To present 16 patients with schizencephaly and neurological involvement, and analyse their characteristics and neuroimages.
Material and methodsThe study included 16 patients, 8 males and 8 females, all of whom were diagnosed with schizencephaly at less than 3 years of age; 2 patients were diagnosed prenatally. Schizencephaly was identified by computerised tomography (CT) in 1 patient and by MR or three-dimensional MR (3DMR) with a 1.5tesla apparatus in the others. Most patients were referred for evaluation because of psychomotor delay, motor disabilities and/or seizures.
ResultsFive patients had bilateral schizencephaly with open lips (2 of them had suffered intrauterine cytomegalovirus infections); 2 showed unilateral schizencephaly with separated lips, 8 presented unilateral schizencephaly with fused lips, and 1 had schizencephaly with open lips on one side and fused lips on the other. Prenatal cytomegalovirus infection was diagnosed in 2 patients. A cerebral malformation that affected the midline was diagnosed by routine ultrasound studies in 2 patients. Eight patients (50%) presented with seizures that were focal, except for one patient who showed secondary generalisation. The latter was the only patient whose disease was refractory to complete seizure control with antiepileptic medication. All patients had some degree of motor deficit, which was either unilateral (hemiparesis) or bilateral (tetraparesis).
Conclusion3DMR imaging was very important in diagnosing of schizencephaly in our patients because it showed the polymicrogyria that covered the area of the cleft and permitted us to rule out porencephaly. Neuronal migration disorders such as heterotopias and, more frequently, cortical dysplasias, were observed in several patients. Half of the patients had epilepsy which was controlled with antiepileptic medication, except in 1 patient.
Presentar 16 pacientes con esquisencefalia y afectación neurológica recalcando sus características clínicas y de imagen.
Material y métodosSon 16 pacientes, 8 varones y 8 mujeres, todos ellos con edades por debajo de los 3 años al hacerse el diagnóstico de esquisencefalia. En dos casos el diagnóstico se hizo prenatalmente y, en los otros 14, a lo largo de los cinco primeros años de vida. El diagnóstico se hizo por tomografía computarizada (TC) en un caso y por RM tridimensional (RM3D) con un aparato de 1,5 T en los otros casos. Los motivos de la consulta fueron retraso psicomotor, trastornos motores y/o crisis epilépticas en la mayoría de los pacientes.
ResultadosLa esquisencefalia era de labios abiertos bilaterales en 5 pacientes (dos de ellos por citomegalia durante la gestación), labios abiertos unilaterales en 2 pacientes, 8 mostraban esquisencefalia unilateral de labios cerrados y 1 tenía esquisencefalia de labios abiertos en un lado y cerrados en otro. En dos pacientes se diagnosticó infección prenatal por citomegalovirus y en otros dos se diagnosticó malformación cerebral central prenatal por ecografía rutinaria durante la gestación. Todos los pacientes presentaban algún tipo de deficiencia motriz uni o bilateral. Ocho pacientes padecían crisis epilépticas (50%) parciales en todos los casos y solo en uno de ellos se generalizaban. Este último caso fue el único en el que las crisis no llegaron a ser controladas. Todos los pacientes presentaban algún tipo de deficiencia motriz, generalmente benigna, unilateral (hemiparesia) o bilateral (tetraparesia).
ConclusiónLa RM3D es muy importante para el diagnóstico de la esquisencefalia ya que permite ver la capa de polimicrogiria que tapiza los labios de la malformación y, por ello, la diferenciación con las cavidades porencefálicas. Alteraciones de la migración, tales como heterotopias y especialmente displasias corticales se observaban en varios pacientes. Un 50% de los pacientes presentaba epilepsia, que fue controlable con medicación en todos los casos menos en uno.
Schizencephaly is a congenital malformation of the cerebral hemispheres consisting of a cleft in the mantle of 1 or both hemispheres, with communication between the lateral ventricle and the subarachnoid space.
The term ‘schizencephaly’ was first used by Yakovlev and Wadsworth in 2 different studies to describe a congenital defect in one or both cerebral hemispheres causing communication between the lateral ventricles and the ipsilateral subarachnoid space. The defect described in the first study was a fused cleft that did not allow cerebrospinal fluid (CSF) to pass (closed-lip schizencephaly1). The second study described a cleft of irregular width allowing CSF to pass between the ventricular cavity and the subarachnoid space. This malformation is also known as open-lip schizencephaly.2 Schizencephalic clefts, both fused (closed lips) and separated (open-lip clefts which may be extremely large), are covered by abnormal grey matter with characteristics of polymicrogyria. Before the advent of magnetic resonance imaging (MRI), especially three-dimensional imaging (3D MRI) which reveals the polymicrogyric layer, the edges of the cavity appeared to be smooth. It was therefore difficult to differentiate porencephalic, postnecrotic, and postsurgical cavities (which have smooth edges due to cicatricial gliosis) from schizencephalic cavities, which are covered by polymicrogyric grey matter.
At present, we can easily distinguish between the 2 types of pathologies by using 3D MRI.3 Although most cases of schizencephaly described to date seem to appear sporadically, other cases are linked to family history,4–8 and some are associated with mutation of the EMX2 gene.9 Schizencephaly belongs to the group of malformations of cortical development which are frequently associated with malformations such as abnormal neurogenesis and neuronal migration and organisation disorders. These malformations lead to microcephaly, lissencephaly-pachygyria, grey matter heterotopia, and schizencephaly.10 The prevalence of schizencephaly ranges between 3%11 and 11%12 compared to the total of congenital malformations of the cerebral mantle and cortex. Most consultations with a paediatric neurologist are requested due to psychomotor developmental delay and seizures.11–14
This study was published in order to analyse 16 paediatric patients who were first examined by the paediatric neurology department at Hospital Universitario La Paz. All patients but one underwent a 3D MRI study.
Material and methodsOur series included 16 patients (8 males and 8 females) (Table 1) aged 0 to 3 years who were referred to our hospital for a neurological examination; multiple motives for referral were cited in most cases. Signs and symptoms were as follows: developmental delay and motor problems (unilateral or bilateral) in almost all patients, with differing degrees of severity; cranial nerve involvement, especially cranial nerve VI (bilateral in several cases), and focal seizures (50% of the children also experienced generalised seizures). The most common cause for referral was when paediatricians requesting imaging studies in primary or secondary care centres discovered abnormalities that many considered to be alarmingly complex. On other occasions, doctors failed to identify the problem correctly and therefore requested either a second or third opinion or a higher-quality neuroimaging study.
Schizencephaly clinical and radiological findings in 16 patients.
| Case | Sex | Age at 1st visit | Reason for visit | Gestation | Birth | Symptoms | EEG | Image | Treatment | Progress |
| 1 | F | NN | Schizen. IU | Cytomegalovirus | Normal | Microcephaly | General hypoact. | Schizen. BLOL; SP absent | Stim.; physio. | Lost to follow-up in 1st year |
| 2 | M | NN | Schizen. IU | Cytomegalovirus | Normal | Microcephaly | Normal | Schizen. RCL; SP absent | Stim.; physio. | Lost to follow-up in 1st year |
| 3 | F | 2 y | PMR; L hemiparesis | Normal | Normal | L hemiparesis | Normal | Schizen. RCL; SP absent | Physio. | L. Hemiparesis; borderline IQ |
| Lost to follow-up at 15 y | ||||||||||
| 4 | M | 3 y | PMR, L hemiparesis, seizures | Normal | Normal | L hemiparesis | BL foc. abnorm. | ROL schizen.; RCL schizen.; RH cort. dyspl.; SP absent; hypopl. CC | Physio.; AED | At age 19 y: L hemiparesis; PMR; AED |
| 5 | F | 2 y | L hemiparesis, seizures | Normal | Normal | Hemiparesis L | Normal | RCL schizen.; R cort. dyspl.; SP absent | Physiot.; AED | At age 14 y: L hemiparesis |
| 6 | M | 2 y | L hemiparesis | Normal | Normal | L hemiparesis | Normal | RCL schizen.; SP absent | Physio. | At age 8 y: Lost to follow-up. L hemiparesis |
| 7 | M | 3 y | L hemiparesis, seizures | Normal | Normal | Hemiparesis L | Normal | RCL; SP absent | Physio. AED | At age 14 y: L hemiparesis |
| 8 | M | 3 y | Tetraparesis; seizures | Risk of miscarriage in 4th and 6th months | Dystocia | Severe PMR; spastic tetraparesis | BL impairments | BLOL schizen.; SP absent | AED, physio.; stim; orthop. | At age 16 y: spastic tetraparesis; severe PMR |
| 9 | F | 1 y | L hemiparesis | Normal | Normal | L hemiparesis | Normal | RCL schizen.; SP absent | Physio.; stim. | At age 10 y: Borderline IQ |
| 10 | M | 6 m | PMR | Risk of miscarriage in 5th m | Normal | PMR | Normal | ROL schizen. | Stim.; physio. | Lost to follow-up at 2 y. Severe PMR |
| 11 | M | 3 y | Spastic tetraparesis; PMR; seizures | Diagnosis (US): CC agenesis, porenceph. | Normal | Tetraparesis, PMR | Hypoact. in RH | BLOL schizen.; SP absent; CC defect | Stim.; physio.; orthop.; botu. toxin | At age 12 y: PMR; orthop. |
| 12 | M | 5 m | Seizures; PMR | Diagnosis (US): HPE | Normal | Tetraparesis; seizures | BL impairments | BLOL schizen.; LCL schizen.; SP absent | AED, stim.; physio.; orthop.; AED | Discharged at age 15 y; PMR; AED |
| 13 | F | 2 y | R hemiparesis; PMR | Normal | Normal | R hemiparesis; PMR | Normal | LOL schizen.; SP absent | Physio.; stim. | Lost to follow-up at 4 y: PMR: R hemiparesis |
| 14 | F | 1 y | PMR; seizures | Normal | Normal | L hemiparesis, seizures | RH focus | RCL schizen.; SP absent | AED; physio. | At age 6 y: seizures controlled with AED; L hemiparesis |
| 15 | F | 8 m | R Hemiparesis; seizures | Normal | Normal | R Hemiparesis; seizures | Normal | LCL schizen. | AED; physio. | Lost to follow-up at 5 y; seizures controlled with AED |
| 16 | F | NN | Cytomegalovirus | Cytomegalovirus | Normal | Microceph.; seizures; PMR | BL impairments | BLOL schizen. | Stim.; physio.; AED | Lost to follow up in 1st year |
F: female; M: male; NN: neonate; y: years; m: months; L: left; R: right; schizen.: schizencephaly; IU: intrauterine; PMR: psychomotor retardation; hypoact.: hypoactivity; gen.: generalised; BLOL: bilateral open-lip; ROL: right open-lip; RCL: right closed-lip; LCL: left closed-lip; stim.: stimulation; physio.: physiotherapy; SP absent: absence of septum pellucidum; IQ: intelligence quotient; BL foc. abnorm.: bilateral focal abnormalities; cort. dyspl.: cortical dysplasia; hypopl.: hypoplasia; CC: corpus callosum; AED: antiepileptic drugs; porenceph.: porencephaly; hypoact.: hypoactivity; LH: left hemisphere; RH: right hemisphere; botu. toxin: botulinum toxin; HPE: holoprosencephaly.
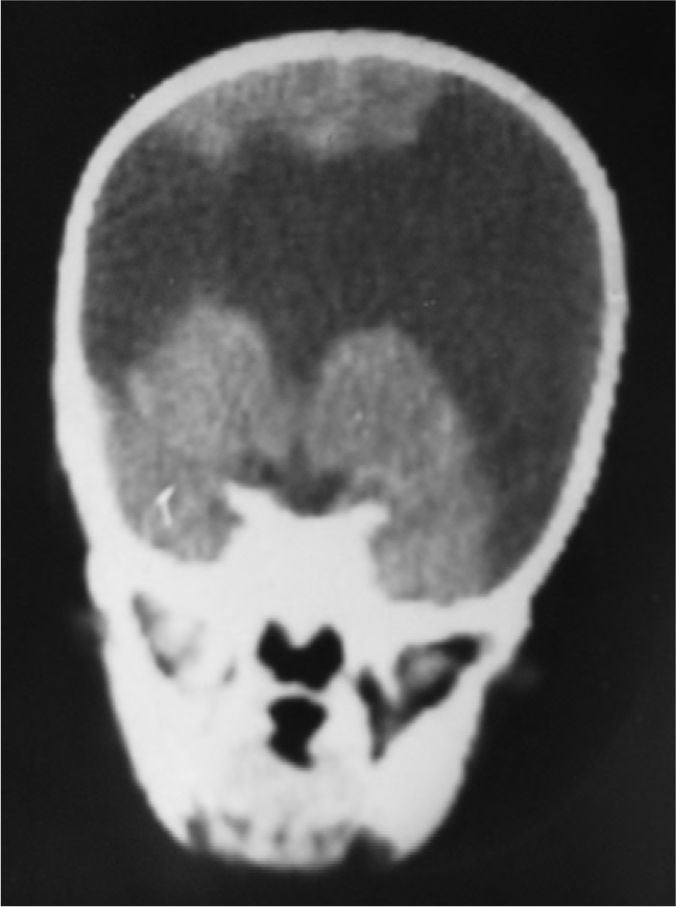
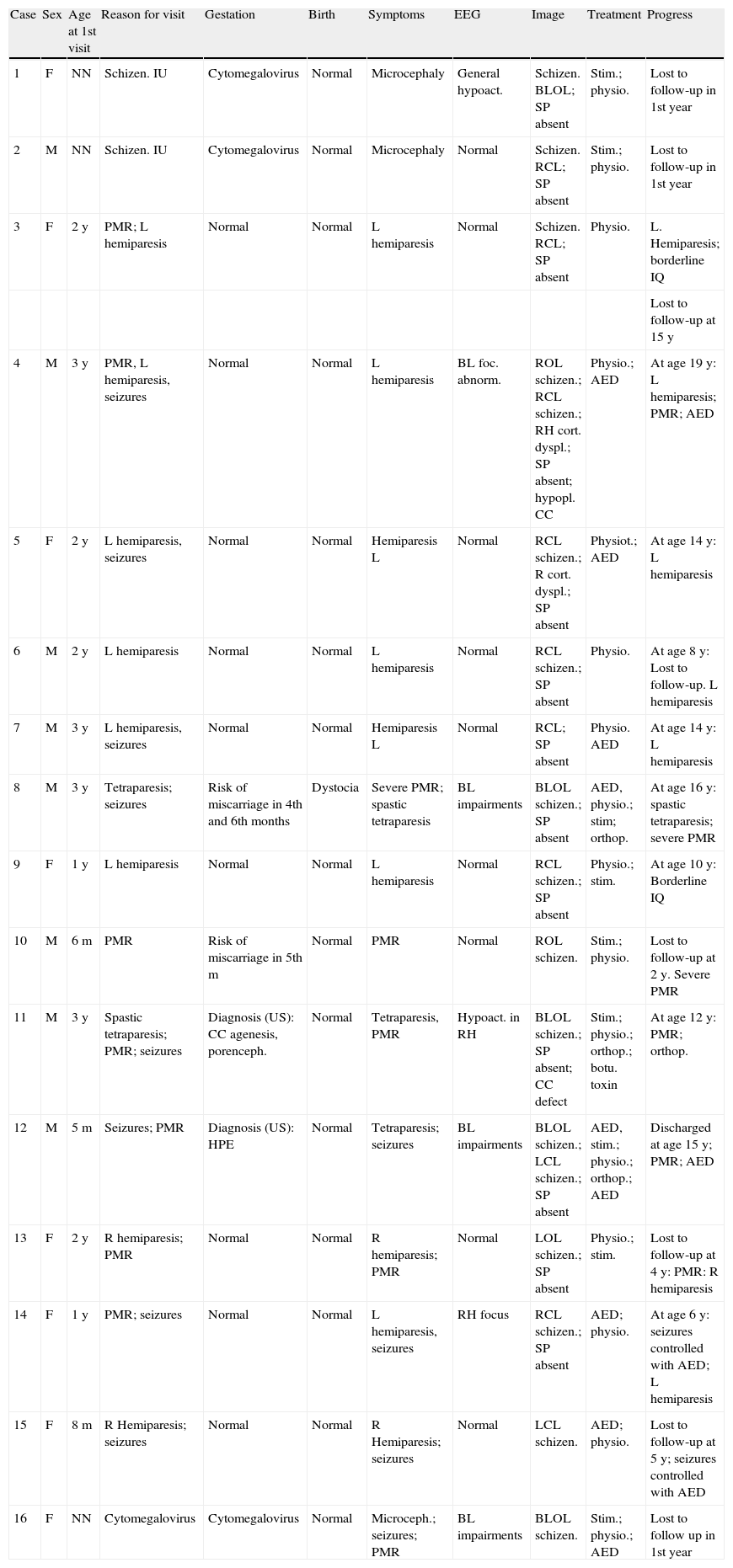
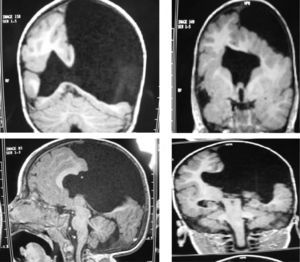
The study protocol included recording medical history (especially prenatal and perinatal); family history of disease and consanguinity between parents/grandparents; psychomotor development prior to the consultation; prior medication and its effect on seizures; findings from the physical examination we performed; results from laboratory analyses (3 patients suffered intrauterine cytomegalovirus infection); EEG study; and 3D MRI study. Schizencephaly was diagnosed based on 3D MRI findings in all but 1 patient (a newborn baby). The computed tomography (CT) scan of that baby revealed obvious bilateral schizencephaly with very open lips, an extremely severe gap in the hemispheric mantle, and total lack of corpus callosum, septum pellucidum, and grey commissure (Fig. 1).
The 3D MRI studies were performed using a GE 1.5tesla scanner, Excite 11 software, 8-channel head coil, T1 weighted 3D SPGR (spoiled gradient echo) sequence along the axial plane of the entire cranium, TE 6ms, TR 20ms, flip angle 30°, RBW 14.71, fov 28, slice thickness 1.4mm, matrix 224×224, nex 1, zip 512, voxel size 0.55mm×0.55mm×1.4mm=0.42mm3. Duration: 9.37min.
ResultsAll patients presented some degree of developmental delay, which was very mild in cases of closed-lip schizencephaly, particularly in those cases with only 1 affected hemisphere (cases 6, 7, 9, 10, 13, 14, and 15). These patients experienced occasional or no seizures and suffered no relapses after beginning treatment with antiepileptic drugs. Eight of the patients experienced occasional epileptic seizures. All but 2 (cases 2 and 15) patients showed focal and generalised EEG abnormalities in the EEG, which were not severe in most cases. Although the most extreme open-lip cases presented the most severe psychomotor symptoms, some of these patients did not experience epileptic seizures or EEG abnormalities after the first years of life (cases 8 and 11).
The only sign of developmental delay in most of the patients with unilateral closed-lip schizencephaly was lack of initiative, difficulty keeping up with children of similar ages during games, mild language acquisition delay, and mild lack of motor coordination on the side of the body opposite to the affected side of the brain. Bilateral lack of motor coordination was also observed when lips (whether open or closed) were not extensive. After localising the schizencephaly with the help of a 3D MRI, some of the patients had to undergo an additional neurological examination in order for us to identify asymmetries in motricity, limb positioning, and myotatic reflexes, as the initial, less detailed neurological examination provided normal results in these areas. Five patients presented bilateral open-lip schizencephaly (cases 1, 8, 11, 12, and 16). Two were caused by intrauterine cytomegalovirus infection and the other 3 cases appeared to be spontaneous. In the 2 patients with bilateral open-lip schizencephaly (lips slightly separated) associated with cytomegalovirus during gestation (cases 1 and 16), developmental delay was more severe than in patients without this profile. It is true that in these patients the lateral ventricles were very wide and schizencephalic clefts were present on both sides. Their follow-up period was short. The patients with schizencephaly of unknown origin (that is, all patients except those whose condition was caused by cytomegalovirus) were monitored over several years and experienced gradual improvements. Although neurological involvement was severe, it was less pronounced than doctors would expect in light of the MRI images. In any case, all patients presented varying degrees of motor and intellectual disability. They all experienced hemiparesis or spastic quadraparesis depending on whether schizencephaly was unilateral or bilateral. Three patients with pronounced open-lip bilateral schizencephaly (cases 8, 11 and 12) had serious speech deficiencies; they were unable to produce speech sounds and were only able to communicate through gestures.
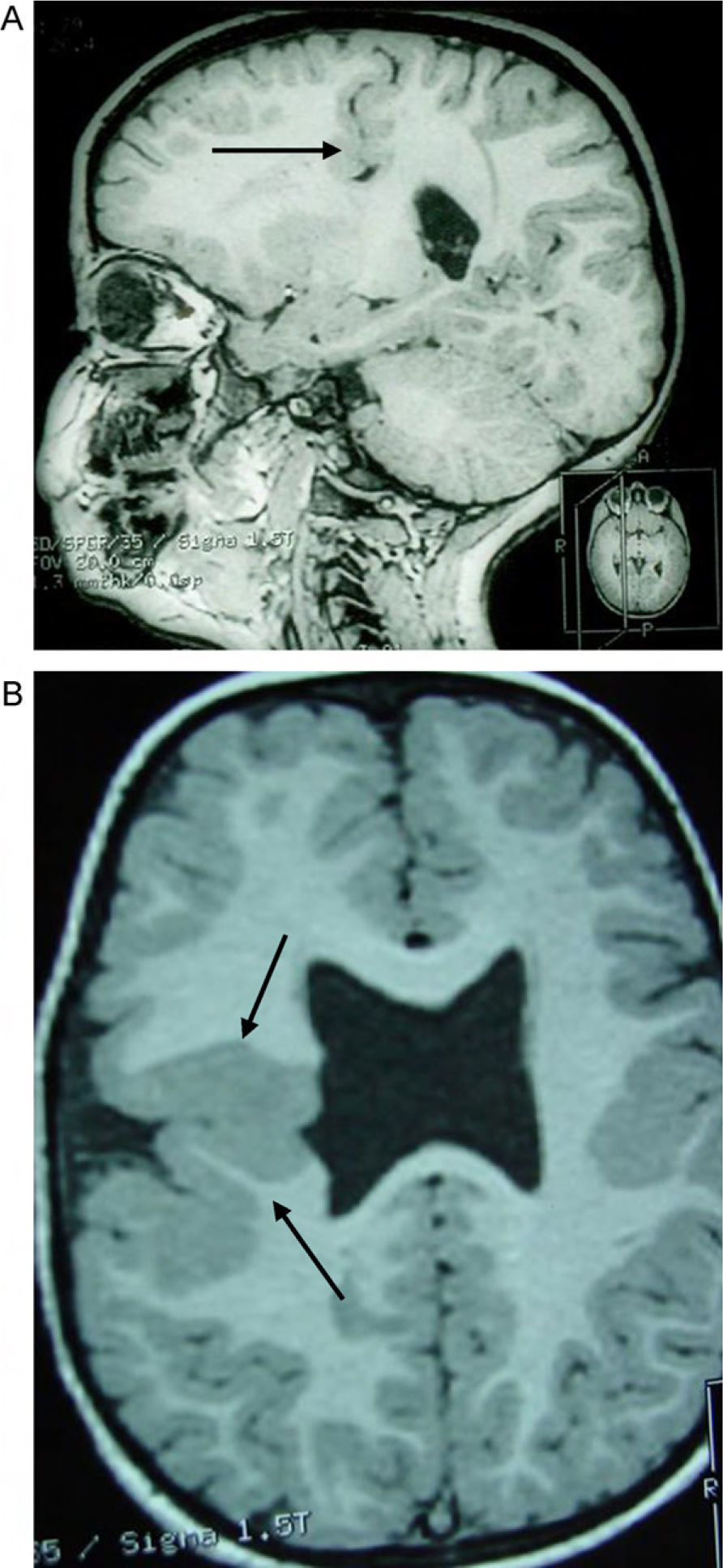
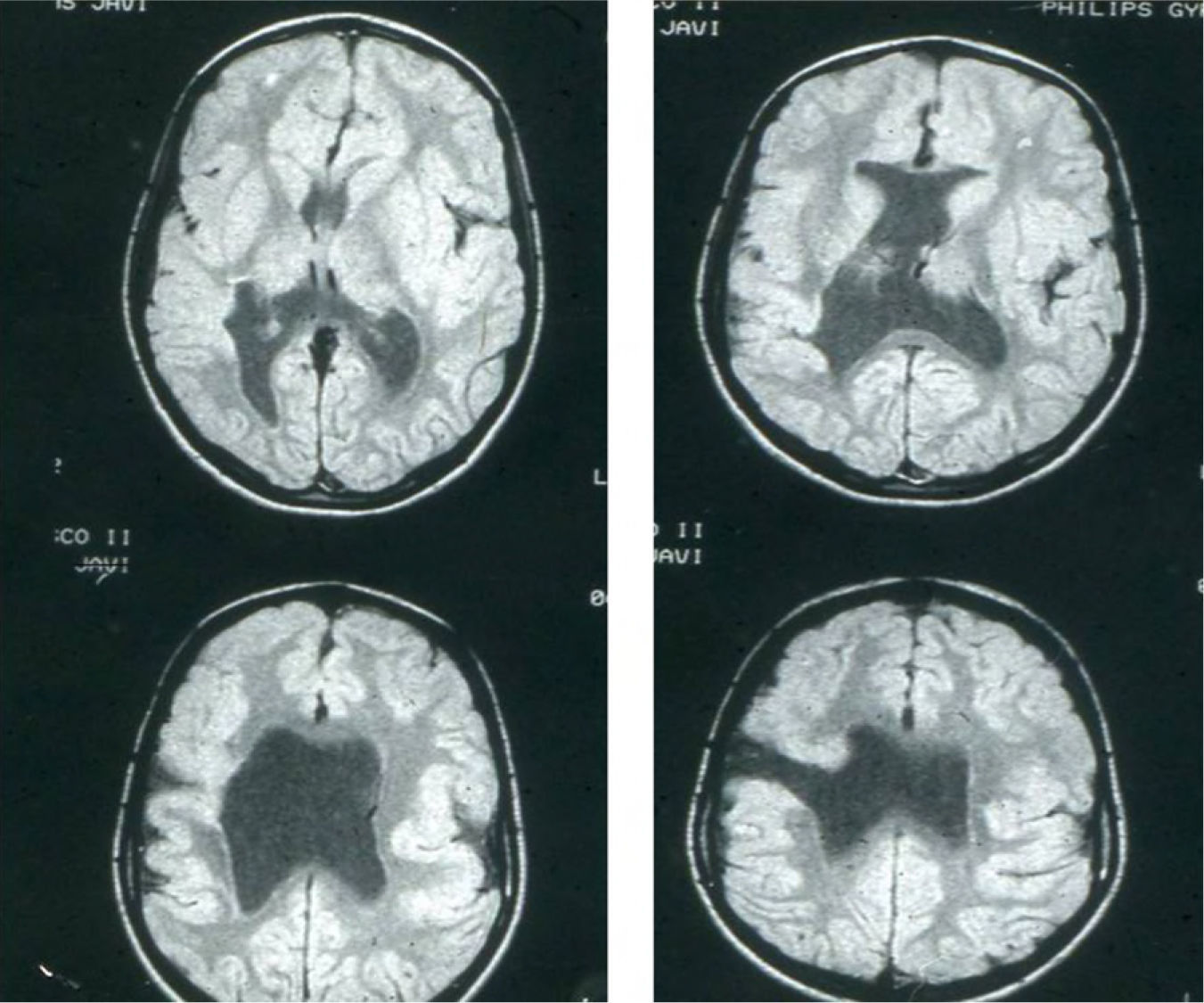
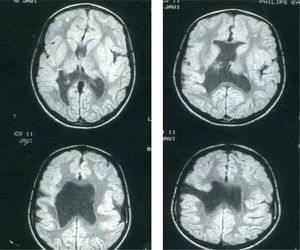
Head circumference did not exceed the 75th percentile in any of the cases, with just one patient reaching that percentile. In 4 patients, circumference was below the 25th percentile (the 3 patients affected by cytomegalovirus and case 8 without CMV). Head circumferences in the rest of the patients fell between the 25th and the 50th percentiles. We did not find any cases of hydrocephalus with elevated intraventricular pressure in the direction of the cerebral parenchyma. The polymicrogyric edges of the open and closed lips were clearly visible using 3D MRI, and also by using other MRI techniques employed to differentiate white matter from grey matter, although findings revealed by those procedures are not as clear as those in 3D MRI. During gestation, 2 patients were diagnosed with cerebral malformations by an ultrasound study; 1 case was diagnosed as holoprosencephaly and the other as porencephaly. After birth, they were all diagnosed with schizencephaly with the aid of a 3D MRI study. The 3 patients with the most extreme cases of open-lip schizencephaly (cases 8, 11, and 12) were males, whereas 5 of the 7 patients with unilateral closed-lip schizencephaly (cases 3, 5, 9, 14, and 15) were females and 2 were males (cases 6 and 7). Performing axial, coronal and even sagittal slices (Figs. 2 and 3) was of vital importance in order to identify the types of schizencephalic lips in each hemisphere and their potential association with heterotopia and cortical/subcortical dysplasia. These defects were typically found in areas adjacent to those with polymicrogyria covering the edges of the lips. They could also be located in other areas of the ipsilateral and/or contralateral cerebral hemisphere cortex. Additionally, they revealed the circumscribed or diffuse location of the different malformations, especially in the cerebral cortex, corpus callosum, and septum pellucidum (Fig. 4), all of which appeared to be more or less affected in all cases. The septum was also completely absent in all cases. A patient with extreme open-lip schizencephaly was the only one whose seizures could not be controlled completely.
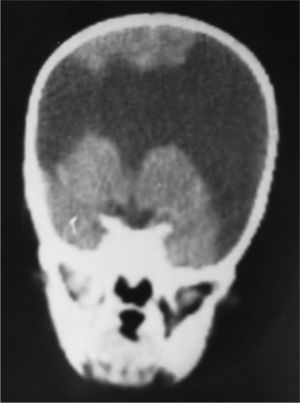
Case 3: unilateral closed-lip schizencephaly in a 2-year-old girl with slight motor impairment on the left side of the body. 3D MRI. (A) Sagittal slice showing closed lips cutting an irregular path from the cortex to the lateral ventricle (arrow). (B) Axial slice showing extensive cortical–subcortical dysplasia in the right hemisphere extending from the sunken area of the cortex to the lateral ventricular wall. The profile is irregular, but this slice does not allow us to see the fused schizencephalic cleft. Small cortical dysplasia with cortical irregularity (arrows).
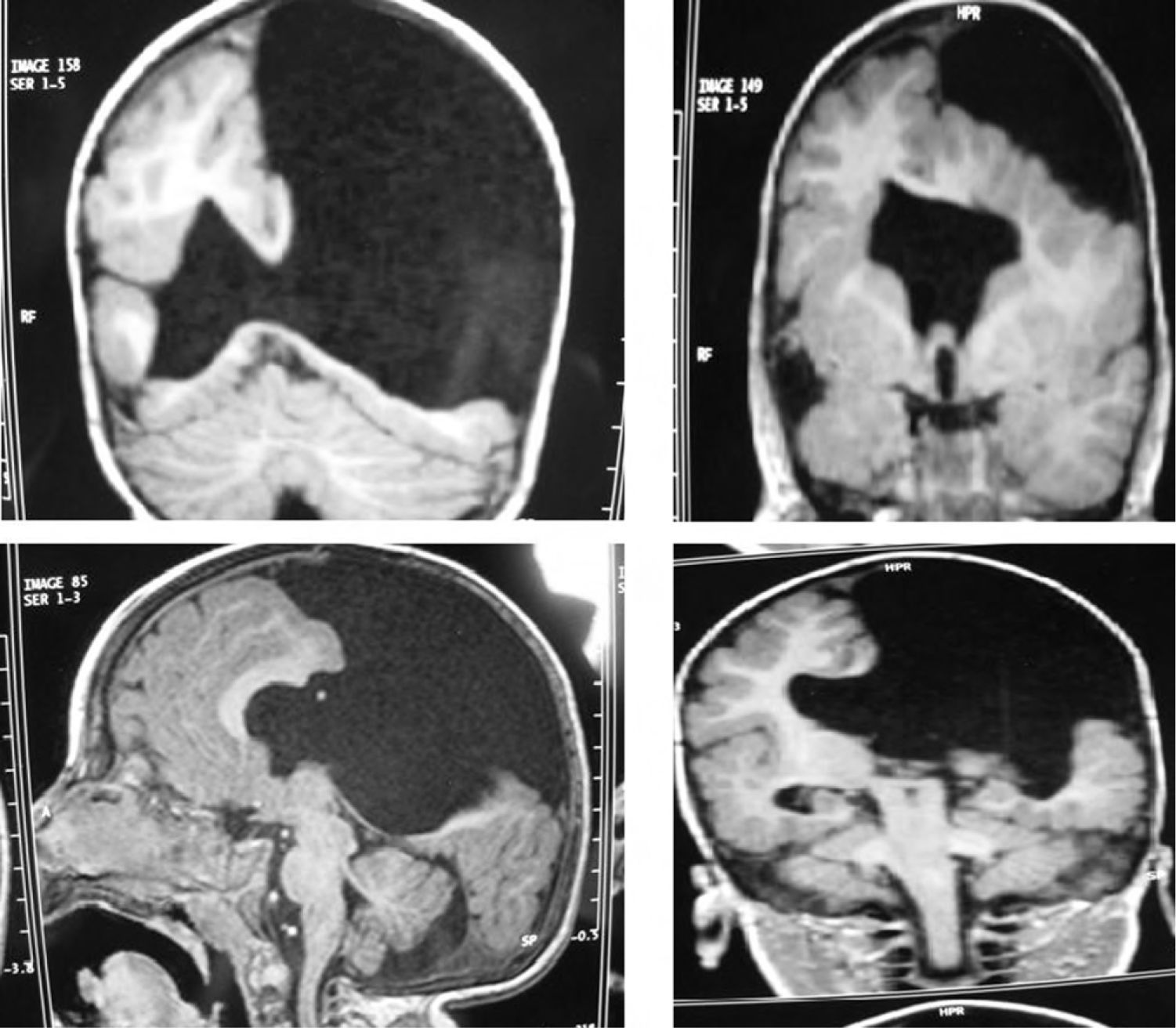
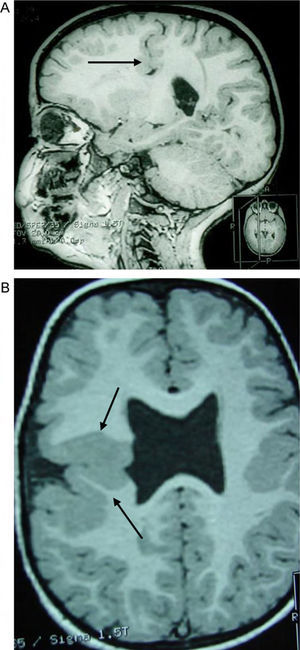
Case 4: unilateral open-lip schizencephaly in a 5-year-old child. Four axial slices at different levels; only 1 shows the schizencephaly in the right hemisphere, while cortical–subcortical dysplasia can be seen in a number of regions in both hemispheres. Septum pellucidum is absent and corpus callosum is very thin.
When discussing schizencephaly, it is important to consider a number of aspects, including its origin, relationship with other types of brain hemisphere pathologies, neurological symptoms (especially those related to motricity, intelligence and epileptic seizures), the most applicable imaging studies, relationship with other neuronal migration and cortical organisation disorders, differential diagnosis, and disease progression.
Schizencephaly seems to originate sporadically in most cases, but some hereditary cases have also been described.4,9 They are occasionally related to mutations of the homeobox gene EMX2, which is located in the 10q26.1 chromosome.9 However, the same gene was also found to be affected in a series of 7 sporadic cases.15 In turn, we could not find mutations in the EMX2 locus in cases with a family history of schizencephaly spanning 3 generations.16 Embryonic malformations are more likely to appear between the third and fourth months of gestation,17 instead of during the first 2 months as Yakovlev hypothesised.1 As researchers have occasionally reported, it is possible to diagnose the condition by using an imaging test during gestation.18 This was performed for 2 patients in our series (cases 11 and 12), both of whom had severe clefts in the cerebral mantle. Three patients suffered intrauterine infection with cytomegalovirus (cases 1, 2 and 16), a finding which has also been described in the literature.19,20 These patients’ ventricular walls showed thin calcifications. Other aetiologies, such as intrauterine vascular problems,21 have also been mentioned as potential causes of the condition. Even if this would be difficult to prove, considering the early stage during which they could cause the condition, such aetiologies may be more common than the literature would suggest. Intrauterine presence of this hemispheric malformation could well have caused premature birth in 20% of the subjects in one of the largest series ever published13; in the general population, the rate is only 10%.22 The 2 patients in our series who were diagnosed during gestation (cases 11 and 12) were born full-term.
In intrauterine imaging studies, large open-lip schizencephaly may be mistaken for wide porencephalic cavities. However, this error can only occur in the final months of gestation, when the imaging study of the fetus is performed using rudimentary methods. This is because polymicrogyric edges of the lips in the entrance of the schizencephalic cavities can be identified perfectly by using 1.5T or 3.0T MRI, or even modern ultrasound systems. Confusion between images of porencephalic cavities and schizencephalic clefts is really a problem of the past. The 2 patients mentioned above were both diagnosed in utero with a single frontal cavity (1 case of holoprosencephaly and 1 case of porencephaly, as a result of the absence of septum pellucidum in both patients). Hypoplasia of the optic nerves has been reported in up to 30% of all schizencephaly cases; these conditions, together with the absence of septum pellucidum, lead to malformations associated with septo-optic dysplasia.23,24
The 3D MRI scan provides clear images of schizencephaly. However, in our experience, it is necessary to obtain every possible type of slice of the brain (especially the sagittal, axial and coronal planes). We also recommend reconstructing the cerebral cortex of both hemispheres and, in some cases, performing oblique slices to obtain complete surface and deep views of the cerebral hemispheres. We found no cerebellar abnormalities. The degree of open-lip schizencephaly differs (small, medium and large) and their characteristics in MRI imaging were described several years ago.22,25 Abnormalities in the 3D MRI, seen as schizencephaly in the cerebral hemispheres, are not usually limited to the fused clefts between cortical spaces and the lateral ventricles in closed-lip schizencephaly or in the more or less separated clefts seen with open lips. Cleft edges are covered by polymicrogyria, which appears from the cortex to the inner ventricle in both forms of the malformation. Cortical or cortical–subcortical areas of dysplasia are also frequently found in regions adjacent to schizencephalic clefts or in other areas. This association has been reported ever since doctors first discovered the importance of MRI imaging for identifying this pathology,26 and it was later demonstrated in large series13,14,22 (only a limited number of patients with this malformation were included in these series, but we also observed this association in most of our patients after performing a complete imaging study). According to some studies, fused and separated clefts connecting the cortical and ventricular spaces largely occur in areas closest to the cortex. In our series, however, we found very heterogeneous locations. The septum pellucidum was absent in all the open-lip and closed-lip schizencephaly cases in our series. We also found abnormalities in the corpus callosum (general narrowing or partial absence in some of the cases, especially in those cases with open-lip malformations).
Clinical abnormalities mainly consisted of unilateral or bilateral motor difficulties (depending on the hemisphere or hemispheres in which the malformation was present) and epileptic seizures (simple partial or complex in most cases).27–29 Occasionally, the patients also presented infantile spasms and tonic, clonic and tonic-clonic seizures. The reported prevalence of epilepsy in this population is between 33%13 and 57%22 and it is related to the type of schizencephaly. Prevalence is higher in patients with open-lip malformations. Although early onset of seizures is more common in cases with open-lip malformations located proximal to the area between the ventricles and the cortex, seizure severity and type do not seem to be related to the location of the defect.9,22 Most cases of seizures can be controlled with medication, and this was true in almost all of our patients. The literature describes cases with medically intractable epilepsy that were successfully treated with surgical resection of the epileptogenic region27 or the temporal lobe which is also involved in adult patients.29 Although the results were considered partially positive, the treatment was never widespread and nearly 20 years have passed since it was last used. As a result, conservative treatment may be the best solution in a large percentage of cases, especially considering that increasingly effective antiepileptic drugs are being made available.
Some studies report a link between hydrocephalus and open-lip schizencephaly.22 Nevertheless, we were unable to observe an increase in the pressure gradient in areas showing increased CSF inside the cerebral parenchyma. We observed no cases of macrocephaly (head circumference above the 75th percentile) among our patients. Between 57%13 and 83%22 of patients in large series present problems that accompany schizencephaly. These mainly manifest as motor impairment on one side of the body or part of it, or on both sides; this is associated to a greater or lesser extent with intellectual, language, or other sensory deficits. Intellectual disabilities among these patients vary greatly, but they are never as severe as the magnitude of the cerebral lesion would lead us to believe.9,28,30,31 Patients with the most severe intellectual and motor disabilities tend to have a wide lesion affecting both hemispheres, the septum pellucidum, and corpus callosum, with a wide space between the parenchymatous regions located between the lips of the schizencephaly.
ConclusionWe can draw a number of conclusions based on this series. The first is that it was impossible to find the cause of either type of malformation in any of the patients except for the 3 who suffered from prenatal cytomegalovirus infection. In these cases, there was a clear relationship between a viral infection during gestation and the malformation of the cerebral mantle. Likewise, we did not determine the reasons explaining the different degrees of hemispheric malformation (some cases had closed lips marking only a very thin furrow; others had large bilateral cavities with central and lateral parenchymatous spaces which could not be linked to any specific pathology). The characteristics of the placenta and umbilical cord, and the absence of haemorrhages, maternal intoxication, etc., did not allow us to formulate a well-founded suspicion of prenatal pathology. We confirmed the diagnosis of the cerebral malformation using 3D MRI; this could also have been performed using other MRI sequences with 1.5T and 3.0T systems. We observed that 50% of the patients had epileptic seizures, which were mainly focal. In general, patients responded well to antiepileptic drugs. Epilepsy control was achieved in all patients but one, who presented a large bilateral open-lip malformation. Both motor and intellectual developmental delays were directly related to the extent of the malformation. All patients with unilateral closed-lips schizencephaly presented relatively mild contralateral hemiparesis and a ‘borderline’ or normal-to-low intellectual level.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pascual-Castroviejo I, et al. Esquisencefalia. Estudio de 16 pacientes. Neurología. 2012;27:491–9.