Increases in brain β-amyloid protein (Aβ) levels have been demonstrated in animal models following oral inoculation of periodontopathogens or their enzyme gingipain. We investigated the association between periodontitis and brain Aβ protein levels in mild cognitive impairment (MCI).
MethodsAn observational study was designed. All participants underwent a periodontal examination and an amyloid-PET scan. Subsequently, the following groups were established: MCI and suspected Alzheimer disease (AD) (MCI/Aβ+ group) (n=45); MCI and suspected non-AD pathology (MCI/Aβ– group) (n=59); cognitively healthy elderly individuals with negative PET-amyloid scan results (non-MCI/Aβ– group) (n=60).
ResultsPatients with moderate-severe periodontitis had a higher risk of abnormal accumulation of Aβ in the brain, with an odds ratio (OR) of 3.30 (95% confidence interval [CI], 1.30–8.26) when comparing patients from the MCI/Aβ+ and MCI/Aβ– groups, and an OR of 4.94 (95% CI, 1.65–14.84) when comparing the MCI/Aβ+ group against the non-MCI/Aβ– group.
ConclusionsOur findings suggest that periodontal disease may be associated with anomalous accumulation of cerebral Aβ protein in older people, independently of cognitive impairment.
Se ha demostrado un aumento en los niveles cerebrales de la proteína β-amiloide (Aβ) en modelos animales tras la inoculación oral de patógenos periodontales o su enzima gingipaína. Investigamos la asociación entre periodontitis y niveles cerebrales de la proteína Aβ en el deterioro cognitivo leve (DCL).
MétodosDiseñamos un estudio observacional. Todos los participantes se sometieron a un examen periodontal y a un estudio PET amiloide, tras lo cual establecimos los siguientes grupos: DCL y sospecha de enfermedad de Alzheimer (EA) (grupo DCL/Aβ+; n=45); DCL y sospecha de enfermedad no Alzheimer (grupo DCL/Aβ–; n=59); individuos de edad avanzada cognitivamente sanos con resultados negativos en PET amiloide (grupo no-DCL/Aβ–; n=60).
ResultadosLos pacientes con periodontitis moderada/grave presentaban un riesgo mayor de acumulación de Aβ en el cerebro, con una odds ratio (OR) de 3,30 (intervalo de confianza del 95% [IC 95%]: 1,30-8,26) al comparar los pacientes de los grupos DCL/Aβ+ y DCL/Aβ–, y una OR de 4,94 (IC 95%: 1,65-14,84) al comparar el grupo DCL/Aβ+ con el grupo no-DCL/Aβ–.
ConclusionesNuestros resultados sugieren que la enfermedad periodontal puede estar relacionada con una acumulación de proteína Aβ en el cerebro de las personas de edad avanzada, independientemente del deterioro cognitivo.
Dementia, and Alzheimer disease (AD) in particular, currently represents a public health problem with high economic and social costs for the population and no short-term solutions.1 Current treatment strategies require understanding of the underlying mechanisms involved in its pathogenesis. Recent studies have recognized the involvement of certain key biological processes occurring years before the onset of the disease symptoms, such as the abnormal accumulation of β-amyloid (Aβ) and Tau proteins, and neuroinflammation resulting from microglial activation.2 These processes contribute to neuronal damage, particularly under circumstances such as advanced age, neuroinflammation, blood vessel alterations, and certain genetic variants (e.g., carriers of the APOE ɛ4 allele).3 However, further research is needed to elucidate at what point a brain damaged by an abnormal accumulation of Aβ protein has an impact on patients’ clinical signs and symptoms, both in early stages of mild cognitive impairment (MCI) and later in AD.3
In recent years, periodontal disease (PD), and more specifically periodontitis, has emerged as a possible risk factor for AD.4 Periodontitis is a local infectious condition with the potential to induce a chronic inflammatory state and an inappropriate immune response. Periodontopathogens and their virulence factors (e.g., lipopolysaccharide, proteases, fimbriae) can travel across the blood–brain barrier.5 A considerable body of scientific evidence supports the link between periodontitis and AD, and between periodontitis and cognitive impairment, based on both cross-sectional and prospective studies6–8 and studies that analyze biomarkers of inflammation9 or the periodontal microbiota.10 However, despite the fact that periodontal diseases could be a biologically plausible risk factor for AD, the strength of the available evidence is insufficient to confirm causality.11
The biological mechanism that supports the relationship between periodontitis and the inflammatory brain response to periodontopathogenic flora is not well understood.4,12 Three etiological theories have been proposed to explain this relationship: the Aβ hypothesis, the microbiome-infection hypothesis, and the inflammation–host response hypothesis.11 The initiation and progression of AD probably involves interactions between all 3 of these mechanisms.4 However, this study will focus on the possible role of periodontitis and amyloid pathology in the brain due to its critical role in AD diagnosis.
Studies of wild-type and genetically susceptible mice report that oral inoculation of a periodontal pathogen (i.e., Porphyromonas gingivalis [PG]) or its enzyme (gingipain) is followed by increases in Aβ, Tau protein tangles, and inflammatory brain mediators, microglial activation and proliferation, activation of amyloid precursor-cleaving enzymes, neurodegeneration, and loss of synaptic connections.13–16 However, there is very little evidence of the potential role of periodontitis on the accumulation of Aβ in the human brain. Only 2 studies in humans have explored this association in cognitively normal elderly adults.17,18 Whereas the study by Kamer et al.17 found an association between periodontal disease and Aβ load, Adam et al.18 concluded that elevated Aβ accumulation was not associated with baseline PD status. Therefore, it is difficult to draw conclusions from these previous studies as a result of their contradictory findings. In addition, these studies were solely focused on cognitively healthy elderly adults, who may or may not progress to AD. This demonstrates the need for a better understanding of the association between PD and brain Aβ accumulation in participants with and without MCI, due to the potential for MCI to progress to AD. To the best of our knowledge, no previous study has addressed this association in patients with MCI.
Based on the hypothesis that chronic periodontitis is involved in the pathological process that culminates in AD onset, our study attempted to determine whether there is an association between periodontitis and brain Aβ protein accumulation in elderly patients with cognitive impairment.
MethodsDesign, study population, and settingWe designed a cross-sectional study including 164 participants: 104 patients with MCI and 60 cognitively healthy individuals. All participants underwent an oral-periodontal examination and an amyloid-PET scan, with a maximum interval of one month between the 2 studies.
Elderly individuals with MCI were recruited at the cognitive and behavioral neurology unit of Hospital Virgen de las Nieves in Granada (Spain). Neurologists requested an amyloid-PET scan when AD was suspected. We applied the diagnostic criteria for MCI described by Petersen et al.19 These criteria were applied with such neuropsychological tests as the Fototest,20 among others. Cognitively healthy elderly adults were recruited from the AGUEDA cohort, which includes cognitively healthy elderly adults (65–80 years of age) from the same reference population as the cases. The AGUEDA project (ClinicalTrials.gov identifier: NCT05186090; submission date: December 22, 2021) aims to study the effects of a 24-week resistance exercise program on executive function in cognitively healthy elderly adults. Among other parameters, Aβ accumulation is analyzed before and after the exercise program. For this study, the PET scan and periodontal examination were conducted before the exercise program (baseline).
After complete evaluation of all study participants, 3 groups were established: group 1, elderly adults with MCI and suspected AD (MCI/Aβ+ group) (n=45); group 2, elderly adults with MCI and suspected non-AD pathology (MCI/Aβ− group) (n=59); and group 3, cognitively healthy elderly adults and with negative amyloid-PET results (non-MCI/Aβ− group) (n=60) (Fig. 1). All study participants signed informed consent forms. Ethical approval was obtained from the Ethics Committee of the University of Granada (reference # 2684/CEIH/2022).
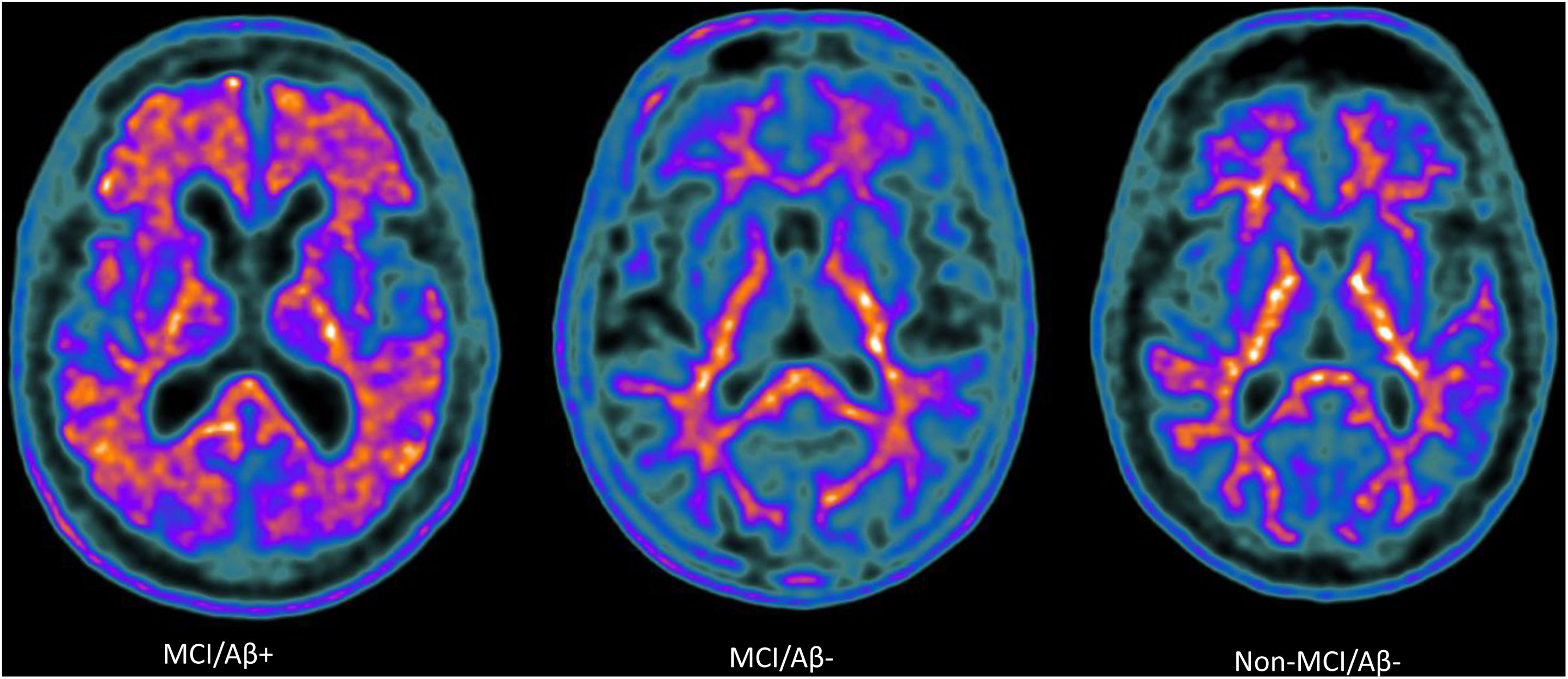
Amyloid-PET scans ([18F]Florbetaben) of representative patients from each group: MCI/Aβ+ participant (left) showing intense amyloid burden in most cortical areas (positive finding); MCI/Aβ− (center) and non-MCI/Aβ− participants (right) showing normal uptake in the white matter (negative finding).
Participants with MCI had to meet the following inclusion criteria: 1) patients who, according to the recommendations of the Spanish Society of Neurology,21 required an amyloid-PET scan for the study of cognitive impairment of possible amyloid origin; 2) patients who gave their informed consent. The inclusion criteria for the cognitively healthy participants were: 1) individuals without cognitive impairment or episodic memory loss, and without severe alterations in a study of daily living activities; 2) individuals who gave their informed consent. To identify cognitively normal participants in the latter group, the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) tests were used, based on previous studies that proposed standardized cut-off points adjusted for education level and age in the Spanish population.22
The following patients were excluded from the study: individuals younger than 45 years of age; individuals with metabolic disorders (hypothyroidism; vitamin B12 or folic acid deficiency), known psychiatric disease (schizophrenia or depression), stroke with limiting disability, or neurological disease (e.g., parkinsonian syndrome, epilepsy); individuals with a history of psychotropic substance abuse; individuals with difficulty or inability undergoing an oral and periodontal examination; and individuals who had received periodontal treatment in the last 3 months.
Due to the availability of patients with MCI (n=45 in group 1 and n=59 in group 2), we decided to establish a similar sample size for cognitive healthy elderly adults (n=60). With these sample sizes, the statistical power analysis performed with Sample Power 2.0 (SPSS Inc.; Chicago, IL), and using the t test for independent samples, with a power of 80% and an alpha error of 5%, it is possible to detect standardized differences between the values 0.5 (moderate difference) and 0.8 (substantial difference) in quantitative variables (e.g., periodontal variables), according to Cohen's scale.23
Exposure variablesThe main exposure variable was periodontitis. For its evaluation and definition, the clinical attachment loss (CAL), bleeding on probing (BP), and dental plaque index (DPI) were taken during the periodontal exam. CAL is a clear reflection of periodontal damage accumulated over the years. It is measured as the sum of probing depth and distance between the enamel-cement junction and gingival margin. BP reflects the current periodontal activity and is evaluated by counting the number of bleeding sites after periodontal examination. Oral hygiene performed by the subject was analyzed using the DPI according to the Sillness and Löe plaque index.24 These 3 determinations were used independently as potential predictor variables.
Periodontitis severity was defined according to the percentage of explored sites with CAL>3mm, classifying patients into 2 groups: i) subjects with healthy periodontium/mild periodontitis (0–33% of sites with CAL>3mm); and ii) subjects with moderate/severe periodontitis (34–100% of sites with CAL>3mm.25
Outcome variablesThe main outcome variable was the amyloid-PET assessment. The protocol used in the nuclear medicine department of Hospital Virgen de las Nieves was developed in accordance with international recommendations.21,26 In summary, the exploration started with the intravenous injection of the radiopharmaceutical ([18F]Florbetaben [FBB]), at a maximum dose of 8mCi [300MBq]) and the subsequent acquisition of the Images 90min post-injection (between 90 and 110min). The images obtained were reported by visual assessment according to the manufacturer's instructions and international guidelines.26,27 FBB uptake was interpreted by a nuclear medicine physician blinded to the clinical information, as follows: Aβ+ (loss of gray-white matter contrast; regional cortical tracer uptake at any of the cortical target regions: lateral temporal, frontal, posterior cingulate-precuneus, and parietal); Aβ− (good gray-white matter contrast; no tracer uptake at target regions); or inconclusive for amyloid plaque presence. Inconclusive cases were excluded from the study.
Statistical analysisStatistical analysis was performed with SPSS for Windows, version 20.0 (IBM; Armonk, NY). The methods used are explained at the bottom of each table. Briefly, for bivariate analysis, we used the chi-square test for categorical/dichotomous variables, the Mann–Whitney U test for ordinal variables, and the t test for quantitative variables. To analyze the association between periodontitis and the 3 comparison groups, we built 3 multiple logistic regression models (i.e., MCI/Aβ+ group vs MCI/Aβ− group; MCI/Aβ+ group vs non-MCI/Aβ− group; and MCI/Aβ− group vs non-MCI/Aβ− group). Together with periodontitis, we forced the variables age, sex, and education level as well-known confounding factors. Other variables present in Table 1 were forced if they showed statistically significant differences in any of the 3 comparisons between groups. No variable from Table 2 was included because they are all conceptually related to periodontitis.
Socio-demographic and background variables.
| Variable | MCI/Aβ+ group (n=45) | MCI/Aβ− group (n=59) | Non-MCI/Aβ− group (n=60) | Comparison (p value)a | ||
|---|---|---|---|---|---|---|
| MCI/Aβ+ vs MCI/Aβ− | MCI/Aβ+ vs non-MCI/Aβ− | MCI/Aβ− vs non-MCI/Aβ− | ||||
| Reason for PET, n (%) | – | .399 | – | – | ||
| Single-domain amnestic CI | 8 (18.2) | 17 (29.8) | ||||
| Multiple-domain amnestic CI | 31 (70.5) | 35 (61.4) | ||||
| Single-domain non-amnestic CI | – | 1 (1.8) | ||||
| Multiple-domain non-amnestic CI | 5 (11.4) | 4 (7.0) | ||||
| Missing data (n) | (1) | (39) | ||||
| Sex, n (%) | .693 | .085 | .213 | |||
| Women | 33 (73.3) | 40 (67.8) | 33 (55.0) | |||
| Men | 12 (26.7) | 19 (32.2) | 27 (45.0) | |||
| Age (years) | .907 | <.001 | <.001 | |||
| Range | 48–84 | 48–75 | 65–80 | |||
| Mean (SD) | 64.1 (7.6) | 63.9 (5.9) | 70.4 (3.8) | |||
| Education, n (%) | .460 | <.001 | <.001 | |||
| Primary | 20 (52.6) | 23 (62.2) | 12 (21.1) | |||
| Secondary | 11 (28.9) | 8 (21.6) | 16 (28.1) | |||
| University | 7 (18.4) | 6 (16.2) | 29 (50.9) | |||
| Missing data (n) | (7) | (22) | (3) | |||
| Smoking, n (%) | .014 | .022 | .334 | |||
| Never | 21 (70.0) | 13 (37.1) | 22 (37.3) | |||
| Former smoker | 5 (16.7) | 13 (37.1) | 32 (54.2) | |||
| Current smoker | 4 (13.3) | 9 (25.7) | 5 (8.5) | |||
| Missing data (n) | (15) | (24) | (1) | |||
| Family history of dementia, n (%) | .990 | .002 | <.001 | |||
| No | 17 (48.6) | 23 (46.0) | 49 (81.7) | |||
| Yes | 18 (51.4) | 27 (54.0) | 11 (18.3) | |||
| Missing data (n) | (10) | (9) | (0) | |||
| Cardiovascular history, n (%) | ≈1 | .149 | .099 | |||
| No | 20 (45.5) | 25 (44.6) | 37 (61.7) | |||
| Yes | 24 (54.5) | 31 (55.4) | 23 (38.3) | |||
| Missing data (n) | (1) | (3) | (0) | |||
| History of diabetes, n (%) | .263 | .931 | .172 | |||
| No | 37 (84.1) | 42 (75.0) | 52 (86.7) | |||
| Yes | 7 (15.9) | 14 (25.0) | 8 (13.3) | |||
| Missing data (n) | (1) | (3) | (0) | |||
Aβ: β-amyloid protein; CI: cognitive impairment; MCI: mild cognitive impairment; PET: positron emission tomography; SD: standard deviation.
Periodontal variables.
| Variable | MCI/Aβ+ group (n=45) | MCI/Aβ− group (n=59) | Non-MCI/Aβ− group (n=60) | Comparison (p value)a | ||
|---|---|---|---|---|---|---|
| MCI/Aβ+ vs MCI/Aβ− | MCI/Aβ+ vs non-MCI/Aβ− | MCI/Aβ− vs non-MCI/Aβ− | ||||
| Periodontitis classification, n (%) | .040 | .008 | .649 | |||
| Healthy/mild periodontitis | 19 (42.2) | 38 (64.4) | 42 (70.0) | |||
| Moderate/severe periodontitis | 26 (57.8) | 21 (35.6) | 18 (30.0) | |||
| No. teeth, n (%) | .738 | .038 | .010 | |||
| 0 (edentulous) | 5 (11.1) | 7 (11.9) | 1 (1.7) | |||
| 1–10 | 5 (11.1) | 6 (10.2) | 7 (11.7) | |||
| 11–20 | 10 (22.2) | 16 (27.1) | 7 (11.7) | |||
| 21–32 | 25 (55.6) | 30 (50.8) | 45 (75.0) | |||
| Mean (SD) | 18.6 (9.5) | 18.0 (9.9) | 22.4 (8.1) | |||
| CALb | .264 | .006 | .054 | |||
| Range | 1.52–9.50 | 1.37–7.35 | 1.06–6.00 | |||
| Mean (SD) | 3.36 (1.42) | 3.04 (1.27) | 2.60 (1.07) | |||
| % teeth with CAL>3mmb | .133 | .036 | .621 | |||
| Range | 0.0–100 | 0.0–100 | 0.0–100 | |||
| Mean (SD) | 37.2 (32.7) | 26.5 (34.1) | 23.5 (29.0) | |||
| % teeth with BPb | .992 | .099 | .078 | |||
| Range | 0.0–100 | 0.0–100 | 0.0–100 | |||
| Mean (SD) | 37.1 (34.9) | 37.0 (35.9) | 25.9 (28.6) | |||
| Dental plaque indexb | .262 | .297 | .896 | |||
| Range | 0.00–3.00 | 0.00–3.00 | 0.00–3.00 | |||
| Mean (SD) | 1.52 (1.08) | 1.26 (1.12) | 1.29 (1.09) | |||
Aβ: β-amyloid protein; BP: bleeding on probing; CAL: clinical attachment loss; MCI: mild cognitive impairment; SD: standard deviation.
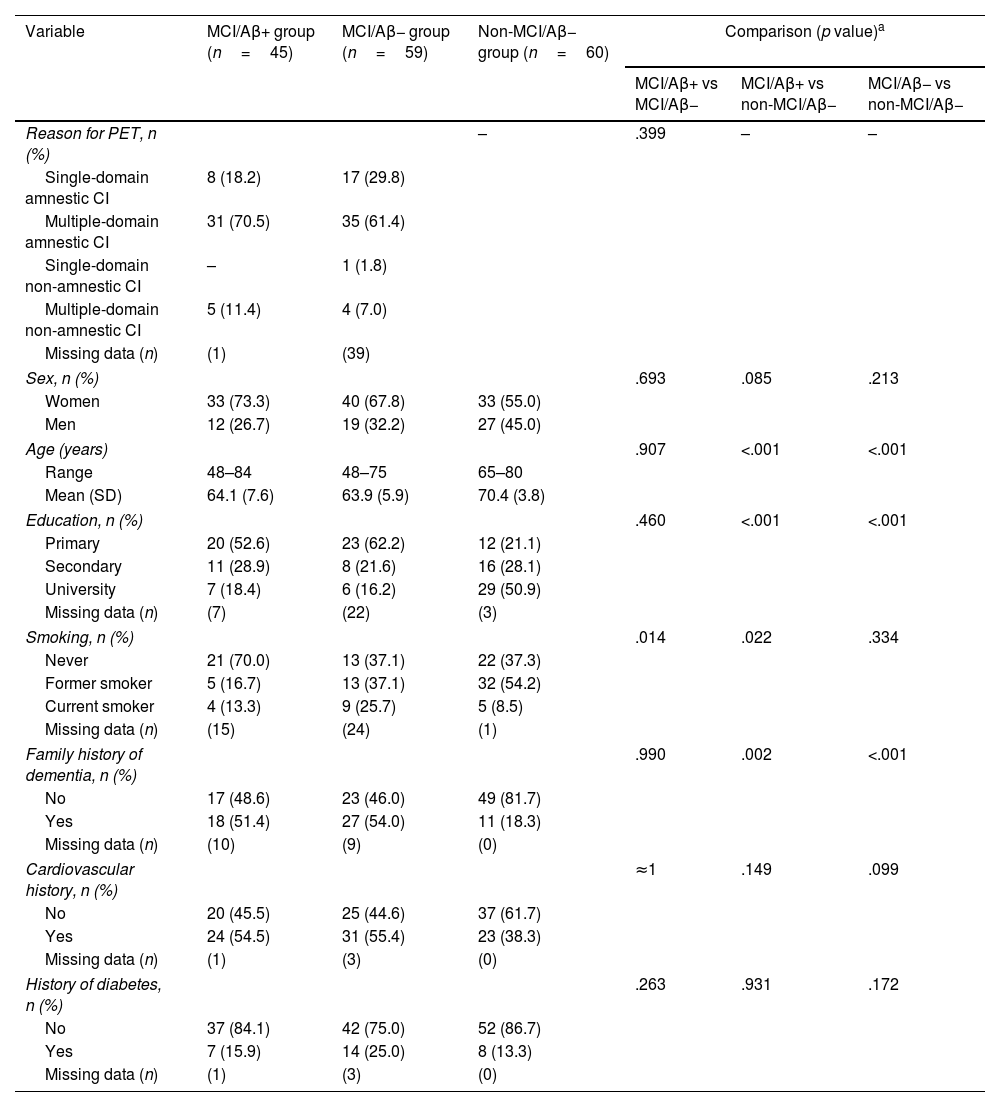
A total of 164 participants were included in the study. Of the 104 elderly adults with MCI who underwent amyloid-PET scan, results were positive in 45 (43.2%) (MCI/Aβ+ group: mean [standard deviation; SD] age 64.1 [7.6] years, 73.3% women) and negative in 59 (56.7%) (MCI/Aβ− group: mean age 63.9 5.9] years, 67.8% women). Sixty cognitively healthy and amyloid-PET negative elderly adults were included in the study (non-MCI/Aβ− group: mean age 70.4 [3.8] years, 55% women). The characteristics of the sample are presented in Table 1. Multidomain amnestic cognitive impairment was the most frequent presentation in both the MCI/Aβ+ group (70.5%) and the MCI/Aβ− group (61.4%). Among others, significant differences between the MCI/Aβ+ and non-MCI/Aβ− groups included age (P<.001), level of education (P<.001), smoking (P=.022), and family history of dementia (P=.002).
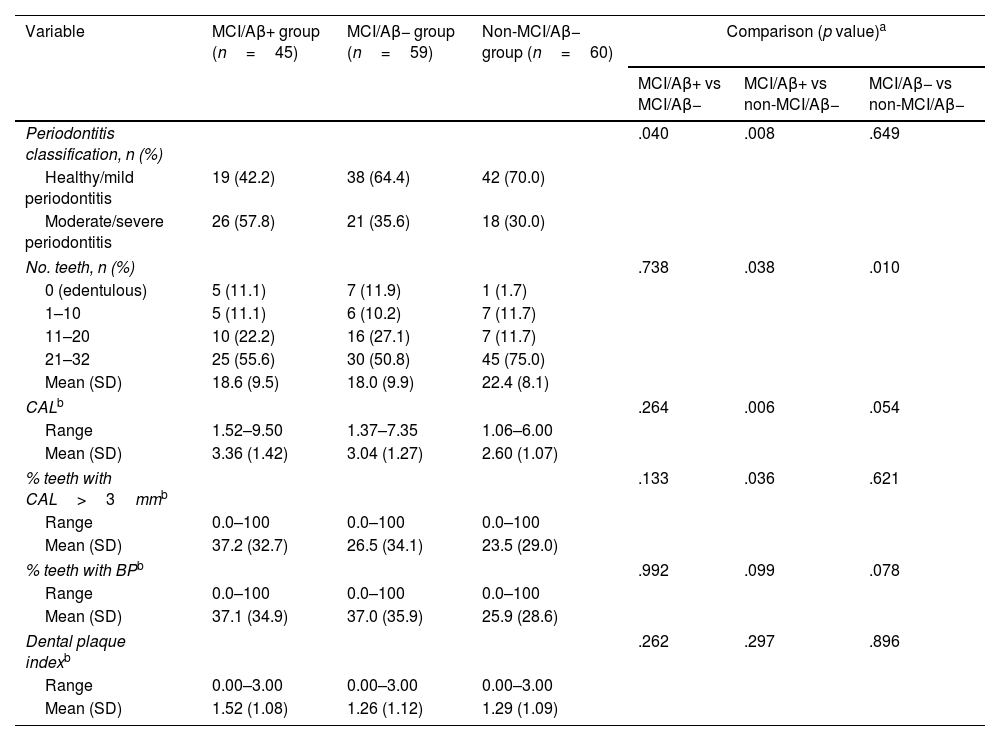
Table 2 shows the periodontal health status of the sample. Members of the MCI/Aβ+ group presented a significantly higher frequency of moderate or severe periodontitis, compared to both the MCI/Aβ− group and the non-MCI/Aβ− group, despite the fact that the latter participants were older. However, there were no significant differences between the MCI/Aβ− and non-MCI/Aβ− groups, suggesting that the differences observed were due to Aβ pathology, rather than cognitive status. In relation to the clinical variable used to classify periodontal disease (CAL), expressed in terms of mean CAL or percentage of teeth with a mean epithelial attachment loss of more than 3mm, we observed differences when comparing members of the MCI/Aβ+ group against those in the MCI/Aβ− group, and a trend when comparing the MCI/Aβ+ group against the non-MCI/Aβ− group (Table 2). No differences were observed in relation to oral hygiene (assessed by the DPI) or BP (indicative of active gingival inflammation).
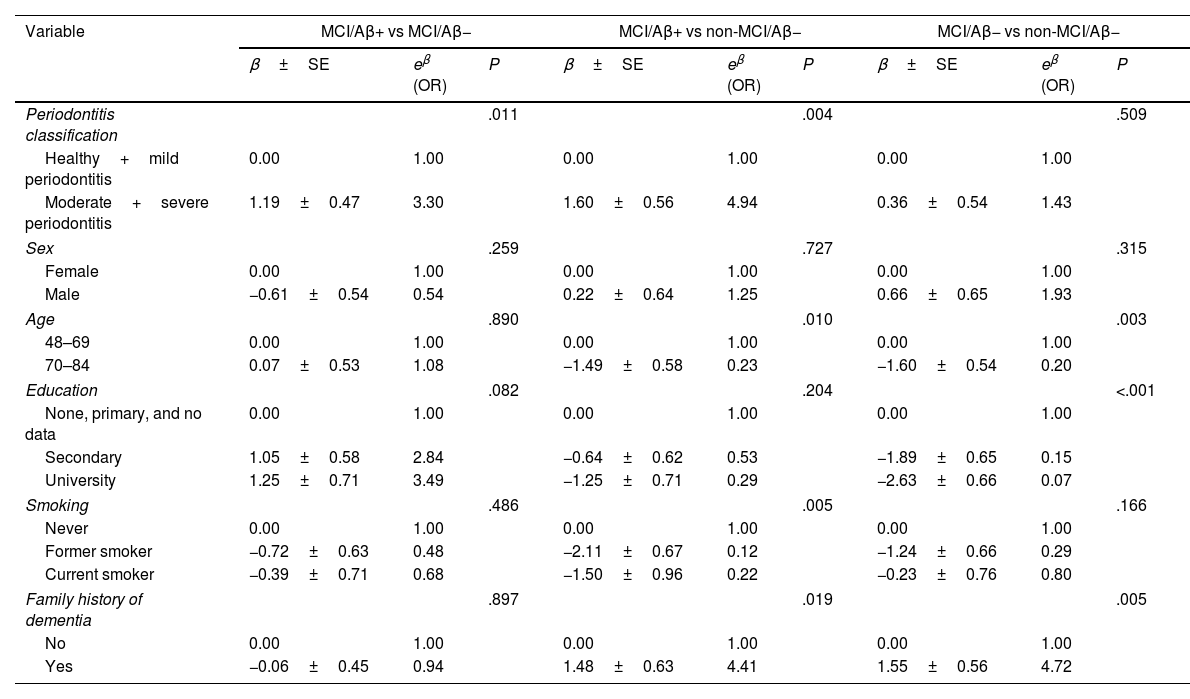
The multivariate analysis (Table 3) shows how, compared to participants with healthy periodontium or mild periodontitis, patients with moderate–severe periodontitis presented a threefold increase in the risk of abnormal Aβ accumulation in the brain (OR: 3.30; 95% CI, 1.30–8.26), in the comparison of members of the MCI/Aβ+ and the MCI/Aβ− groups. When the MCI/Aβ+ group was compared against the non-MCI/Aβ− group, the OR was 4.94 (95% CI, 1.65–14.84). Comparison of the MCI/Aβ− and non-MCI/Aβ− groups revealed no significant differences, indicating the importance of Aβ pathology versus cognitive status. The multivariate models constructed were adjusted for sex, age, education level, smoking, and family history of dementia.
Multivariate logistic regression modelsa.
| Variable | MCI/Aβ+ vs MCI/Aβ− | MCI/Aβ+ vs non-MCI/Aβ− | MCI/Aβ− vs non-MCI/Aβ− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β±SE | eβ (OR) | P | β±SE | eβ (OR) | P | β±SE | eβ (OR) | P | |
| Periodontitis classification | .011 | .004 | .509 | ||||||
| Healthy+mild periodontitis | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| Moderate+severe periodontitis | 1.19±0.47 | 3.30 | 1.60±0.56 | 4.94 | 0.36±0.54 | 1.43 | |||
| Sex | .259 | .727 | .315 | ||||||
| Female | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| Male | −0.61±0.54 | 0.54 | 0.22±0.64 | 1.25 | 0.66±0.65 | 1.93 | |||
| Age | .890 | .010 | .003 | ||||||
| 48–69 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| 70–84 | 0.07±0.53 | 1.08 | −1.49±0.58 | 0.23 | −1.60±0.54 | 0.20 | |||
| Education | .082 | .204 | <.001 | ||||||
| None, primary, and no data | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| Secondary | 1.05±0.58 | 2.84 | −0.64±0.62 | 0.53 | −1.89±0.65 | 0.15 | |||
| University | 1.25±0.71 | 3.49 | −1.25±0.71 | 0.29 | −2.63±0.66 | 0.07 | |||
| Smoking | .486 | .005 | .166 | ||||||
| Never | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| Former smoker | −0.72±0.63 | 0.48 | −2.11±0.67 | 0.12 | −1.24±0.66 | 0.29 | |||
| Current smoker | −0.39±0.71 | 0.68 | −1.50±0.96 | 0.22 | −0.23±0.76 | 0.80 | |||
| Family history of dementia | .897 | .019 | .005 | ||||||
| No | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | |||
| Yes | −0.06±0.45 | 0.94 | 1.48±0.63 | 4.41 | 1.55±0.56 | 4.72 | |||
Aβ: β-amyloid protein; MCI: mild cognitive impairment; OR: odds ratio; SE: standard error.
After adjusting for age, sex, education level, and periodontitis. The remaining variables from Table 1 were forced if they showed statistically significant differences in any of the 3 comparisons between groups. No other variable from Table 2 was included because they are all conceptually related to periodontitis.
This study demonstrates for the first time the association between PD and abnormal brain Aβ pathology in elderly patients with different cognitive statuses. Participants with severe or moderate periodontitis and MCI had a higher risk of Aβ pathology than did those with mild periodontitis or healthy periodontium plus MCI.
Two papers have addressed this question with similar approaches to our research, and report contradictory results. Kamer et al.,17 in a study with 38 cognitively healthy elderly adults, observed that measurements of periodontal disease (i.e., CAL>3mm and the different definitions of periodontitis used) were associated with amyloid accumulation in brain areas that are prone to amyloid accumulation in patients with AD (P=.002). Our study examined the association between periodontitis and Aβ accumulation in early stages of AD, where even if MCI was present, it could not be affirmed before the amyloid-PET scan that MCI was due to an amyloid disease such as AD. Severe periodontitis was significantly associated with positive amyloid-PET scan findings, i.e., patients finally diagnosed with AD. Moreover, in our work, 2 of the comparison groups comprised elderly adults with newly diagnosed MCI. When we compared the groups of subjects with MCI and positive amyloid-PET results versus those with MCI and negative amyloid-PET scans, the differences observed were significant, with a higher percentage of patients with moderate or severe periodontitis in the former group. The same occurred when patients with MCI and positive amyloid-PET findings were compared against cognitively healthy subjects; however, no differences were observed when the 2 groups with negative amyloid-PET scan findings were compared (one group with MCI and the other with cognitively healthy individuals). These results are consistent with the idea that periodontitis and the subsequent state of chronic local and systemic inflammation could be one of the factors triggering abnormal protein accumulation in the brain. The lack of significant differences between the MCI/Aβ− and non-MCI/Aβ− groups suggests that the differences detected in our study are associated with Aβ pathology rather than cognitive status, as stated by Kamer et al.17 in their study with cognitively healthy participants. Also, as in those authors’ work, we did not observe an association with other periodontal clinical variables such as gingival bleeding or pocket depth, which are indicative of current inflammation rather than chronicity.17
The second study, by Adam et al.,18 included participants from the ARIC cohort, with a mean follow-up of 14 years of 248 cognitively healthy elderly adults; the authors concluded that elevated Aβ accumulation determined by PET imaging was not associated with baseline PD status, although there was a trend toward an association among APOE ɛ4 allele carriers. The authors acknowledged that there may have been a selection bias, since older patients with poorer periodontal status and greater numbers of missing teeth were less likely to attend the appointment for amyloid-PET. Therefore, underestimation of their results is a possibility. Although our study did not follow a longitudinal design, we did retrospectively know the exposure to periodontopathogens, since we evaluated the results of such exposure with clinical periodontal variables such as CAL.
Currently, the diagnosis of AD is based on a combination of clinical and biomarker analysis. AD is considered a continuum of progressive cognitive decline, with changes occurring in biomarkers many years before the manifestation of dementia.3 For research and clinical purposes, these biomarkers were classified as A (amyloid), T (phosphorylated Tau), and N (neurodegeneration): the ATN framework.28 According to the inflammatory hypothesis, cerebral Aβ protein accumulation may have a direct and/or indirect cytotoxic effect on neurons or on the interconnections between them.29 This situation triggers a local inflammatory response, driven primarily by microglial cells, which proliferate and release inflammatory mediators that can damage neurons, interneuronal connections, and the blood–brain barrier.30 Finally, the inflammatory response induces a hyperreactive state being unable to clear misfolded or damaged neuronal proteins,31 and furthermore enhances the aggregation of neuronal proteins such as Aβ1–42.32 As mentioned above, numerous studies have highlighted the relevance of the low-grade inflammation and periodontal bacterial invasion in the association between periodontal inflammation and cognitive impairment.11,33 However, the role of periodontal infection in the initiation of the biological events mentioned above or in local inflammation is not fully clarified.
The microbiome-infection hypothesis may also partially explain these facts. Some authors propose that infectious agents like herpes simplex virus 1, Chlamydia pneumonia, spirochetes, or even PG34 may reach the central nervous system and remain there in latent form. In a murine model, it was observed that the effects of translocated PG in the brain may inhibit the adaptive immune responses, resulting in impaired clearance or induction of Aβ35 and Tau protein tangles.13 For several reasons, these pathogens or the local inflammation they induce may damage neurons and cause progressive synaptic dysfunction. The presence of Aβ, which initially appears to be only a defense mechanism,36 might act as an antimicrobial peptide37 and may potentially be beneficial in early periodontal pathogenic exposure.15 However, under prolonged chronic periodontal infection, these antimicrobial mechanisms may lead to the overproduction of Aβ and the aggregation of toxic gingipains (toxic PG-derived proteases) that degrade Aβ, resulting in neuroinflammation.15 Our study confirms these results. We have shown that more severe periodontitis is associated with greater risk of abnormal cerebral Aβ accumulation. Furthermore, according to our global hypothesis, the presence of periodontitis at the time of the examination is the result of prolonged exposure to periodontopathogens, which would explain why we detected significant differences in periodontal clinical measurements of chronicity but not in measurements of current inflammation.
Our study has certain limitations. Firstly, we did not obtain information on the APOE genotype of most of the participants. Accordingly, information on a possible confounding or modulating variable of the association might be missing, as proposed by some authors.15 Secondly, the cross-sectional design of the study and the fact that it was adapted to a clinical setting prevent us from determining the direction of the association. However, 2 facts suggest that periodontitis could be a risk factor for cerebral Aβ accumulation: i) due to the very nature of chronic periodontitis, we know that the onset of the pathophysiological processes causing the loss of final epithelial attachment in the periodontium began several years earlier, probably before the onset of the symptoms of cognitive impairment; ii) as explained above, the fact that we observed a clear association effect when we used periodontal measurements reflecting chronicity of exposure, rather than current inflammation, supports the idea that periodontitis occurred prior to the accumulation of Aβ in the brain. Although cross-sectional study designs make it difficult to control risk factors between PD and AD,38 we adjusted for age, sex, smoking, education level, and family history of dementia. Finally, we considered edentulous participants to have severe PD, although the exact cause of tooth loss was unknown. It is possible that the causes may have included dental caries, trauma, or surgical procedures, all of which have no or very modest evidence in the literature linking them to increased risk of AD.18
Finally, our data do not allow us to confirm whether the increased risk of having a greater amyloid burden is due to the extent and severity of periodontal disease itself, or to an altered immunological state in the host that influences both the severity of PD and the deposition of amyloid protein. Therefore, follow-up studies should be designed to confirm the progression of neurological pathology in this population, according to the severity and extent of periodontal disease. Likewise, it would be important to consider local (instead of global) cortical Aβ deposition in order to detect earlier Aβ stages and their relationship with periodontal diseases.
ConclusionsIn conclusion, we observed an association between severe/moderate periodontal disease and the presence of cerebral Aβ protein in subjects with MCI (A+, according to the ATN framework for the biological diagnosis of AD), after adjusting for age, sex, education level, smoking, and family history of dementia.
FundingThe assessment of the cognitively healthy group was partially supported by the AGUEDA study (Ref: I+ D+ iRTI2018-095284-J-I00; 2018). For the remaining comparison groups, no specific grant was received from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interestThe authors have no conflicts of interest to declare that are relevant to the content of this article.
The authors would like to thank all the participants for providing the data used in this manuscript, and would especially like to thank all the staff at the Department of Nuclear Medicine at Hospital Universitario Virgen de las Nieves in Granada (Spain) who processed and made available the PET data used in this manuscript.




![Amyloid-PET scans ([18F]Florbetaben) of representative patients from each group: MCI/Aβ+ participant (left) showing intense amyloid burden in most cortical areas (positive finding); MCI/Aβ− (center) and non-MCI/Aβ− participants (right) showing normal uptake in the white matter (negative finding). Amyloid-PET scans ([18F]Florbetaben) of representative patients from each group: MCI/Aβ+ participant (left) showing intense amyloid burden in most cortical areas (positive finding); MCI/Aβ− (center) and non-MCI/Aβ− participants (right) showing normal uptake in the white matter (negative finding).](https://static.elsevier.es/multimedia/02134853/unassign/S0213485324001294/v1_202410310612/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

