Despite the highly favorable prognosis, mortality occurs in nearly 2% of patients with cerebral venous thrombosis (CVT), in which decompressive craniectomy (DC) may be the only way to save the patient's life. The aim of this report is to describe the risk factors, neuroimaging features, in-hospital complications and functional outcome of severe CVT in patients treated with DC.
Materials and methodsConsecutive malignant CVT cases treated with DC from a retrospective third-level hospital database were analyzed. Demographic, clinical, and functional outcomes were analyzed.
ResultsTwenty-six patients were included (20 female, age 35.4±12.1 years); 53.8% of the patients had acute CVT, with neurological focalization as the most common symptom in 92.3% of the patients. Superior sagittal sinus thromboses were found in 84.6% of cases. Bilateral lesions were present in 10 patients (38.5%). Imaging on admission showed a parenchymal lesion (venous infarction±hemorrhagic lesion)>6cm measured along the longest diameter in 25 patients (96.2%). Mean duration of clinical neurological deterioration was 3.5 days; eleven patients (42.3%) died during hospitalization.
ConclusionIn patients with severe forms of CVT, we found higher mortality than previously reported. DC is an effective life-saving treatment with acceptable functional prognosis for survivors.
A pesar del pronóstico favorable en pacientes con trombosis venosa cerebral (TVC), cerca de un 2% de estos pacientes fallecen, para los cuales la craniectomía descompresiva (CD) puede ser una opción terapéutica. El objetivo de este artículo es describir los factores de riesgo, las características de las neuroimágenes, complicaciones hospitalarias y evolución funcional, de pacientes con TVC severa tratados con CD.
Materiales y métodosSe analizaron características demográficas, clínicas y funcionales de casos consecutivos de TVC severa tratados con CD, a partir de una base de datos retrospectiva de un hospital de tercer nivel.
ResultadosVeintiséis pacientes fueron incluidos (20 mujeres, media de edad 35,4±12,1 años); un 53,8% de los pacientes presentaron una TVC aguda, con manifestaciones neurológicas focales como el síntoma más frecuente en el 92,3% de los casos. La trombosis del seno sagital superior estuvo presente en el 84,6% y se presentaron lesiones bilaterales parenquimatosas en 10 pacientes (38,5%). La imagen al ingreso demostró lesiones parenquimatosas (infarto venoso±lesión hemorrágica)>6cm (medida en el mayor diámetro de la misma), en 25 pacientes (96,2%). La duración media del deterioro neurológico fue de 3,5 días; 11 pacientes (42,3%) murieron durante la hospitalización.
ConclusiónEn pacientes con formas severas de TVC encontramos una mayor mortalidad que la publicada previamente; la CD podría ser una opción terapéutica en ese grupo de pacientes.
Cerebral venous thrombosis (CVT) is a rare type of cerebrovascular disease, accounting for approximately 0.5% of all types of strokes.1 The estimated annual incidence ranges from 1 to 12 cases per million adults per year.1–3 However, in Mexican hospital registries, data suggest that its prevalence is higher, ranging from 3 to 6%.4
The clinical course of CVT is highly variable and unpredictable2; most patients with CVT have a benign prognosis, and most surviving patients recover completely or have only mild limitations in functional outcomes.5 One of the main explanations for this involves the parenchymal lesions in CVT: venous infarcts differ significantly from arterial infarcts in having more edema and less necrosis, explaining greater potential for recovery.6,7 Despite the highly favorable prognosis, mortality occurs in nearly 2% of patients.8 In severe cases of “malignant CVT” with supratentorial parenchymal hemorrhagic lesions, severe cerebral edema and brain herniation, decompressive surgical approaches may be the only way to save the patient's life.9,10
Decompressive craniectomy (DC) is recommended in selected patients with medically intractable mass effects, elevated intracranial pressure and brain herniation. Multiple small observational studies suggest that DC may improve survival with acceptable outcomes even in patients with severe clinical conditions.9–13 However, no randomized trials regarding the benefits of DC in patients with CVT have been reported, and hospital series have shown some discrepancies in outcome rates (from 51 to 61% good functional outcome) and mortality (5–31%) after DC. The aim of this report is to describe the risk factors, neuroimaging features, in-hospital complications and functional outcome of severe CVT in patients treated with DC.
Patients and methodsThis is a retrospective analysis of a systematic database, prospectively recruiting consecutive Mexican mestizo patients with confirmed CVT who were treated from January 1990 to January 2019 in two tertiary care teaching centers in Mexico City (the National Institute of Neurology and Neurosurgery and the National Institute of Medical Sciences and Nutrition). Our standardized database systematically collects demographic and clinical data, imaging studies including computed tomography (CT) and computed tomography venography (CTV), magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) or digital subtraction angiography (DSA), laboratory studies, in-hospital, discharge and outpatient follow-up information data, as well as complications and functional outcomes.
From our database, we selected cases that, due to their severity, were treated with DC. We reviewed their clinical and imaging characteristics, as well as factors that could be associated with the decision to implement DC, functional prognosis, such as time from symptom onset to DC, and time from arrival at the hospital to DC. For each patient, clinical, laboratory and imaging data were reviewed by two neurologists (AA, FS).
Time of onset to diagnosis was defined as acute (<7 days), subacute (7–14 days), and chronic (>14 days).
The indications for surgery were severe cerebral edema and sufficient mass effect to threaten cerebral herniation (according to neurologist/neuroradiologist evaluation compared to baseline brain image), third nerve dysfunction or progressive neurological deterioration on the Glasgow coma score (a decrease of more than 4 points from baseline) caused by local brain edema or venous infarction with midline shift or obliteration of basal cisterns and not attributable to seizures, and an association with CT evidence of space-occupying lesions causing midline shifts or obliteration of the basal cisterns despite maximal medical therapy and the provision of intensive care. Decision for DC was evaluated on each single case, according to concomitant neurological and neurosurgical evaluation, and the degree of neurological worsening during hospitalization.
Motor deficit at onset was defined as mild (affected segment[s] with active movement against some resistance), moderate (affected segment[s] with active movement against gravity) and severe (affected segment[s] only with palpable or visible contraction).
In all cases, a large hemicraniectomy was performed, with special effort to extend the decompression toward the temporal skull base. The dura was opened widely to ensure maximal decompression; no internal decompression was performed in our sample of cases. In all cases, patients were treated concomitantly with anticoagulants (heparin or enoxaparin followed by oral anticoagulants [vitamin K antagonists]) and 24-h suspension of any previous anticoagulation therapy prior to the surgical procedure, which was restarted after the surgery, according to neuro-intensive care specialists and vascular neurologist criteria after 24h of surgery, if no contraindication for such therapeutic intervention.
Follow-up was performed in person at the stroke clinic, by telephone interview or by medical chart review. Follow-up visits were performed at 3, 6 and 12 months after discharge, and outcome was expressed on the modified Rankin Scale (mRS). The primary end-point of this analysis was disability at discharge, 3 months and last follow-up, assessed by the modified Rankin Scale (mRS); a score of 0–2 on this scale was considered a favorable functional outcome.
Exclusion criteria for the study included: patients<18 years (both institutions attend adult neurological cases), absence of medical records to collect the pre-defined clinical/imaging variables and absence of follow-up information.
Data analysis of descriptive statistics was performed with the statistical package SPSS Version 21.0 software. Categorical variables are expressed as counts and percentages, while continuous variables are expressed as the means±standard deviations (SDs) or medians and interquartile ranges (IQRs) depending on the normality of the distribution. Associations between prognostic variables and outcomes were calculated using Fisher's exact test for categorical analysis. All p values<0.05 were considered significant.
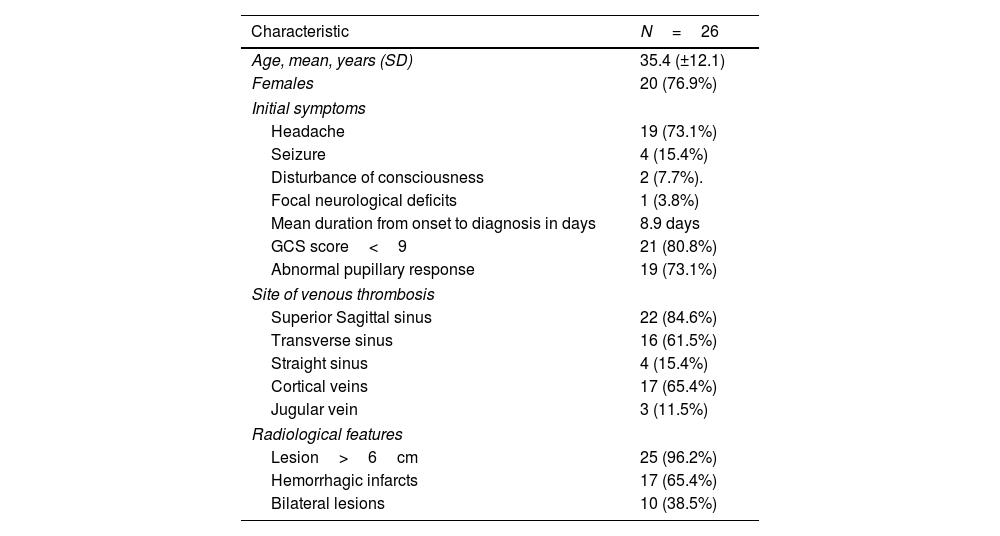
ResultsGeneral characteristicsThe registry included 471 patients; among these, 26 patients (5.5%) required DC. The mean age of the patients was 35.4±12.1 years (range 18–57 years), and 20 were female (76.9%). The baseline characteristics of these patients are shown in Table 1.
Baseline characteristics.
| Characteristic | N=26 |
|---|---|
| Age, mean, years (SD) | 35.4 (±12.1) |
| Females | 20 (76.9%) |
| Initial symptoms | |
| Headache | 19 (73.1%) |
| Seizure | 4 (15.4%) |
| Disturbance of consciousness | 2 (7.7%). |
| Focal neurological deficits | 1 (3.8%) |
| Mean duration from onset to diagnosis in days | 8.9 days |
| GCS score<9 | 21 (80.8%) |
| Abnormal pupillary response | 19 (73.1%) |
| Site of venous thrombosis | |
| Superior Sagittal sinus | 22 (84.6%) |
| Transverse sinus | 16 (61.5%) |
| Straight sinus | 4 (15.4%) |
| Cortical veins | 17 (65.4%) |
| Jugular vein | 3 (11.5%) |
| Radiological features | |
| Lesion>6cm | 25 (96.2%) |
| Hemorrhagic infarcts | 17 (65.4%) |
| Bilateral lesions | 10 (38.5%) |
In four patients (15.4%), CVT was associated with a recent surgery; furthermore, one patient had protein S deficiency (3.8%) and another patient had a prothrombin mutation. Three patients (11.5%) presented with a history of extracranial deep venous thrombosis. One patient (3.8%) had a CVT related to an active neoplasm (gastric cancer). Anemia on admission was present in 6 patients (23.1%). Female-specific risk factors were the most common: pregnancy and puerperium were present in 14 patients (53.8%), oral contraceptive use in 1 (3.8%), and hormone replacement therapy in two (7.7%).
Clinical features on presentationIn 14 patients (53.8%), the presentation of neurological symptoms was acute (<7 days). Headache was the most common index symptom (73.1%), followed by seizures (15.4%). A Glasgow Coma Score of <9/15 at admission was seen in 21 patients (80.8%). In the acute phase, 8 patients (30.8%) had clinical neurological deterioration; meanwhile, 12 (46.2%) presented with this symptom over the next 7 days (see in-hospital evolution). The mean duration of symptoms at the time of diagnosis was 8.9 days (range 1–40 days).
Neuroimaging findingsImaging studies in the CVT patients included CTV in 25 patients (96.2%), MRV in 18 patients (69.2%) and DSA in 15 patients (57.7%). The most common venous sinus involved was the superior sagittal sinus in 22 patients (84.6%). There were 17 patients with intracranial hemorrhage (65.4%) and 4 with isolated venous infarctions (15.4%). One patient had subarachnoid bleeding. Bilateral lesions were present in 10 patients (38.5%). Admission imaging showed a parenchymal lesion size>6cm measured at the longest diameter in 25 patients (96.2%). The location of the parenchymal lesion was deep in 3 (11.5%) and superficial in 23 (88.5%).
In-hospital clinical evolution and surgeryNeurological focalization was the most common symptom in 92.3% of the severe CVT patients who underwent DC. One patient presented with neurological focalization on admission; however, during in-hospital evolution, it developed in 23 additional patients: hemiparesis in 19 patients (73%), quadriparesis in 4 patients (15.4%) and monoparesis in one patient (3.8%). Eight patients (30.8%) had sensitive deficits, and two patients (7.7%) had aphasia. Twelve patients (46.2%) had cranial nerve compromise (mainly 6th nerve palsy).
Headache was present in 88.5% of patients and was the initial symptom in 19 patients. Epilepsy was present in 65.4% of patients; it was the first symptom in 4 patients (15.4%) and appeared during in-hospital follow-up in 13 (50%) patients. Generalized seizures were observed in 14 patients (82.4%).
The mean duration of initial symptoms at the time of clinical deterioration was 8.4 days (range 1–47 days). Eight patients (30.8%) had signs of herniation at admission, and 12 patients (46.2%) had signs during the clinical course.
The mean duration of clinical neurological deterioration at the time of surgery was 3.5 days (range 0–14 days). In two patients, bilateral DC was performed.
At discharge, 16 patients (61.6%) presented with motor deficits (8 mild and 8 moderate), 3 patients (11.5%) presented with sensitive deficits, 4 patients (15.4%) presented with aphasia and 4 patients (15.4%) presented with epilepsy.
Few post-surgical complications were reported in the entire sample of DC cases: surgical wound infection (2 cases) sinking skin flap (1 case), post-surgical bleeding (2 cases: parenchymal hemorrhage 1 case and subdural hematoma in 1 case). No paradoxical herniation, post-surgical meningitis/cerebritis or hydrocephalus were recorded. One patient underwent a re-intervention to extent the craniectomy area due to extension of brain edema.
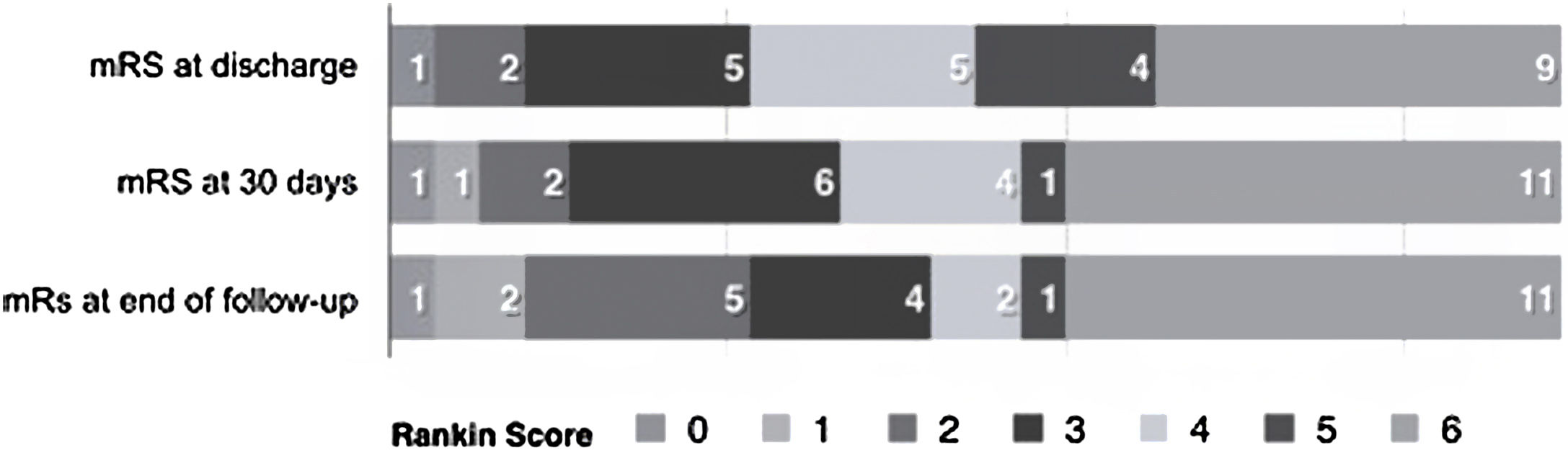
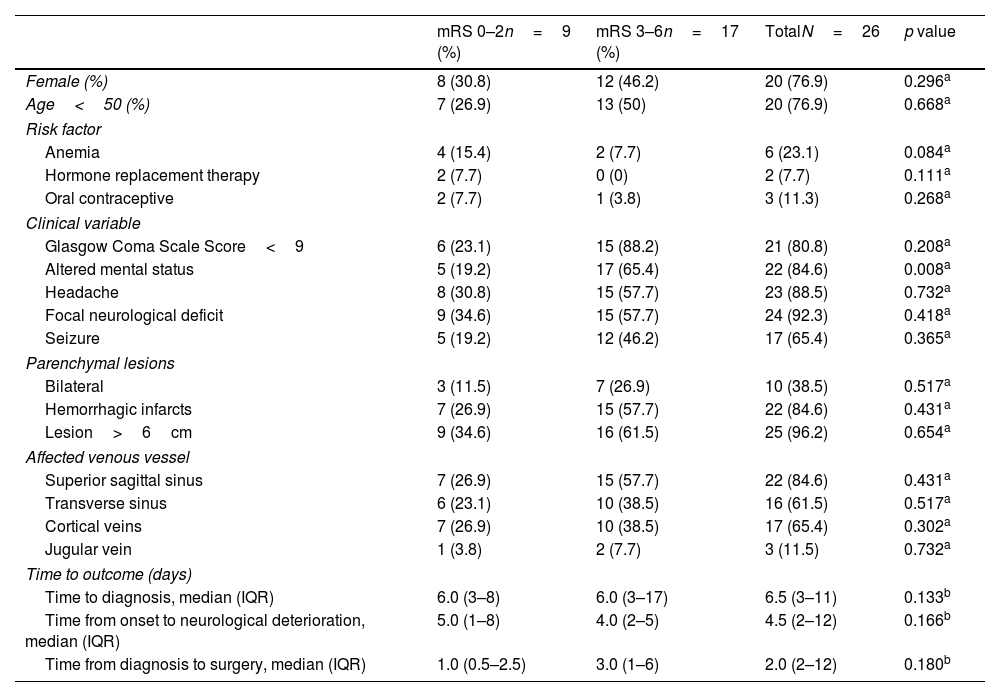
Follow-up and functional statusPatients were followed up for a median of 52.3 months (range 1–279 months). At the final follow-up, 13 patients (50%) presented with motor deficits (8 mild and 5 moderate), 2 patients (7.7%) with sensitive deficits, 2 patients (7.7%) with aphasia, and 9 patients (34.6%) with epilepsy. None of the patients had CVT recurrence during follow-up. Table 2 summarizes the different variables according to the dichotomic mRS score.
General features and risk factors according to functional outcome.
| mRS 0–2n=9 (%) | mRS 3–6n=17 (%) | TotalN=26 | p value | |
|---|---|---|---|---|
| Female (%) | 8 (30.8) | 12 (46.2) | 20 (76.9) | 0.296a |
| Age<50 (%) | 7 (26.9) | 13 (50) | 20 (76.9) | 0.668a |
| Risk factor | ||||
| Anemia | 4 (15.4) | 2 (7.7) | 6 (23.1) | 0.084a |
| Hormone replacement therapy | 2 (7.7) | 0 (0) | 2 (7.7) | 0.111a |
| Oral contraceptive | 2 (7.7) | 1 (3.8) | 3 (11.3) | 0.268a |
| Clinical variable | ||||
| Glasgow Coma Scale Score<9 | 6 (23.1) | 15 (88.2) | 21 (80.8) | 0.208a |
| Altered mental status | 5 (19.2) | 17 (65.4) | 22 (84.6) | 0.008a |
| Headache | 8 (30.8) | 15 (57.7) | 23 (88.5) | 0.732a |
| Focal neurological deficit | 9 (34.6) | 15 (57.7) | 24 (92.3) | 0.418a |
| Seizure | 5 (19.2) | 12 (46.2) | 17 (65.4) | 0.365a |
| Parenchymal lesions | ||||
| Bilateral | 3 (11.5) | 7 (26.9) | 10 (38.5) | 0.517a |
| Hemorrhagic infarcts | 7 (26.9) | 15 (57.7) | 22 (84.6) | 0.431a |
| Lesion>6cm | 9 (34.6) | 16 (61.5) | 25 (96.2) | 0.654a |
| Affected venous vessel | ||||
| Superior sagittal sinus | 7 (26.9) | 15 (57.7) | 22 (84.6) | 0.431a |
| Transverse sinus | 6 (23.1) | 10 (38.5) | 16 (61.5) | 0.517a |
| Cortical veins | 7 (26.9) | 10 (38.5) | 17 (65.4) | 0.302a |
| Jugular vein | 1 (3.8) | 2 (7.7) | 3 (11.5) | 0.732a |
| Time to outcome (days) | ||||
| Time to diagnosis, median (IQR) | 6.0 (3–8) | 6.0 (3–17) | 6.5 (3–11) | 0.133b |
| Time from onset to neurological deterioration, median (IQR) | 5.0 (1–8) | 4.0 (2–5) | 4.5 (2–12) | 0.166b |
| Time from diagnosis to surgery, median (IQR) | 1.0 (0.5–2.5) | 3.0 (1–6) | 2.0 (2–12) | 0.180b |
IQR: interquartile range.
Eleven patients (42.3%) who underwent DC died during hospitalization; Causes of death were related to septic shock (due to sepsis outside CNS) in two cases, and post-surgical cardiac conditions in one case; the rest of cases were deceased due to the primary severe CVT.
At the last follow-up, 9 (34.6%) had a favorable prognosis (mRS=0–2), whereas 5 (23%) had an unfavorable outcome (mRS score=3–5), see Fig. 1.
DiscussionThis is the first report addressing survival and functional status in patients who underwent DC in severe CVT cases in Latin America. As previously stated, mortality and poor functional outcome are uncommon conditions for most CVT patients, but identifying those cases that could undergo neurological clinical deterioration in the acute phase is a challenging task that should be performed based on validated clinical signs/symptoms.14,15 From previous studies, certain variables, such as coma, dilated fixed pupils and bilateral parenchymal lesions, are more likely to result in poor functional outcome for severe CVT patients16; this observation was not found in our patients, with nonsignificant differences between patients with good vs. bad functional outcomes regarding their clinical symptoms. Our findings confirm that CVT is a highly pleomorphic disease, not only in its clinical presentation but also in its evolution and resolution.
The pathophysiological basis of parenchymal damage in severe CVT is mainly determined by the effect of venous and capillary pressure due to the occlusion of a cortical vein and the breakdown of the blood-brain barrier, which results in localized brain edema with subsequent progression to venous infarction and hemorrhagic transformation.17 Decompressive surgery may interrupt this vicious cycle by reducing the intracapillary pressure, thus resolving venous congestion, improving cerebral perfusion, allowing time for cortical collateral vein drainage (6), and improving transtentorial herniation.
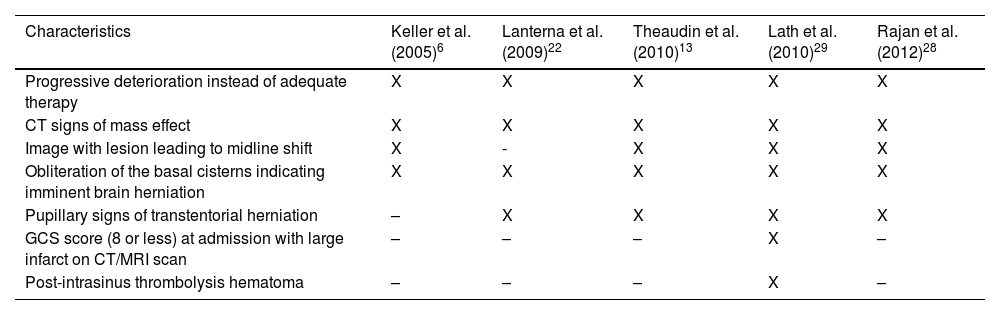
DC and hematoma evacuation are typically reserved for patients with CVT with large parenchymal lesions causing herniation. Early surgery and younger patients are predictors of a good outcome after DC; however, there is no clear and strong evidence from current guidelines for determining whether to choose hemicraniectomy, bilateral craniectomy or hematoma evacuation.18,19 Coutinho et al interviewed 91 physicians20 (85% neurologists with >5 years of experience) regarding treatment options in patients with CVT and impending cerebral herniation due to focal cerebral lesions; 93% of them responded that DC is a therapeutic option, and 43% indicated that they had previously used this treatment. There is no consensus on the specific indications for surgery (apart from the medical judgment of the treating physicians based on clinical evaluation), and the main indications observed from DC in severe CVT series are described in Table 3. Since 2006, the European Federation of Neurological Societies Guidelines on the treatment of CVT has recommended decompressive craniectomy as an alternative option in patients with deterioration, especially in the presence of large intracerebral hemorrhages.2 Given the low incidence of CVT, few studies with only small sample sizes have been reported. However, recent guidelines for CVT cases18,21 recommend decompressive surgery for patients with acute CVT and parenchymal lesion(s) with impending herniation to prevent death.
Indications for surgery.
| Characteristics | Keller et al. (2005)6 | Lanterna et al. (2009)22 | Theaudin et al. (2010)13 | Lath et al. (2010)29 | Rajan et al. (2012)28 |
|---|---|---|---|---|---|
| Progressive deterioration instead of adequate therapy | X | X | X | X | X |
| CT signs of mass effect | X | X | X | X | X |
| Image with lesion leading to midline shift | X | - | X | X | X |
| Obliteration of the basal cisterns indicating imminent brain herniation | X | X | X | X | X |
| Pupillary signs of transtentorial herniation | – | X | X | X | X |
| GCS score (8 or less) at admission with large infarct on CT/MRI scan | – | – | – | X | – |
| Post-intrasinus thrombolysis hematoma | – | – | – | X | – |
Decompressive surgery is defined in terms of external and internal decompression. External decompression is defined as a hemicraniectomy with duraplasty (diameter≥12cm), and internal decompression consists of resecting the infarcted brain tissue and possibly draining cerebral hematomas6,13; this last surgical intervention (evacuation) need not be considered except if the condition is associated with a progressive and severe neurological deficit.9,19 According to some case reports, external decompression is primarily indicated in the event of diffuse hematomas infiltrating edematous, potentially still viable, eloquent brain tissue, whereas internal decompression is primarily indicated in the event of localized hematomas in infarcted, noneloquent brain parenchyma.6,22
Few trials have explored the rationale of DC versus standard medical treatment in severe CVT patients with a high mortality rate in the medical group (100%) based on a retrospective analysis.23 Recently, cases with DC and surgical thrombectomy,24 combined IV rtPA thrombolysis for malignant CVT,25 or endovascular mechanical thrombectomy26 have been described, showing plausible therapeutic options that should be explored in clinical trials. One of the main problems for the rationale of these types of study is the difficulty of establishing those patients in the acute phase who could benefit from this type of intervention, basically defined as severe CVT refractory to standard anticoagulation or with development of new worsening intracranial hypertension or anticoagulation27; nevertheless, no clear predictor variables are consistent in the studies. In the present study, “altered metal status” was one of the variables with significant association for those patients with worst functional outcome, more than the sum of the GCS; recently, the sub classification of the types of the different terms related to level of consciousness have proved a better prediction capacity in terms of death and functional prognosis for severe CVT, which could explain this finding.14 The utility of score systems that determine those patients with a higher risk of developing acute clinical neurological deterioration, such as the CVT grading scale, could be a practical help in clinical decision-making when defining those patients who could benefit from more aggressive treatment.14
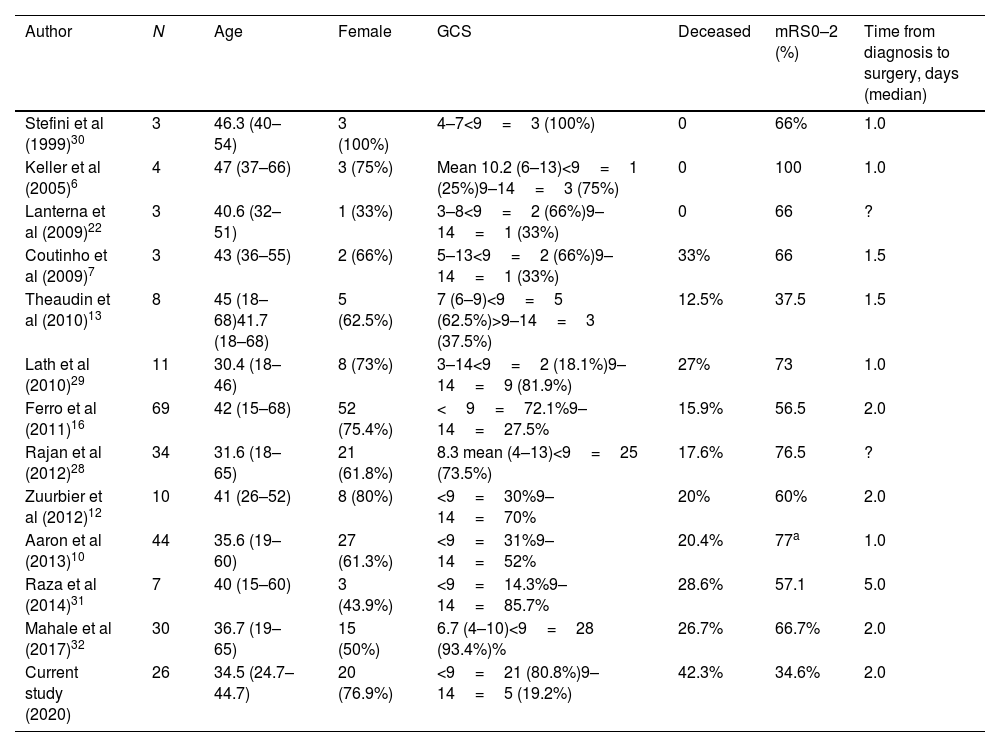
Mortality, as stated previously, is uncommon in CVT cases, but those with malignant CVT have an increased mortality (from 12.5 to 33%) in many case series (see Table 4). Our study demonstrated a mortality of 42.3%, which is higher than expected. One of the possible explanations for this is that when these patients arrive at a tertiary center, the time between the onset of the first symptoms and the diagnosis could be overestimated. Additionally, the availability of more advanced technical resources (CTV, MRV or DSA) over the last decade has changed, with the possibility of more severe cases at the beginning of our database. Unfortunately, this observation was not totally proven. Finally, other clinical covariables could also influence the functional outcome in a critical patient, who may present a higher risk of infectious and metabolic complications during hospitalization.
Studies on decompressive surgery for CVT.
| Author | N | Age | Female | GCS | Deceased | mRS0–2 (%) | Time from diagnosis to surgery, days (median) |
|---|---|---|---|---|---|---|---|
| Stefini et al (1999)30 | 3 | 46.3 (40–54) | 3 (100%) | 4–7<9=3 (100%) | 0 | 66% | 1.0 |
| Keller et al (2005)6 | 4 | 47 (37–66) | 3 (75%) | Mean 10.2 (6–13)<9=1 (25%)9–14=3 (75%) | 0 | 100 | 1.0 |
| Lanterna et al (2009)22 | 3 | 40.6 (32–51) | 1 (33%) | 3–8<9=2 (66%)9–14=1 (33%) | 0 | 66 | ? |
| Coutinho et al (2009)7 | 3 | 43 (36–55) | 2 (66%) | 5–13<9=2 (66%)9–14=1 (33%) | 33% | 66 | 1.5 |
| Theaudin et al (2010)13 | 8 | 45 (18–68)41.7 (18–68) | 5 (62.5%) | 7 (6–9)<9=5 (62.5%)>9–14=3 (37.5%) | 12.5% | 37.5 | 1.5 |
| Lath et al (2010)29 | 11 | 30.4 (18–46) | 8 (73%) | 3–14<9=2 (18.1%)9–14=9 (81.9%) | 27% | 73 | 1.0 |
| Ferro et al (2011)16 | 69 | 42 (15–68) | 52 (75.4%) | <9=72.1%9–14=27.5% | 15.9% | 56.5 | 2.0 |
| Rajan et al (2012)28 | 34 | 31.6 (18–65) | 21 (61.8%) | 8.3 mean (4–13)<9=25 (73.5%) | 17.6% | 76.5 | ? |
| Zuurbier et al (2012)12 | 10 | 41 (26–52) | 8 (80%) | <9=30%9–14=70% | 20% | 60% | 2.0 |
| Aaron et al (2013)10 | 44 | 35.6 (19–60) | 27 (61.3%) | <9=31%9–14=52% | 20.4% | 77a | 1.0 |
| Raza et al (2014)31 | 7 | 40 (15–60) | 3 (43.9%) | <9=14.3%9–14=85.7% | 28.6% | 57.1 | 5.0 |
| Mahale et al (2017)32 | 30 | 36.7 (19–65) | 15 (50%) | 6.7 (4–10)<9=28 (93.4%)% | 26.7% | 66.7% | 2.0 |
| Current study (2020) | 26 | 34.5 (24.7–44.7) | 20 (76.9%) | <9=21 (80.8%)9–14=5 (19.2%) | 42.3% | 34.6% | 2.0 |
Certain limitations should be acknowledged. First, this is a retrospective analysis of a consecutive group of CVT patients; therefore, we have the biases of an observational study. Second, the sample size of severe CVT cases was small, which is understandable given the typically good functional outcome in CVT patients. Third, the type of surgery, the duration of the procedure and the clinical variables used to determine whether to perform DC were established according to the clinical judgment of the treating physician and not to a formal or standardized protocol, which should be addressed in a clinical trial with clear clinical and imaging variables to determine whether this sort of procedure should be performed.
In conclusion, patients with severe forms of CVT, we found higher mortality than previously reported. DC is an effective life-saving treatment with a good functional prognosis for survivors. Clear indications for this technique should be explored in clinical trials or prospective registries.
FundingThis study did not receive any special funding.
Conflicts of interestThe authors confirm that there are no relevant conflicts of interest.