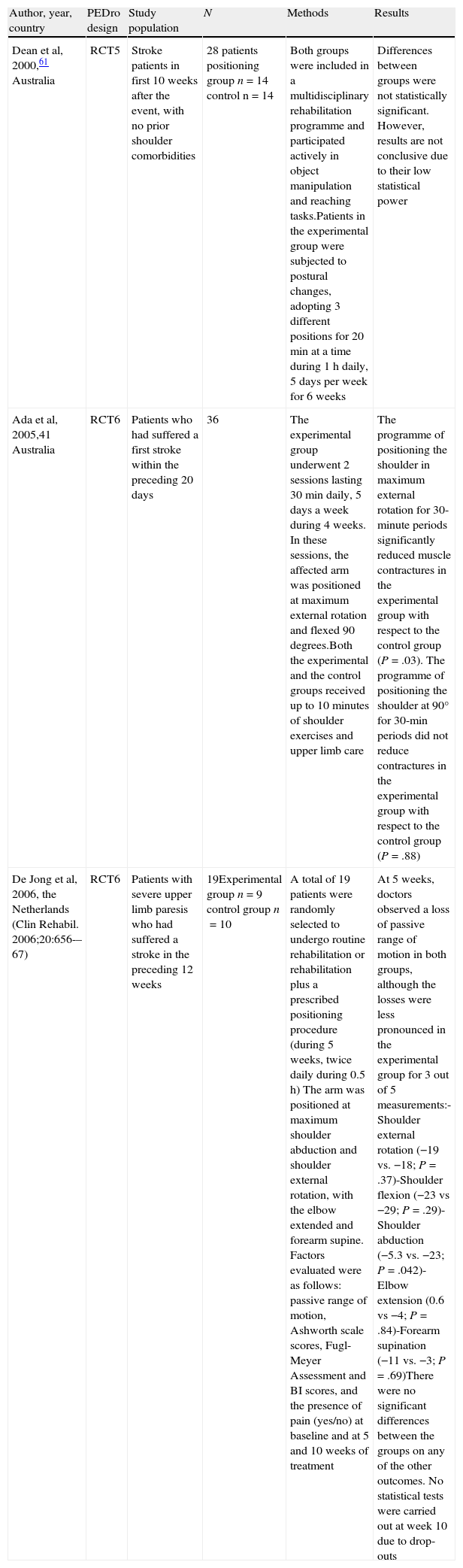
Positioning hemiplegic shoulderMaintaining the upper limb in the correct position is fundamental to treating PHS.37 Careful positioning of the shoulder serves to minimise subluxation and eventually, muscle contractures as well. Poor postures may have a negative effect on symmetry, balance and body image. Gilmore et al38 suggest that careful and correct arm positioning can prevent PHS (Table 2).
Posture indicative of shoulder pain in stroke patients.
| Author, year, country | PEDro design | Study population | N | Methods | Results |
| Dean et al, 2000,61 Australia | RCT5 | Stroke patients in first 10 weeks after the event, with no prior shoulder comorbidities | 28 patients positioning group n=14 control n=14 | Both groups were included in a multidisciplinary rehabilitation programme and participated actively in object manipulation and reaching tasks.Patients in the experimental group were subjected to postural changes, adopting 3 different positions for 20min at a time during 1h daily, 5 days per week for 6 weeks | Differences between groups were not statistically significant. However, results are not conclusive due to their low statistical power |
| Ada et al, 2005,41 Australia | RCT6 | Patients who had suffered a first stroke within the preceding 20 days | 36 | The experimental group underwent 2 sessions lasting 30min daily, 5 days a week during 4 weeks. In these sessions, the affected arm was positioned at maximum external rotation and flexed 90 degrees.Both the experimental and the control groups received up to 10minutes of shoulder exercises and upper limb care | The programme of positioning the shoulder in maximum external rotation for 30-minute periods significantly reduced muscle contractures in the experimental group with respect to the control group (P=.03). The programme of positioning the shoulder at 90° for 30-min periods did not reduce contractures in the experimental group with respect to the control group (P=.88) |
| De Jong et al, 2006, the Netherlands (Clin Rehabil. 2006;20:656-–67) | RCT6 | Patients with severe upper limb paresis who had suffered a stroke in the preceding 12 weeks | 19Experimental group n=9 control group n=10 | A total of 19 patients were randomly selected to undergo routine rehabilitation or rehabilitation plus a prescribed positioning procedure (during 5 weeks, twice daily during 0.5h) The arm was positioned at maximum shoulder abduction and shoulder external rotation, with the elbow extended and forearm supine. Factors evaluated were as follows: passive range of motion, Ashworth scale scores, Fugl-Meyer Assessment and BI scores, and the presence of pain (yes/no) at baseline and at 5 and 10 weeks of treatment | At 5 weeks, doctors observed a loss of passive range of motion in both groups, although the losses were less pronounced in the experimental group for 3 out of 5 measurements:-Shoulder external rotation (−19 vs. −18; P=.37)-Shoulder flexion (−23 vs −29; P=.29)-Shoulder abduction (−5.3 vs. −23; P=.042)-Elbow extension (0.6 vs −4; P=.84)-Forearm supination (−11 vs. −3; P=.69)There were no significant differences between the groups on any of the other outcomes. No statistical tests were carried out at week 10 due to drop-outs |
The recommended position for the affected upper limb is abducted, rotated externally and with the shoulder flexed. However, when we reviewed the most popular theories, we found no consensus as to the proper position.39
Slings and other devicesSlings reduce the effect which gravity has on the glenohumeral joint.40 They are often used in early stages following a stroke. Use of slings is controversial because they hold the arm in a flexed position, inhibit shoulder movement, favour the appearance of contractures, and discourage use of the affected arm. However, slings are considered to be the best devices for supporting the paretic limb while the patient is standing or being moved. In their systematic review, Ada et al41 conclude that evidence is insufficient to guarantee that these devices reduce or prevent shoulder subluxation after a cerebrovascular accident. It is not yet clear which type of sling provides the best shoulder support. In recent years, different types of sling have been designed in order to improve the anatomical alignment of the joint, with satisfactory results.42 What we do know is that as tone returns to the dorsal muscles, the risk of shoulder subluxation decreases and use of slings and similar devices may be discontinued.
Strapping the hemiplegic shoulderStrapping the hemiplegic shoulder is used as a method for preventing or reducing shoulder subluxation and may provide a certain level of sensory stimulation. It is often used in combination with other techniques to treat subluxation and shoulder pain. Strapping must be applied by an experienced professional, and reapplied periodically since it can irritate the skin.43
The literature describes 3 different ways of strapping the hemiplegic shoulder.43–45 In any case, it is unclear whether or not strapping reduces pain in PHS.
Physical therapyThe association between spasticity, muscle imbalance and painful frozen shoulder suggests that a treatment approach designed to improve range of motion for a hemiplegic shoulder should lessen pain. However, Kumar et al46 state that aggressive exercises with a wide range of motion provoke much more intense pain than that experienced when doing exercises with a more limited range of motion. Lynch et al47 and Gustafsson and McKenna48 suggest that while active exercises are preferable to passive ones, exaggeratedly aggressive programmes may result in a higher incidence of PHS compared with more moderate exercise programmes (Table 3).
Physical therapy for a hemiplegic shoulder.
| Author, year, country | PEDro | Study population | N | Methods | Results |
| Inaba et al, 1972,62 USA | 7 (RCT) | Patients with hemiplegia who experienced shoulder pain in the range of 0-90 degrees of flexion or abduction of the arm after stroke | 33 | Patients were randomly assigned to 1 of 3 groups:-Range-of-motion (ROM) exercises and positioning-ROM and ultrasound-ROM and mock ultrasound-All patients received ROM exercises during 4 weeks and given a minimum of 15 treatments | No significant differences between the groups were observed in measures of ROM |
| Kumar et al, 1990,46 USA | 5 (quasi-randomised controlled trial) | Inpatients admitted to a neuro-rehabilitation unit after a stroke | 28 | Patients were assigned to 1 of 3 groups:-Rehabilitation programme of range of motion by therapist (ROMT) once a day, 5 days a weekRehabilitation programme with use of skateboard once a day, 5 days a week-Rehabilitation programme with use of overhead pulley once a day, 5 days a week | Significant difference in the incidence of pain reported between the groups. Shoulder pain was more common in the overhead pulley group (63%) than in the ROMT group (8%). Range of motion was significantly lower in patients who developed shoulder pain when compared to those who did not have shoulder pain. Shoulder subluxation was found in 46% of all patients with no significant differences between treatment groups |
| Partridge et al, 1990,63 United Kingdom | 5 (RCT) | Stroke patients | 65 | Patients were randomly allocated to receive either cryotherapy or the Bobath approach 5 days a week for 4 weeks and assessed by a blinded investigator | On exit from the study, a greater percentage of patients treated with the Bobath approach were pain-free or had only occasional pain compared to the cryotherapy group. |
| Poduri et al, 1993,6 USA | – | Patients with stroke experiencing shoulder pain after completing outpatient therapy | Patients were assigned to 1 of 2 groups:-One group of patients received a non-steroidal anti-inflammatory drug (ibuprofen 400–800g three times a day and sulindac 150mg twice a day) 30–60min prior to occupational therapy-A second group of patients received only occupational therapyOccupational therapy: ROME, active assistive and strengthening exercises, and training for daily living activities | A significantly greater proportion of patients receiving the treatment drug prior to therapy experienced pain relief. Flexion and abduction movements and functional recovery were significantly greater in patients taking the non-steroidal anti-inflammatory drug before therapy | |
| Lynch et al, 2005,47 USA. | 6 (RCT) | Stroke patients with significant upper motor impairment | 35 | Patients were randomised to 1 of 2 groups:- Control group (n=16): self range-of-motion exercises under the supervision of a physiotherapist-Experimental group (n=19): continuous passive motion treatments with the use of a device (25min sessions, 5 days per week until discharge)All patients received rehabilitation therapies for 3.5h per day | There were no significant differences between groups for any of the outcome measures assessed (Modified Ashworth scale, Fugl-Meyer, FIM) |
Electrical neuromuscular stimulation consists of superficial application of electrical current, causing muscle contraction and increased muscle recruitment. The two most commonly used methods are functional electrical stimulation (FES) and transcutaneous electrical nerve stimulation (TENS). The second technique uses a lower intensity and higher frequency than the first. The supraspinatus and deltoid muscles are the most commonly treated for PHS.49 Treatment should be administered 6h per day, 5 days per week for a duration of 6 weeks, at frequencies of 35–50Hz.49 In 11 studies offering a specific assessment of the effects of electrical stimulation as treatment for shoulder pain, the majority recorded an improvement. The results suggest that ENS associated with conventional treatment may effectively reduce pain in the affected shoulder and improve upper limb function.23
However, results from the largest trial with the most rigorous methodology suggest that ENS treatment may in fact be associated with deterioration of arm function, especially in patients with severe paralysis. Further studies are needed in order to examine the effects of functional electrical stimulation in PHS.
InjectionsIntra-articular injections of steroids, intramuscular botulinum toxin injections and other agents have been used to treat muscle spasticity, correct imbalances and alleviate PHS.
We found 4 randomised controlled trials, all of good quality, which studied the efficacy of botulinum toxin in treating PHS (as monotherapy or combined with TENS). The subscapularis muscle was the most common injection site. Two of these trials51,52 reported that treatment provided pain relief, while the others53,54 found no differences. Snels et al55 observed that intra-articular injections with steroids produced no improvement, whether in terms of pain or passive movement of the PHS.
One trial compared triamcinolone acetonide with botulinum toxin, but results were hard to interpret since subjects in both arms improved.56 Despite differences not being statistically significant, the authors of this trial suggest that results of botulinum toxin treatment are better and that its effects are longer-lasting.
It seems obvious, however, that complementing botulinum toxin type A injections with physical therapy is necessary in order to produce the desired results,57 especially if the goal is to achieve functional mobility.
Other approachesSurgerySince muscle spasticity has been identified as a cause of PHS, treatment designed to correct this imbalance may be able to relieve the pain. Braun et al7 resected the tendons of the subscapularis and pectoralis muscles, and patients took part in an intensive physical therapy programme during the postoperative period which lessened pain and increased range of movement in stroke patients with PHS. However, 6 months later, patients once again experienced pain and discomfort.
AromatherapyThe use of aromatherapy acupressure in PHS treatment was investigated in a single randomised trial,58 which found a significantly higher level of pain reduction in the aromatherapy group. The authors hypothesised that the decrease in shoulder pain might result from an enhancement of the parasympathetic response through the effects of smell and touch that promote relaxation, and which had already been shown to modify pain perception.
ConclusionsShoulder pain after a stroke is a common complication which neurologists must prevent and treat. Proper treatment will have an effect on the stroke patient's future functional state.
An exhaustive review of PHS revealed the following evidence:
Evidence regarding causes of PHS- •
Shoulder subluxation tends to occur soon after a stroke.
- •
Painful hemiplegic shoulder is associated with subluxation of the shoulder joint and spasticity, but not with scapular rotation.
- •
It seems that the subscapularis and pectoralis major play important roles, since they engage in more tonic activity. This creates a muscular imbalance in the shoulder.
- •
The appearance of PHS is associated with a poor functional outcome.
- •
There is a moderate level of evidence (Level 1b) suggesting that prolonged positioning does not negatively influence range of motion or pain.
- •
Limited evidence (Level 2) suggests that slings may prevent subluxation associated with painful hemiplegic shoulder. There is also limited evidence (Level 2) suggesting that one device or method may be better than another.
- •
There is conflicting evidence (Level 4) that strapping the hemiplegic shoulder reduces the development of pain. Moderate evidence (Level 1b) suggests that strapping a hemiplegic shoulder does not improve its functional range of motion.
- •
There is moderate evidence (Level 1b) suggesting that vigorous exercise with overhead pulleys causes intense pain and should be avoided. There is moderate evidence (Level 1b) that within a neuro-rehabilitation programme, gentle exercises guided by a physiotherapist reduce pain in the hemiplegic shoulder. There is conflicting evidence (Level 4) as to whether or not electrical stimulation reduces pain, improves function and treats post-stroke shoulder subluxation.
- •
Moderate evidence (Level 1b) suggests that corticosteroid injections do not improve either pain or range of motion in hemiplegic patients. There is limited evidence (Level 2) suggesting that botulinum toxin reduces pain in the hemiplegic shoulder. There is conflicting evidence (Level 4) that botulinum toxin injected into the subscapularis muscle reduces pain in the spastic shoulder and improves passive range of motion. Moderate evidence (Level 1b) suggests that intra-articular steroid injections do not improve either pain or passive range of motion in cases of hemiplegic shoulder.
- •
Despite there being evidence-based findings, PHS is a poorly understood entity. More studies will be needed in order to research and assess its cause or causes and determine which specific treatment plans are necessary.
The authors have no conflicts of interest to declare.
Please cite this article as: Murie-Fernández M, et al. Hombro doloroso hemipléjico en pacientes con ictus: causas y manejo. Neurología. 2012; 27:234–44.