Many factors influence the satisfaction and quality of life of informal caregivers of non-responder patients with Alzheimer disease (AD). Among these include, the course of the disease, cognitive impairment and behavioural disturbances of the patient, the level of family support and caregiver inherent factors such as time commitment, psychological status and awareness of the disease. The aim of this work is to determine the profile of informal caregivers of non-responder AD patients and to evaluate the different factors that affect their quality of life, burden and overall satisfaction with treatment.
Patients and methodsWe carried out a prospective and multicentre study in Spain that included a total of 249 AD patients unresponsive to anticholinesterase treatment, and their informal caregivers. We evaluated caregivers’ quality of life with the SF-36 questionnaire and their associated burden with the Zarit scale, both validated for Spain. The severity and progression of the disease was quantified according to Clinical Dementia Rating (CDR) and Mini-Mental State Examination (MMSE).
ResultsCaregiver burden showed a significant increase with the time elapsed since the start of the study, while treatment satisfaction increased slightly with this factor. Caregiver burden is highly correlated with CDR scale on patient symptoms, both in the initial visit (p<.0001) and final visit (p=.0001). Caregiver satisfaction with treatment was mainly affected by the degree of change in cognitive deterioration experienced by the patient between the two visits (p=.021).
ConclusionsOverall satisfaction with the treatment stated by the caregiver does not correlate with compliance to treatment, but it does so with the changes in patient's cognitive impairment, a factor that also influences caregiver's burden.
Numerosos factores influyen en la satisfacción y la calidad de vida del cuidador informal del paciente con enfermedad de Alzheimer (EA) no respondedor. Entre ellos, destacan el curso de la enfermedad, el deterioro cognitivo y los trastornos conductuales de los pacientes, el grado de apoyo familiar y los factores inherentes al cuidador (tiempo de dedicación, estado psicológico y conocimiento de la enfermedad). El objetivo del trabajo fue determinar el perfil del cuidador informal del paciente con EA no respondedor, así como evaluar los diferentes factores que intervienen en su calidad de vida, carga soportada y satisfacción global con el tratamiento.
Pacientes y métodosSe llevó a cabo un estudio epidemiológico, prospectivo, multicéntrico y nacional que incluyó a 249 pacientes con EA no respondedores al tratamiento anticolinesterásico y a sus cuidadores. Se evaluó la calidad de vida del cuidador según el cuestionario de salud Short Form-36 (SF-36) y la carga asociada según escala de sobrecarga del cuidador Zarit, ambas validadas para España. La gravedad y la evolución de la patología se cuantificaron según el Clinical Dementia Rating (CDR) y el estado cognitivo mediante el Mini-Mental State Examination (MMSE).
ResultadosLa sobrecarga del cuidador mostró un incremento significativo en función del tiempo transcurrido desde el inicio del estudio, mientras que la satisfacción con el tratamiento aumentaba ligeramente con este mismo factor. La sobrecarga del cuidador resulta altamente correlacionada con el inventario CDR sobre sintomatología del paciente, tanto en visita inicial (p<0,0001) como final (p=0,0001). La satisfacción del cuidador con el tratamiento se vio afectada por el grado de cambio en el deterioro cognitivo padecido por el paciente entre las dos visitas (p=0,021).
ConclusionesLa satisfacción global con el tratamiento que declara el cuidador no se correlaciona con el cumplimiento terapéutico pero sí con los cambios en el deterioro cognitivo del paciente, factor que también influye sobre la carga soportada.
Alzheimer's disease (AD) is currently the most common form of dementia in the elderly. Its clinical manifestations include memory loss, language impairment and visuospatial and behavioural deficits. Severe motor abnormalities and abnormal gait also appear in the latter stages of the disease.1 The prevalence of AD increases with age, reaching around 5–10% involvement in the age group between 60 and 65 years and increasing to 45–50% of those aged between 85 and 90 years.2
Although numerous parameters related to quality of life and caregiver burden are included in the evaluations concerning the effectiveness of treatments for AD,2 no study has prospectively assessed the evolution of the burden for informal caregivers of patients with AD who do not respond adequately to treatment for dementia according to clinical practice. There are many factors that influence the burden and the perceived quality of life for the informal caregiver of a patient with AD. An aggressive disease course directly causes the caregiver a greater burden.3 Another factor to be taken into account is the level of perception of the disease by the caregiver and the patient. Important differences between the perception of cognitive impairment and behavioural alterations of patients by caregivers or by patients themselves have been found, with this perception being more pronounced in the case of the caregiver.4
In general, patients with lower awareness of their memory loss and behavioural disorders represent a greater direct burden.5 These previous results were replicated by Seltzer et al.,6 who also found a greater caregiver burden in patients who were unaware of their memory loss; this was independent of the current state of dementia and other socio-demographic variables.
The inherent subjectivity of assessing the severity of the disease according to the patient's opinion also decisively influences the effectiveness of treatment. A prospective study found that patients with higher self-perception of their cognitive difficulties obtained better results during subsequent rehabilitation and, therefore, suggested that clinicians could select these patients a priori as the best candidates for such palliative therapy.7 Consequently, patients’ opinion of their evolution seems to be especially useful for the physician in selecting the treatment to be applied. However, the influence of the subjective opinion of the caregiver on the response to treatment has not been evaluated so far, and this is more evident in the case of informal caregivers than in the case of health professionals. Overall, the perception of treatment by an informal caregiver is influenced by essentially the mode of clinical presentation of the disease, by the symptoms and their severity, by the family/social support received8 and by factors inherent to the caregivers themselves, such as time devoted, psychological condition, knowledge of the disease and cultural and professional status.9,10
The starting hypothesis for this study was that the pathological features of AD patients who do not respond to treatment may influence the burden of caregivers and, consequently, the information which they, in turn, give the physician.11 The latter will use this information to determine the consequent clinical management of the patient and the overall associated costs.12 To assess this hypothesis, we carried out a prospective study of a cohort of patients with AD who did not respond to standard therapy so as to homogenise the cohort and given that the standard symptomatic treatment for patients with mild to moderate AD is based on cholinesterase inhibitors,1,13,14 we selected only those patients who did not respond to this therapeutic approach.
The main objective of the study was to describe a baseline profile for informal caregivers of these patients, as well as the evolution of this profile over time in relation to the evolution of non-responder AD patients. Secondary objectives defined were to observe and identify the factors that influenced the quality of life for caregivers and which could be taken into account in subsequent support programs.
Patients and methodsThis prospective, multicentre epidemiological study was conducted on a cohort of patients with AD who did not respond to treatment with cholinesterase inhibitors. Patients and their informal caregivers were included in the study over a period of 11 months following a criterion of consecutive sampling performed at private and hospital outpatient clinics. The study protocol was submitted for independent evaluation by a clinical research ethics committee (UASP of Hospital Clínic i Universitari, Barcelona).
We selected patients with Alzheimer's disease according to DSM-IV diagnostic criteria for dementia and NINCDS-ADRDA for AD, with a score from mild to moderately severe as assessed by the Folstein Mini-Mental State Examination (MMSE: 10–26),15 with an informal caregiver, in treatment with cholinesterase inhibitors for at least 3 months prior to inclusion, who did not respond adequately to treatment and who had given written informed consent. We defined non-responders as subjects who met one or more of the following conditions: (a) an individual who did not follow, according to medical criteria, the treatment schedule set by the specialist; (b) who did not tolerate it due to adverse reactions incompatible with the maintenance of the prescribed dosing regime, and (c) who fulfilled the clinical criteria established by Dantoine et al.16 (increase of more than 2 points on the MMSE scale in the past 6 months or more than 3 points in the past year). Exclusion criteria were: diagnosis of dementia other than AD, severe AD (MMSE<10) and patients/caregivers who–according to medical criteria–were not suitable for participation in the study.
As well as a follow-up visit (6 months), we also carried out at the time of inclusion an evaluation of the clinical state of dementia through the Clinical Dementia Rating (CDR) scale,17 cognitive impairment (MMSE) and functional status (Barthel index18). We assessed the quality of life through the SF-36 questionnaire adapted into Spanish in 1995,19 which explores 8 dimensions of the health status: physical function, social function, physical problems, emotional problems, mental health, vitality, pain and perception of health (each scored from 0 to 100). Through a combination of these dimensions, it was also possible to calculate physical and mental health summary scores: Physical Component Summary (PCS) scale and Mental Component Summary (MCS) scale.
Caregiver burden was calculated using the Zarit scale20 validated in Spanish, which quantifies the subjective experience of burden perceived and associated with care. This scale ranges between 22 and 110 points, considering the scores between 47 and 55 as mild burden and between 56 and 110 as intense burden. We calculated the degree of anxiety of the caregivers (Hamilton scale21) and their satisfaction with aspects related to treatment according to a satisfaction questionnaire prepared ad hoc (Appendix). We described therapeutic management and compliance with treatment (through the Morisky–Green test22). In addition, we completed the Neuropsychiatric Inventory (NPI) on behavioural and neuropsychiatric symptoms of the patient.23 In parallel, we designed an ad hoc questionnaire to determine whether the dissatisfaction or overburden that the informal caregiver reported to the researcher influenced, and in which cases, decisions about clinical management and treatment.
ResultsCharacteristics of the populationThe final patient population consisted of 249 patients with mild to moderate AD who did not respond to cholinesterase inhibitor treatment. Five patients were not included in the study: 4 patients were not being medicated with cholinesterase inhibitor treatment and 1 patient did respond to treatment, so they did not meet all inclusion criteria and were not part of the final population assessed. Along with the patients, their 249 informal caregivers were also included in the study.
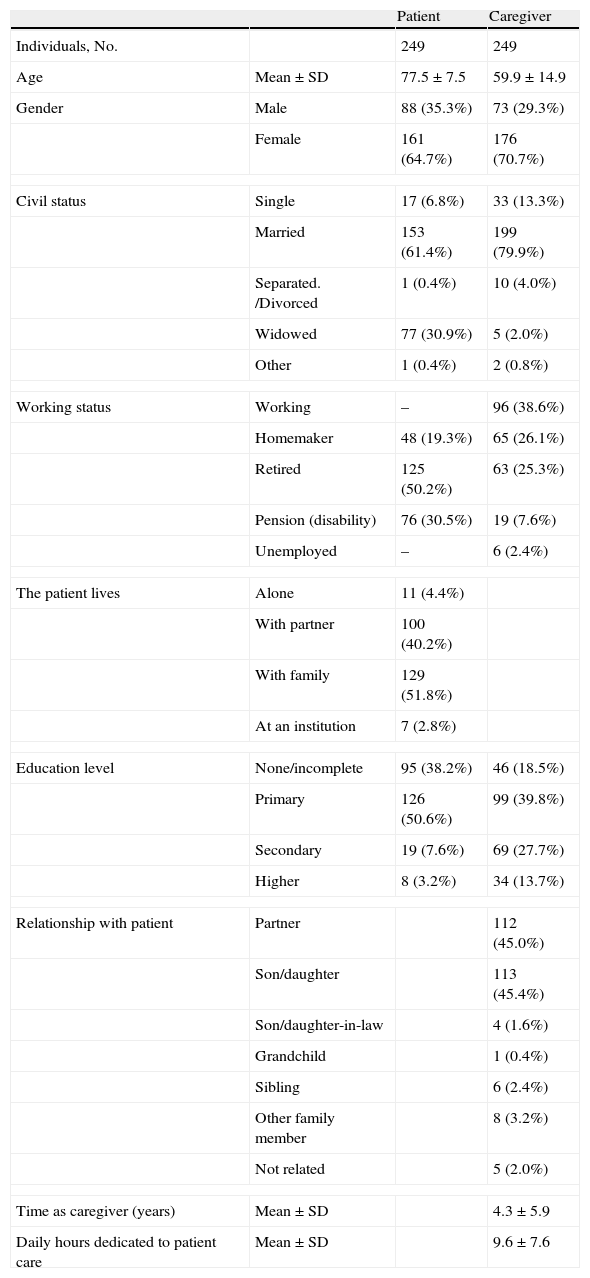
The baseline and demographic characteristics of the study population at the time of inclusion are listed in Table 1. The mean age±standard deviation (SD) of the patients with AD included in the analysis was 77.5±7.5 years and the proportion of women in the group reached 64.7% (n=161). The most common comorbidities presented by the AD population were hypertension (59.0%), lipid metabolism disorders (39.0%), depression (37.8%) and arthritic processes associated with advanced age (26.1%).
Biodemographic data for each patient and their informal caregiver.
| Patient | Caregiver | ||
| Individuals, No. | 249 | 249 | |
| Age | Mean±SD | 77.5±7.5 | 59.9±14.9 |
| Gender | Male | 88 (35.3%) | 73 (29.3%) |
| Female | 161 (64.7%) | 176 (70.7%) | |
| Civil status | Single | 17 (6.8%) | 33 (13.3%) |
| Married | 153 (61.4%) | 199 (79.9%) | |
| Separated. /Divorced | 1 (0.4%) | 10 (4.0%) | |
| Widowed | 77 (30.9%) | 5 (2.0%) | |
| Other | 1 (0.4%) | 2 (0.8%) | |
| Working status | Working | – | 96 (38.6%) |
| Homemaker | 48 (19.3%) | 65 (26.1%) | |
| Retired | 125 (50.2%) | 63 (25.3%) | |
| Pension (disability) | 76 (30.5%) | 19 (7.6%) | |
| Unemployed | – | 6 (2.4%) | |
| The patient lives | Alone | 11 (4.4%) | |
| With partner | 100 (40.2%) | ||
| With family | 129 (51.8%) | ||
| At an institution | 7 (2.8%) | ||
| Education level | None/incomplete | 95 (38.2%) | 46 (18.5%) |
| Primary | 126 (50.6%) | 99 (39.8%) | |
| Secondary | 19 (7.6%) | 69 (27.7%) | |
| Higher | 8 (3.2%) | 34 (13.7%) | |
| Relationship with patient | Partner | 112 (45.0%) | |
| Son/daughter | 113 (45.4%) | ||
| Son/daughter-in-law | 4 (1.6%) | ||
| Grandchild | 1 (0.4%) | ||
| Sibling | 6 (2.4%) | ||
| Other family member | 8 (3.2%) | ||
| Not related | 5 (2.0%) | ||
| Time as caregiver (years) | Mean±SD | 4.3±5.9 | |
| Daily hours dedicated to patient care | Mean±SD | 9.6±7.6 | |
The mean age of caregivers amounted to 59.9±14.9 years and the female representation in this group increased to 70.7% of the population (n=176). In most cases, the informal caregiver was a son/daughter (45.4%) or partner (45.0%). At the time of inclusion, caregivers had been looking after patients with AD for a mean of 4.3±5.9 years, spending a mean of 9.6±7.6h per day.
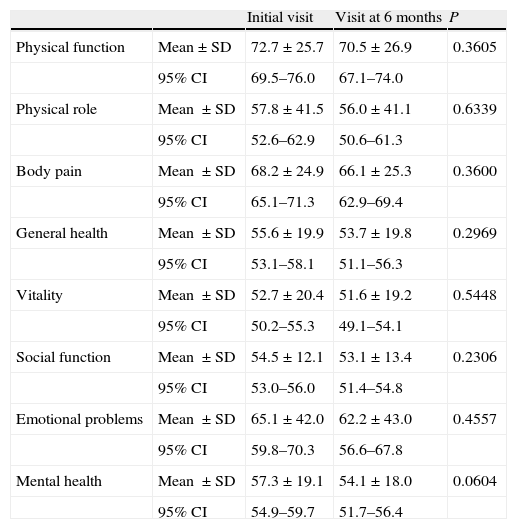
Quality of life of informal caregiversThe quality of life of informal caregivers was quantified through the SF-36 questionnaire. Table 2 shows the results for the different dimensions of this quality of life scale in both the initial (V.0) and final (6 months [V.6]) visits. It reveals certain dimensions in which the deterioration affecting the caregiver tended to increase, without resulting significant in any case. These include: the transformed mental health scale (which went from a value of 57.3±19.1–54.1±18.0), the transformed emotional role scale (from 65.1±42.0 to 62.2±43.0) and the transformed physical function scale (from 72.7±25.7 to 70.5±26.9). Some dimensions were less affected in the course of 6 months, but always showed a negative trend. This is the case, for example, of the transformed vitality scale (from 52.7±20.4 in V.0 to 51.6±19.2 in V.6) and the transformed social function scale (from 54.5±12.1 to 53.1±13.4).
Mean score by informal caregiver for the different dimensions of the SF-36 quality of life scale at baseline (V.0) and end (V.6) visits.
| Initial visit | Visit at 6 months | P | ||
| Physical function | Mean±SD | 72.7±25.7 | 70.5±26.9 | 0.3605 |
| 95% CI | 69.5–76.0 | 67.1–74.0 | ||
| Physical role | Mean ±SD | 57.8±41.5 | 56.0±41.1 | 0.6339 |
| 95% CI | 52.6–62.9 | 50.6–61.3 | ||
| Body pain | Mean ±SD | 68.2±24.9 | 66.1±25.3 | 0.3600 |
| 95% CI | 65.1–71.3 | 62.9–69.4 | ||
| General health | Mean ±SD | 55.6±19.9 | 53.7±19.8 | 0.2969 |
| 95% CI | 53.1–58.1 | 51.1–56.3 | ||
| Vitality | Mean ±SD | 52.7±20.4 | 51.6±19.2 | 0.5448 |
| 95% CI | 50.2–55.3 | 49.1–54.1 | ||
| Social function | Mean ±SD | 54.5±12.1 | 53.1±13.4 | 0.2306 |
| 95% CI | 53.0–56.0 | 51.4–54.8 | ||
| Emotional problems | Mean ±SD | 65.1±42.0 | 62.2±43.0 | 0.4557 |
| 95% CI | 59.8–70.3 | 56.6–67.8 | ||
| Mental health | Mean ±SD | 57.3±19.1 | 54.1±18.0 | 0.0604 |
| 95% CI | 54.9–59.7 | 51.7–56.4 |
Together, the 8 dimensions of the scale can be grouped broadly into the physical component and the mental component of quality of life. The physical component showed a very slight, non-significant, decrease between the two visits (going from 45.4±9.4 to 45.1±9.4). The mental component of the quality of life scale showed a more pronounced overall decline, although still not significant, during the 6 months of the study (from 39.1±10.2 in V.0 to 37.9±9.9 in V.6).
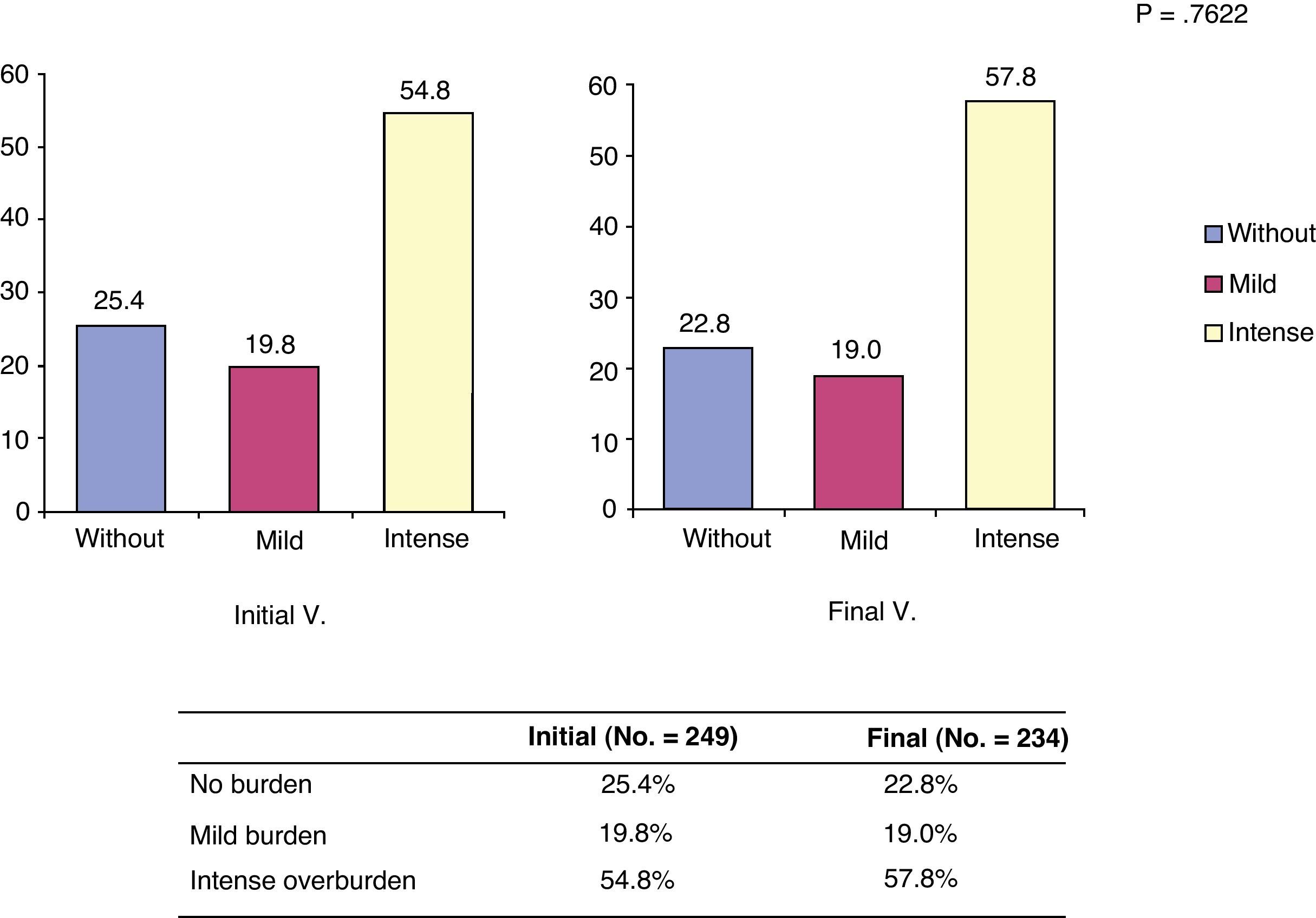
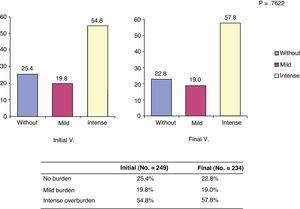
Burden of caregiversThe Zarit caregiver burden scale showed mean scores of 57.8±15.6 at the start of the study and 59.1±15.4 at 6 months. These observed differences were not significant (P=.359). It seems that the burden increased gradually with time and generally became settled in the first section considered as heavy burden. Overall, the perception of heavy burden was common both in V.0 and V.6, although this increased by 3 percentage points over time, as shown in Fig. 1.
Distribution of patients according to burden categories at the start and end of the study. *The burden categories considered were: without (no burden: score less than 47 on the Zarit scale); mild (mild burden: score between 47 and 55 on the Zarit scale); intense (intense burden: score between 55 and 110 on the Zarit scale).
Judging from our ad hoc questionnaire, the overall satisfaction expressed by caregivers with respect to treatment tended to increase slightly over time, so that 50% of caregivers were satisfied or very satisfied at baseline versus 52.4% at 6 months. However, this trend was not significant.
Among the aspects that varied least between visits were: ease of use of the last treatment (87.5% considered it easy or very easy in V.0 and 87.8% in V.6), ease of administration (82.7% in V.0 and 81.6% in V.6) and the degree of influence of this treatment on daily life (73.4% in V.0 and 73% in V.6 considered that it never or rarely interfered).
Measures such as ease of administration ranged more widely (easy or very easy for 87.9% in V.0 and 89.6% in V.6; P=.568).
However, the aspect that changed the most was the influence of treatment on personal life (67% of caregivers in V.0 and 73.8% in V.6 believed that it never or rarely interfered; P=.208).
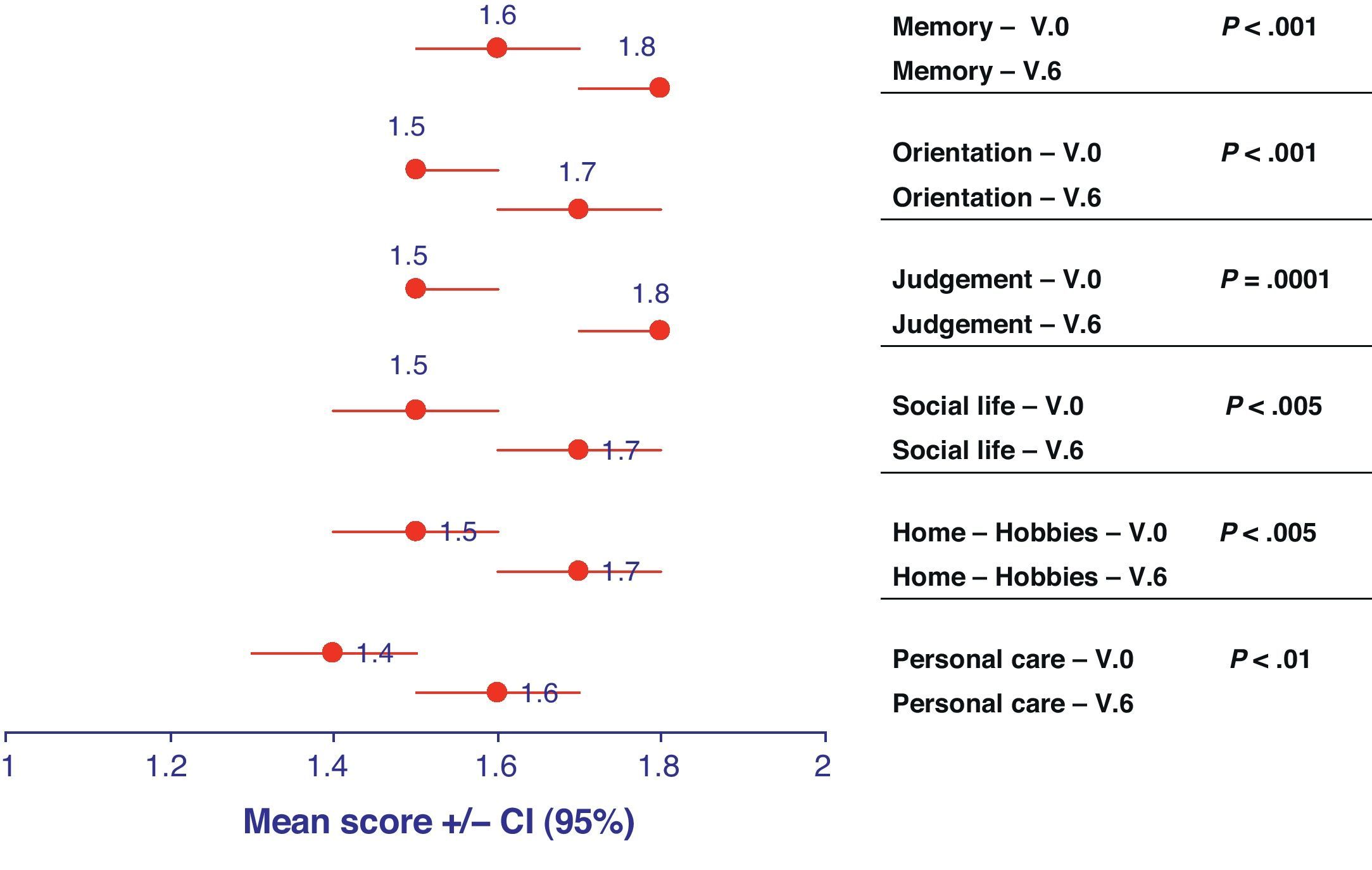
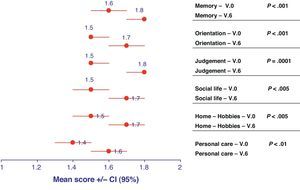
Clinical classification of dementia and status of patientsThe Clinical Dementia Rating (CDR) scale made it possible to categorise the degree of dementia into 5 possible stages (scored from 0 to 3, depending on severity). All categories evaluated using the CDR classification of patients with AD increased slightly over time, ending closer to the category of moderate dementia than that of mild dementia after 6 months (Fig. 2). The decline suffered by the category “judgement and problem solving” was especially significant, as it went from a score of 1.5 (95% CI, 1.5–1.6) in V.0 to 1.8 (95% CI, 1.7–1.8) in V.6 (P=.0001).
Representation of the means and confidence intervals (CI) for each of the descriptors included in the Clinical Dementia Rating (CDR) at the initial visit (V.0) and final visit (V.6). The statistical significance for the differentiation of each of these descriptors between baseline visit and at 6 months is shown on the right side.
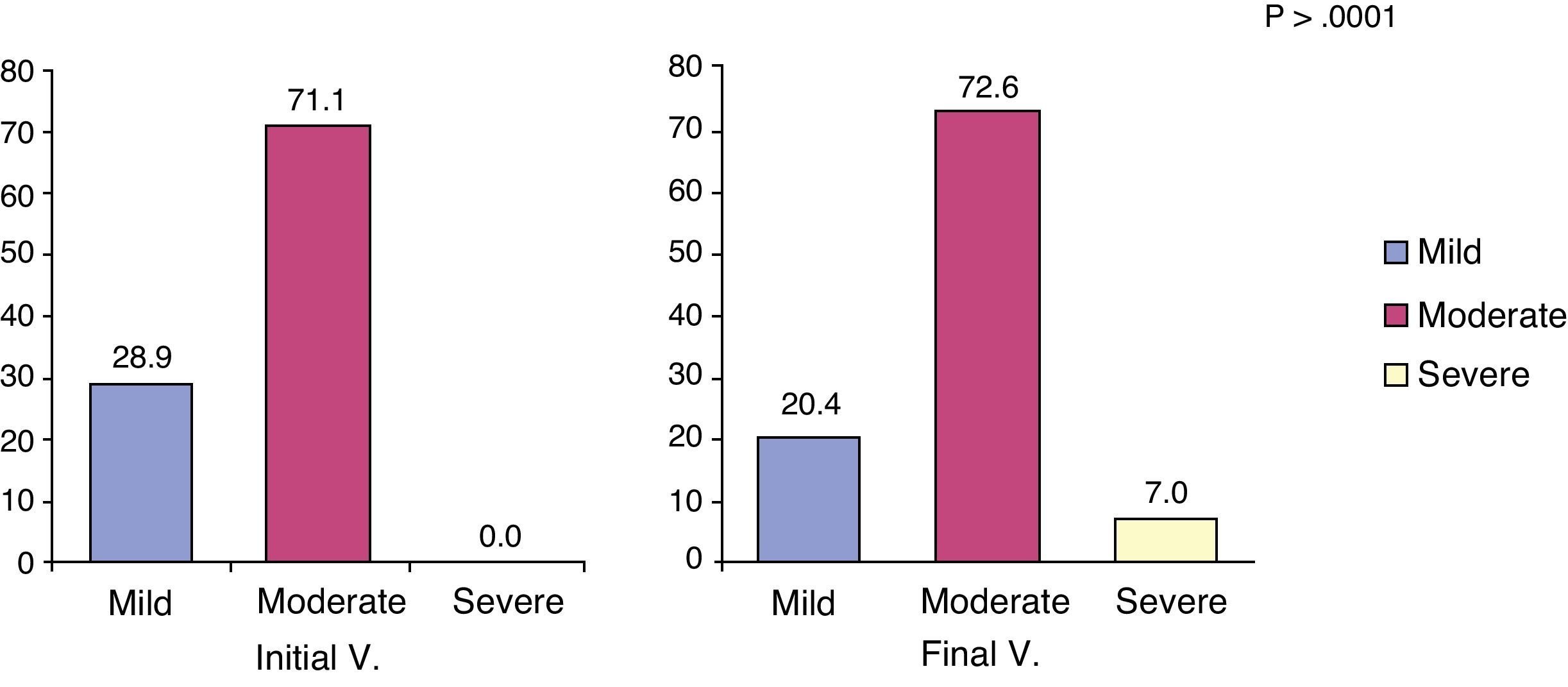
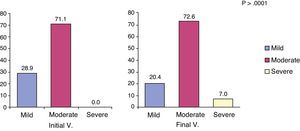
The MMSE scale was used to evaluate cognitive impairment and its severity in the patient. This analysis in patients with AD found that cognitive status decreased from an initial value of 17.0 (95% CI, 16.5–17.4) to a final value of 15.8 (95% CI, 15.2–16.3). Although not found in the V.0, some records began to be considered as severe dementia after 6 months follow-up (specifically in 7% of patients; P<.001) (Fig. 3).
Finally, the Neuropsychiatric Inventory (NPI) quantified the presence of neuropsychiatric and behavioural symptoms in patients with AD. On average, the scores for the frequency and severity aspects of each category were usually relatively low. Consequently, the mean result in V.0 was 12.4 (95% CI, 10.9–14.0) and increased, without being significant, to 13.1 (95% CI, 11.2–15.1) in V.6.
Correlation between cognitive status of patients and caregiver profileOne of the secondary objectives considered in the original design of this study was to evaluate the possible relationship between the caregivers’ quality of life (as well as burden sustained) and the patients’ degree of cognitive impairment and symptoms. In general, for most dimensions in the quality of life scale (SF-36), the correlation with cognitive impairment during the 6 months was not statistically significant. Only 1 of the quality of life dimensions was highly correlated with cognitive impairment of the patients with AD. This was the mental health scale, a dimension highly correlated with the MMSE score of each patient, both at baseline (P=.0051) and at the end of the study (P=.013). Only one other dimension presented a similar trend, although it was not significant: the dimension of emotional role of the caregiver after 6 months (P=.0535).
Similarly, we assessed the relationship between the quality of life and neuropsychiatric symptoms of patients (according to the NPI) using Spearman's correlation test. The quality scale dimensions that were correlated with the NPI were: bodily pain at final visit (this decreased with increasing NPI score; P=.0005), the vitality of the caregiver in both the initial and final visits (this decreased with increasing NPI score; P<.001 for both visits), the emotional role of the caregiver in the initial visit (which worsened as the NPI score of the patient increased; P<.005), and the overall mental health of the caregiver at the initial and final visits (which decreased in direct proportion to the increase in NPI score; P<.0001 in both visits).
The relationship between the burden sustained by the informal caregiver and patient status was especially evident when its correlation with the CDR inventory on patient symptoms was tested, both in the initial visit (P<.0001) and the final visit (P=.0001). Similarly, we observed that the caregiver burden, calculated using the Zarit scale, correlated significantly with the overall sum of the NPI. The Spearman correlation coefficients for these two variables, both at the beginning and end of the study period, were highly statistically significant (P<.0001). We also evaluated in detail the association of overburden with different symptoms linked to the progress of dementia in patients as observed through the NPI. According to this detailed analysis, most significant associations could be observed at the start of the study, specifically those between caregiver burden and the following symptoms in the patient: delirium, agitation, depression, anxiety, apathy, disinhibition and sleepiness (P<.05, in all cases). During the visit at 6 months, this association of overburden with NPI components remained significant only for agitation and increased activity of patients (P<.05, in both cases).
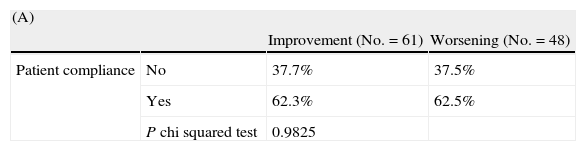
Relationship between caregiver satisfaction and patient compliance and cognitive impairmentTable 3 indicates the distribution of the cases where the caregiver manifested changes in satisfaction with regard to patient compliance (A) and to the degree of cognitive impairment (B). Notably, satisfaction with the treatment as expressed by caregivers was not associated with treatment adherence as assessed using the Morisky–Green test (P=.982) (Table 3A).
Changes in the overall caregiver satisfaction with aspects of treatment for Alzheimer's disease between baseline and final visits: (A) depending on the degree of patient compliance (Morisky–Green test) and (B) depending on the patient's degree of cognitive impairment (MMSE scale).
| (A) | |||
| Improvement (No.=61) | Worsening (No.=48) | ||
| Patient compliance | No | 37.7% | 37.5% |
| Yes | 62.3% | 62.5% | |
| P chi squared test | 0.9825 | ||
| (B) | |||
| Improvement (No.=60) | Worsening (No.=48) | ||
| MMSE difference | |||
| Decrease between −20 and −10 | 1.7% | 4.2% | |
| Decrease between −10 and 0 | 51.7% | 56.3% | |
| MMSE difference=0 | 8.3% | 22.9% | |
| MMSE increase between 0 and 10 | 38.3% | 16.7% | |
| P Fisher exact test | 0.0213 | ||
Nevertheless, this satisfaction with the treatment was affected significantly by the degree of change in cognitive impairment experienced by the patient between the two visits as assessed by the MMSE (P=.021) (Table 3B). This analysis was performed considering 4 categories of change in the MMSE as noted in each case: decrease between −20 and −10 points, between −10 and 0 points, unchanged and MMSE increase between 0 and 10 points. Caregiver satisfaction improved with treatment in only those cases where an improvement was also observed in the cognitive impairment scale of patients (higher MMSE score).
DiscussionThe aim of this study was to clarify the quality of life and satisfaction profiles of informal caregivers of patients with AD who did not respond to cholinesterase inhibitor treatment in Spain. The results indicated that some components of quality of life were particularly affected with the passage of time, even allowing for short periods such as the 6 months of the study. Among these components, those most affected by dedication to patient care were the ones related to the caregivers’ mental health and emotional role. These results were consistent with those obtained by previous studies, indicating that the continued commitment of caregivers to these patients has consequences for their behaviour, emotional stability and personal relations.24,25
Furthermore, our results showed how 6 months of caring for a non-responding AD patient tended to increase the perception of intense burden experienced by the caregiver. Likewise, the proportion of caregivers who did not perceive this burden decreased slightly. Recent studies have shown that only specific support programs and actions (the transfer of patients into a nursing home, as an extreme) are able to avoid these tendencies and the onset of symptoms associated with depression and stress in caregivers.26 These support strategies include the role played by aid groups among caregivers, since this is an empowering tool for efficient patient management that also facilitates the establishment of cohesion and socialisation techniques to combat a progressive worsening of mental health.27
Another factor that should apparently be influenced by the burden and quality of life perceived by informal caregivers is the manifested degree of satisfaction with the treatment received by patients with AD. It is interesting to note that, over time, the perception of caregivers about the ease of treatment and its administration did not worsen. Overall, treatment satisfaction increased slightly over the 6 months of study. This trend was also observed in studies with Spanish informal caregivers, especially in cases where the treatment was based on donepezil monotherapy.28
This study detected significant differences in the development and clinical course of dementia in patients during the study period. In turn, these differences could be correlated with the ongoing quality of life of caregivers. This progression of the pathology was observable from the various measurements of the classification of dementia and patient status–clinical dementia rating (CDR), MMSE and NPI. Through these tools, it was possible to observe a continuing trend towards worsening of associated symptoms. In fact, these are well-established scales that have proven to be good predictors of the progressive course of the neuropathology associated with Alzheimer's disease, especially in the case of the CDR.29
Following the objectives set out in the initial study design, it was considered that the degree of correlation of the status of patients with AD with changes in caregivers’ perceived burden and quality of life could provide useful information for an appropriate design of support programs for family and informal caregivers. These should have an impact on the dimensions of personal life most affected by the progressive course of the disease, as well as the pharmaceutical and economic burdens associated to both patients and their caregivers.30 In this sense, the study showed that most of the quality of life dimensions evaluated for caregivers were not affected by cognitive impairment (MMSE). The main notable exception was the dimension of informal caregiver mental health. The emotional role of informal caregivers also had a worsening trend, without being significant. These involvement patterns for the progressive symptoms of Alzheimer's disease in stress, depression and general mental health of caregivers had been shown previously in studies evaluating the factors associated with cognitive deterioration.25 Nevertheless, other studies seemed to show a preferential association of caregiver depression and mental health with personal factors exclusively, rather than those linked to the course of the disease.10 In this general context, it is not trivial to disregard the effect of the perceived positive aspects of patient care, resulting from the intense personal relationships required. The majority of caregivers (73%) were able to declare at least one positive aspect of their work, and this assessment varied depending on the burden, depression and inconvenience.31 This direct relationship should be decisive when establishing support programs tailored to each caregiver's individual circumstances.
Our results also showed the relationship between quality of life of caregivers and neuropsychiatric symptoms suffered by AD patients. The main components of quality of life of caregivers influenced by the neuropsychiatric impairment of patients were bodily pain, vitality, emotional role and mental health. Other studies have shown the relationship between neuropsychiatric symptoms and quality of life of patients32–34; however, works that analyse the influence of these symptoms on caregiver quality of life components are not common.35
Finally, we should mention that the quality of life assessed for informal caregivers could be affected by their satisfaction with the treatments prescribed to patients. In fact, our analysis showed a direct correspondence between this satisfaction with treatment and the degree of cognitive impairment suffered by patients, while adherence to treatment showed no connection with the satisfaction expressed by caregivers. This conclusion supports recent evidence pointing to the fact that an intervention in the form of cognitive and motor stimulation of patients may offer other benefits beyond slowing disease progression in patients,36 such as significant improvements in the burden experienced by caregivers, their evolution, their overall satisfaction with treatment and the aspects they value as caregivers.37
Overall, this result seems to suggest that both patients and informal caregivers would benefit from social support programs as personalised as possible, especially those addressing the needs arising from the limitations of patients to communicate and those aiding them with the difficulties associated with the burden, stress and anxiety associated with AD patient care.38 Overburdened caregivers suffering from psychological disorders arising from their position could lead to premature admission of patients with AD at institutions and, consequently, to increased health spending destined to deal with the problems of both patients and caregivers. These two joint arguments justify the development and implementation of such personalised educational strategies for informal caregivers of patients with AD, to mitigate the stress, depression and psychological alterations associated with their dedication.39,40
Conflict of interestsThis study received funding from Novartis Pharmaceuticals, Inc.
We wish to thank Emili González-Pérez, from the Science Department of Trial Form Support, Spain, for his help in preparing this manuscript.
Working group of the IMPACT study: José Marey (H. Juan Canalejo, La Coruña), Eduardo Aguera (H. U. Reina Sofía, Córdoba), Enrique Arriola (Fundación Matía, San Sebastián, Vizcaya), Manuel Menéndez (H. Álvarez-Buylla, Mieres, Asturias), Laureano Jesús Cacho (H. U. de Salamanca, Salamanca), Antonio del Olmo (H. Doctor Peset, Valencia), Fernando Castellanos (H. Virgen del Puerto, Plasencia, Cáceres), Carlos Marsal (H. Virgen de la Salud, Toledo), Tomás Ojea (H. R.U. Carlos Haya, Málaga), José Rubí (H. del Poniente, El Ejido, Almería), Alberto Villarejo (H. Doce de Octubre, Madrid), Rosario Vela (H. de Torrevieja, Alicante), Eloy Rodríguez (H. Marqués de Valdecilla, Santander, Cantabria), M. del Carmen Pérez (H. Doctor Negrín, Las Palmas de Gran Canaria, Las Palmas), José Bueno (H. U. Señora de la Candelaria, Santa Cruz de Tenerife), Miguel Moya (H. Puerta del Mar, Cádiz), Eloisa Navarro y Miguel Ángel García (EAP Vicente Soldevilla, Madrid), Eva Cuartero (H. U. Virgen de Valme, Sevilla), Elena Muñoz (H. Verge de la Cinta, Tortosa, Tarragona), Miguel Baquero (H. La Fe, Valencia), M. José García (H. Ernest Lluch, Calatayud, Zaragoza), María Dolores Martínez (H. La Magdalena, Castellón), Luis Cabello (H. Santa María del Rosell, Cartagena, Murcia), Ana Espino (H. Son Llàtzer, Palma, Mallorca), M. Pilar Sanz (H. M. de Mataró, Mataró, Barcelona), Raquel Sánchez-Valle (H. Clínic i Universitari, Barcelona), M. Teresa Avellán and M. Dolores López (H. Geriàtric Torribera, Santa Coloma de Gramenet, Barcelona).
Please cite this article as: Molinuevo JL, Hernández B. Perfil del cuidador informal asociado al manejo clínico del paciente con enfermedad de Alzheimer no respondedor al tratamiento sintomático de la enfermedad. Neurología. 2011;26:518–27.