To ascertain the opinions of an Epilepsy Expert Group and prepare a consensus document on the definition of drug-resistant epilepsy (DRE) according to the International League Against Epilepsy (ILAE) and the different healthcare levels for the patient with epilepsy in Spain.
Materials and methodsThe study was conducted using the Delphi method, by means of successive rounds of questionnaires. A scientific committee prepared a preliminary document and fourteen associated questions, which were sent by e-mail to the panel of experts. They included items related to the concept of DRE, health care levels and the route between these levels for patients with DRE.
ResultsA total of 41 experts answered the questionnaire. They agreed regarding the necessity and applicability of the DRE definition according to the ILAE, the need for an expert panel on epilepsy, specialist epilepsy clinics, and clinical epilepsy units stratified depending on the level of activities they carried out. There was moderate consensus on the resources and activity of the clinical units of reference and there was no consensus on the referral of patients who have suffered an epileptic seizure to an epilepsy clinic.
ConclusionsThe expert panel agreed with the definition of DRE according to the ILAE and on referring patients with DRE for a detailed study in an epilepsy clinic or epilepsy clinical unit. They highlighted the need for video-EEG monitoring in the study of patients with DRE and the need to propose other forms of treatment in selected patients.
Conocer la opinión de un colectivo de expertos en epilepsia y elaborar un consenso sobre la definición de epilepsia resistente a fármacos (ERF) según la Liga Internacional Contra la Epilepsia (ILAE) y los distintos niveles asistenciales al paciente con ERF en España.
Material y métodosEl estudio fue realizado utilizando el método Delphi, mediante dos rondas sucesivas de cuestionarios. Un comité científico confeccionó un documento preliminar y catorce preguntas relacionadas y fueron remitidos por correo electrónico al panel de expertos. Se incluían ítems relacionados con el concepto de ERF, niveles asistenciales e itinerario entre dichos niveles de los pacientes con ERF.
ResultadosContestaron el cuestionario 41 expertos. Se alcanzó acuerdo sobre la necesidad y aplicabilidad de la definición de ERF según la ILAE, necesidad de la existencia del experto en epilepsia, consulta específica de epilepsia y unidades clínicas de epilepsia con diversa estratificación, según la graduación de actividades que se realicen. Existió moderado consenso con la dotación y actividad de las unidades clínicas de referencia y no hubo consenso sobre la remisión de pacientes que han presentado una crisis epiléptica a una consulta de epilepsia.
ConclusionesEl panel de expertos estuvo de acuerdo con la definición de ERF según la ILAE y en remitir a todo paciente con ERF a un estudio pormenorizado a una consulta de epilepsia o unidad clínica de epilepsia. Se resalta la necesidad de la monitorización vídeo-EEG en el estudio del paciente con ERF y el proponer otras formas terapéuticas en pacientes seleccionados.
Epilepsy is one of the most frequent neurological diseases, with a prevalence of 5–7 cases per 1000 inhabitants, depending on the age group.1 It is estimated that there are between 240000 and 340000 epilepsy sufferers in Spain. Some of these patients have seizures which cannot be controlled through pharmacological treatment; this is known as difficult-to-control, refractory, or drug-resistant epilepsy (DRE).2 Population-based prevalence studies on DRE in subjects aged 16 and older found between 0.94 and 1.36 cases per 1000 inhabitants,3 depending on the DRE concept applied. Figures were similar in the paediatric population.4 Extrapolation of these figures shows that between 45000 and 65000 people suffer from this condition in Spain. Patients generally experience lower quality of life, several associated morbidities, and a higher probability of early death compared to patients presenting controlled epilepsy. As a result, patients with DRE must receive rapid and personalised attention so as to provide the correct diagnosis, treatment, and support in a timely fashion.5
The International League Against Epilepsy (ILAE) has recently reached a consensus on its definition of DRE, describing it as epilepsy in which seizures of any type remain uncontrolled after treatment with two well-tolerated drugs, properly selected and assiduously taken, either in monotherapy or combination therapy. Epilepsy is considered to be uncontrolled when seizures occur within 1 year of beginning treatment or when the seizure-free interval increases, but does not reach 3 times the interseizure interval before beginning treatment. According to this consensus, all DRE patients should be rapidly and thoroughly evaluated in an epilepsy centre or unit in order to obtain a clear diagnosis and provide the best pharmacological treatment, or consider alternative treatment, the prime example being surgery.6
An opinion article published in the current issue of Neurología7 provides a detailed analysis of the ILAE's definition of DRE and proposes stratified and integrated healthcare networks including different clinical epilepsy units (CEU); some such units have already been constituted in Spain. Establishing a consensus to stratify levels of care based on solid, detailed criteria and considering maximum effectiveness and efficiency would be an excellent initiative for Spain. Its purpose would be to provide the best possible care to patients suffering from either controlled or drug-resistant epilepsy. The article mentions that although we do not know exactly how many CEUs will be needed, there should be enough units to guarantee rapid and easy access to all patients with epilepsy.7
The objective of the current study is to ascertain the opinion of a group of adult and paediatric neurologists specialising in epilepsy, and reach a consensus on the need for and applicability of the ILAE's definition of DRE and of the different levels of care proposed in the article mentioned above.7 To that end, we used the Delphi method to determine and integrate experts’ opinions on a particular topic and attempted to reach a consensus by using a set of consecutive questionnaires that were answered anonymously. This procedure allowed us to eliminate the difficulties and inherent biases stemming from face-to-face meetings, such as the influence of opinion leaders and lack of anonymity.8,9 In recent years, surveys addressed to experts have been widely used for obtaining recommendations in the field of epilepsy, and most surveys were aimed at producing a therapeutic consensus.10–13 The Delphi method used in these surveys delivers a greater degree of consensus, and therefore provides widely accepted results and recommendations on several epilepsy-related issues characterised by controversy or varying professional criteria.14–19
Materials and methodsStudy designThe study was divided into 3 phases. During the first phase, the Scientific Committee (Casas Fernández C, Gil-Nagel Rein A, Mauri Llerda JA, Salas Puig J, Sánchez Álvarez JC, and Sancho Rieger J) reviewed existing literature on the subject of DRE and the different levels of care providing treatment to patients with epilepsy. All members then met to co-draft a preliminary document. In addition to that document, they formulated and approved a list of questions on the subject of epilepsy that could generate debate. During the second phase, the preliminary document and the questions were sent by e-mail to a group of adult and paediatric neurologists specialising in the diagnosis and treatment of epilepsy. The aim was to obtain their opinion on the items using 2 successive questionnaires. During the last phase, results were analysed and discussed in an additional face-to-face meeting of the Scientific Committee. In this meeting, all members contributed to the drafting of the final document7 and this results report.
With the aim of establishing a consensus among members of the panel of experts, we used the Delphi method, which recognises the value of experts’ opinions, experience, and intuition when there is not sufficient scientific knowledge for establishing recommendations. This method was developed in the early years of the Cold War as a systematic and interactive way by which a group of independent professionals could predict the impact of technology on the war.8 Using this method, a selected panel of experts on a specific topic answered survey questions in 2 or 3 successive rounds from a remote location. After each round, the facilitator provided an anonymous summary of the opinions of all survey-takers, which all participants could then compare with own answers. Each participant then had the opportunity of giving a new answer, which could be the same as or different from the one provided previously. The purpose of using this iterative method is that it reduces the number of total answers and therefore approximates a consensus answer.9
Elaboration and development of the Delphi surveyResults from exhaustive literature searches in PubMed, Embase, and the Cochrane Library regarding the diagnosis, treatment, and action plans for DRE enabled the authors to draft the preliminary document “Drug-resistant epilepsy: recommendations regarding diagnostic and therapeutic performance in Spain”7 plus 14 questions on that subject (Table 1). These questions included items related to the definition of DRE provided by the ILAE, levels of care, and how DRE patients were referred between different levels of care. The document and questions were sent by e-mail to the panel of experts on epilepsy. During the first round of the Delphi process (DR1), experts could choose one of the following answers: agree completely, agree somewhat, neither agree nor disagree, disagree somewhat, or disagree completely. Participants were invited to add general or specific comments about the document and its questions. Fig. 1 shows the first question from DR1 as an example.
Questions submitted to the panel of experts, along with the preliminary draft corresponding to Ref. 7 in the first round of the Delphi process (question 13 appears according to its wording in the second round of the Delphi process, since it was rewritten at the request of the panel of experts and the scientific committee).
| With regard to the definition of drug-resistant epilepsy (DRE) given by the International League Against Epilepsy (ILAE), please give your opinion concerning the following statements: |
| Question 1. The definition is necessary from a clinical and scientific point of view. |
| Question 2. The definition is useful, applicable, and lends clarity to a concept that was previously poorly defined. |
| Question 3. In certain patients who may opt for elective DRE surgery, treatment with another antiepileptic drug, in addition to the 2 drugs required for a diagnosis of DRE, should be tested before proceeding with surgery. |
| With regard to levels of care and referral protocols for DRE patients, please give your opinion concerning the following statements: |
| Question 4. There is no need for all DRE patients to be evaluated in an epilepsy unit (2nd level of care). |
| Question 5. Follow-up for DRE patients should be provided by an epilepsy unit (2nd, 3rd and 4th levels of care). |
| Question 6. Epilepsy units that do not provide surgical treatment are justified (2nd level of care). |
| Question 7. Categorising medical and surgical epilepsy units as either basic units or units of reference is justified (3rd and 4th levels of care). |
| Question 8. The epileptologist is an adult or paediatric neurologist with 3 years of experience in diagnosing, treating, and caring for epilepsy patients. |
| Question 9. In a medical and surgical unit of reference (4th level of care), performing between 15 and 20 surgeries per year is sufficient to guarantee a good level of care. |
| Question 10. A medical and surgical unit of reference (4th level of care) must include 2 neurologists, 2 neurosurgeons, and 2 neurophysiologists. |
| What types of patients should be referred to a specialised epilepsy clinic? |
| Question 11. All patients who have suffered an epileptic seizure should be referred for evaluation and monitoring. |
| Question 12. Patients who do not achieve seizure control after treatment with an initial antiepileptic drug. |
| Question 13. It is not necessary to refer patients who meet DRE criteria. |
| Question 14. Patients whose diagnosis may be unclear. |
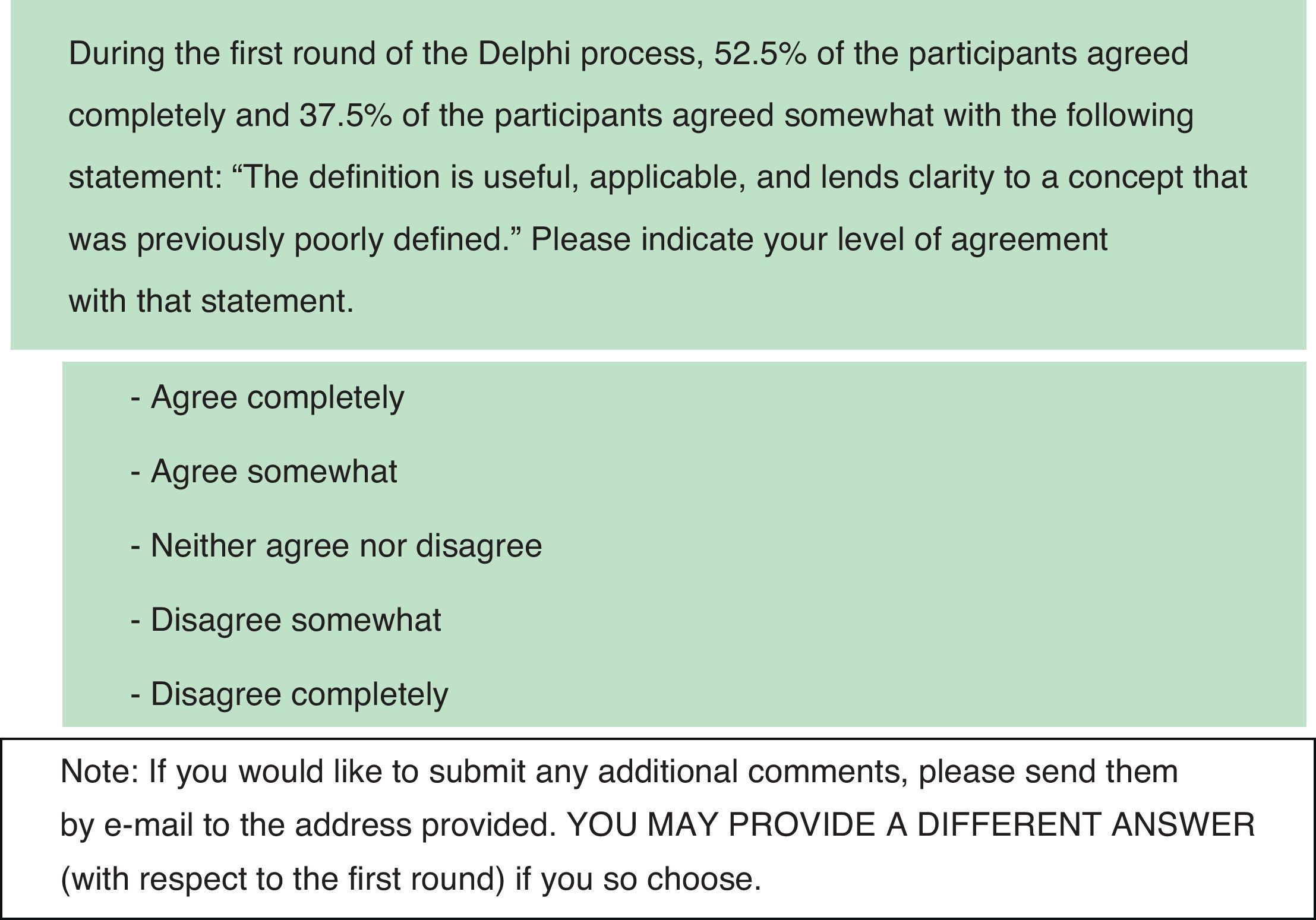
During the second round of the Delphi process (DR2), the survey included the same items, but also included comments gathered during DR1 and a summary of statistics indicating the percentage of participants who chose each answer. Questions for DR2 were presented in the same format as for DR1. Selected questions on DR2 were modified slightly in response to comments received by coordinators during DR1. Concepts and underlying recommendations expressed by the questions remained unchanged except in the case of question 13, which was rewritten at the request of the panel of experts following DR1 and approved by the Scientific Committee. This question was only answered during DR2. All items that achieved a very high degree of consensus during DR1 were eliminated from DR2 (consensus was considered very high when 80% or more of the respondents choose the same answer out of 5 possible answers). For the remaining items, researchers eliminated the percentage data from the 3 answers that had received the least votes and were therefore unlikely to generate positive consensus, even though any of the 5 possible answers could be chosen. For purposes of DR2, consensus was considered very high when 1 of the 5 possible answers received 80% or more of the votes. It was considered high when the total of answers expressing some agreement or disagreement with a statement was equal to or above 80% and the total sum of answers expressing complete agreement or disagreement was higher than 50%. Fig. 2 shows the second question from DR2.
Members of the panel of expertsPotential participants were selected by the Scientific Committee based on their recognised and proven experience in the field of epilepsy. Researchers and adult and paediatric neurologists from all over Spain were included in the study. A total of 60 potential members were invited to participate in the RATE-España consensus, which lasted 8 weeks from April to June 2011. The Scientific Committee received all questionnaires and responded to all comments by e-mail, and it was blinded to the identity of corresponding members. Participants who responded in either of the Delphi rounds are listed in Appendix 1.
AnalysisAnswers received in DR1 were analysed using descriptive and basic statistics generated by SPSS version 17 for Windows to identify any items for which there was positive consensus. During DR1, the answers and comments issued by the panel of experts were summarised by an independent clinical research organisation (Salutis Research S.L.) so as to include the comments and a summary of answer statistics in DR2. When both Delphi rounds were finished, the results were used to expound on and discuss the recommendations that would be included in the final article,7 and to elaborate the RATE-España consensus.
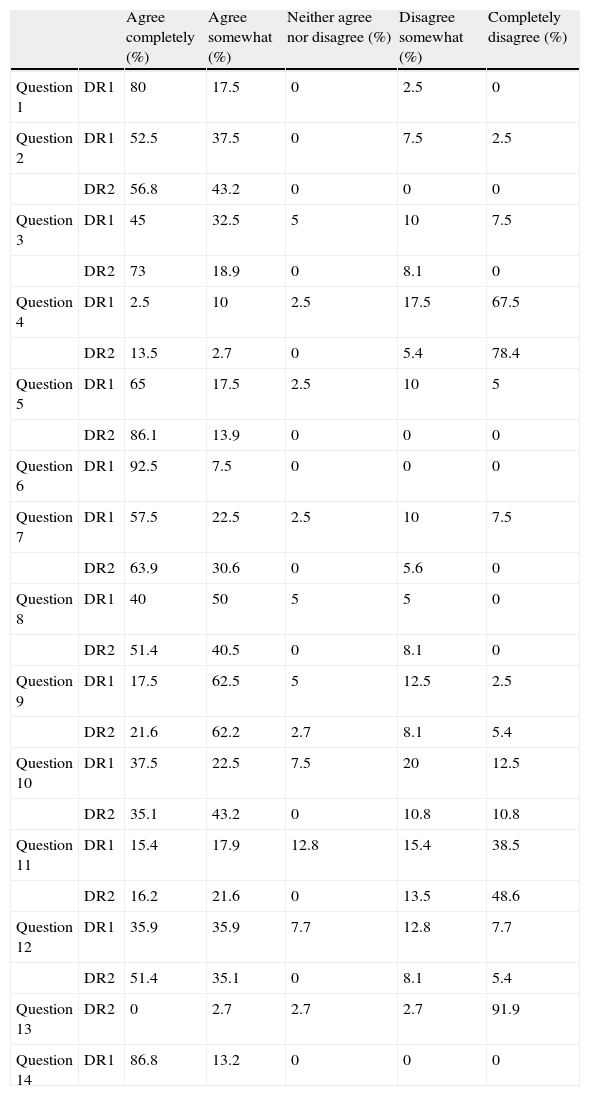
ResultsOf the total of 60 potential participants invited by RATE-España's panel of experts, 41 responded in DR1 and 37 responded in DR2. Table 2 shows the overall results for all questions in both DR1 and DR2.
Overall results of the Delphi survey given as percentages of respondents providing a specific answer. DR1: first round of Delphi process (n=41); DR2: second round of Delphi process (n=37). Question 13 was reformulated for DR2.
| Agree completely (%) | Agree somewhat (%) | Neither agree nor disagree (%) | Disagree somewhat (%) | Completely disagree (%) | ||
| Question 1 | DR1 | 80 | 17.5 | 0 | 2.5 | 0 |
| Question 2 | DR1 | 52.5 | 37.5 | 0 | 7.5 | 2.5 |
| DR2 | 56.8 | 43.2 | 0 | 0 | 0 | |
| Question 3 | DR1 | 45 | 32.5 | 5 | 10 | 7.5 |
| DR2 | 73 | 18.9 | 0 | 8.1 | 0 | |
| Question 4 | DR1 | 2.5 | 10 | 2.5 | 17.5 | 67.5 |
| DR2 | 13.5 | 2.7 | 0 | 5.4 | 78.4 | |
| Question 5 | DR1 | 65 | 17.5 | 2.5 | 10 | 5 |
| DR2 | 86.1 | 13.9 | 0 | 0 | 0 | |
| Question 6 | DR1 | 92.5 | 7.5 | 0 | 0 | 0 |
| Question 7 | DR1 | 57.5 | 22.5 | 2.5 | 10 | 7.5 |
| DR2 | 63.9 | 30.6 | 0 | 5.6 | 0 | |
| Question 8 | DR1 | 40 | 50 | 5 | 5 | 0 |
| DR2 | 51.4 | 40.5 | 0 | 8.1 | 0 | |
| Question 9 | DR1 | 17.5 | 62.5 | 5 | 12.5 | 2.5 |
| DR2 | 21.6 | 62.2 | 2.7 | 8.1 | 5.4 | |
| Question 10 | DR1 | 37.5 | 22.5 | 7.5 | 20 | 12.5 |
| DR2 | 35.1 | 43.2 | 0 | 10.8 | 10.8 | |
| Question 11 | DR1 | 15.4 | 17.9 | 12.8 | 15.4 | 38.5 |
| DR2 | 16.2 | 21.6 | 0 | 13.5 | 48.6 | |
| Question 12 | DR1 | 35.9 | 35.9 | 7.7 | 12.8 | 7.7 |
| DR2 | 51.4 | 35.1 | 0 | 8.1 | 5.4 | |
| Question 13 | DR2 | 0 | 2.7 | 2.7 | 2.7 | 91.9 |
| Question 14 | DR1 | 86.8 | 13.2 | 0 | 0 | 0 |
During DR1, respondents reached a very high degree of consensus on 3 items (questions 1, 6 and 14), expressing agreement in each of those cases. They did not reach a consensus on 11 items, which were reformulated in DR2 in such a way as to allow participants to reconsider their answers in light of other experts’ comments and the statistical summary of answers obtained in DR1.
In DR2, respondents reached a very high degree of consensus on 2 items; 86% of the experts agreed completely with question 5 and 91.9% of the respondents disagreed completely with question 13 (reformulated for DR2). Respondents reached a high degree of consensus on another 6 items in DR2. They answered ‘agree completely’ or ‘agree somewhat’ to 5 items (questions 2, 3, 7, 8, and 12) and ‘disagree completely’ or ‘disagree somewhat’ to 1 item (question 4).
There was a moderate degree of consensus on 2 items (questions 9 and 10), with a trend towards agreement (consensus is considered moderate when the total percentage of answers expressing complete or some agreement was higher than 75% with less than 50% of respondents expressing complete agreement). Lastly, no consensus was obtained for 1 item (question 11) in either DR1 or DR2, as respondents were divided between agreement and disagreement.
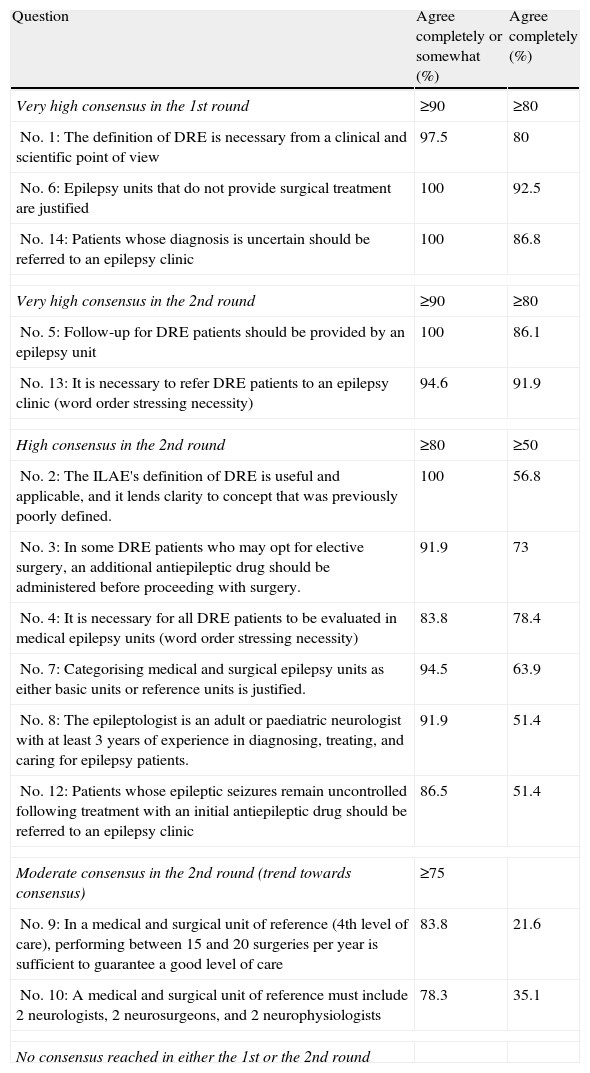
Table 3 shows the summary of results from the RATE-España consensus and the degrees of consensus: very high, high, moderate, and no consensus. In this table, items 4 and 13 were written in a modified format (using a word order that emphasised the necessity of evaluating all DRE patients in a CEU). This also served to prevent respondents from replying mechanically, a risk inherent to all multiple-choice questionnaires.
Degree of consensus obtained from Delphi rounds for the RATE-España consensus.
| Question | Agree completely or somewhat (%) | Agree completely (%) |
| Very high consensus in the 1st round | ≥90 | ≥80 |
| No. 1: The definition of DRE is necessary from a clinical and scientific point of view | 97.5 | 80 |
| No. 6: Epilepsy units that do not provide surgical treatment are justified | 100 | 92.5 |
| No. 14: Patients whose diagnosis is uncertain should be referred to an epilepsy clinic | 100 | 86.8 |
| Very high consensus in the 2nd round | ≥90 | ≥80 |
| No. 5: Follow-up for DRE patients should be provided by an epilepsy unit | 100 | 86.1 |
| No. 13: It is necessary to refer DRE patients to an epilepsy clinic (word order stressing necessity) | 94.6 | 91.9 |
| High consensus in the 2nd round | ≥80 | ≥50 |
| No. 2: The ILAE's definition of DRE is useful and applicable, and it lends clarity to concept that was previously poorly defined. | 100 | 56.8 |
| No. 3: In some DRE patients who may opt for elective surgery, an additional antiepileptic drug should be administered before proceeding with surgery. | 91.9 | 73 |
| No. 4: It is necessary for all DRE patients to be evaluated in medical epilepsy units (word order stressing necessity) | 83.8 | 78.4 |
| No. 7: Categorising medical and surgical epilepsy units as either basic units or reference units is justified. | 94.5 | 63.9 |
| No. 8: The epileptologist is an adult or paediatric neurologist with at least 3 years of experience in diagnosing, treating, and caring for epilepsy patients. | 91.9 | 51.4 |
| No. 12: Patients whose epileptic seizures remain uncontrolled following treatment with an initial antiepileptic drug should be referred to an epilepsy clinic | 86.5 | 51.4 |
| Moderate consensus in the 2nd round (trend towards consensus) | ≥75 | |
| No. 9: In a medical and surgical unit of reference (4th level of care), performing between 15 and 20 surgeries per year is sufficient to guarantee a good level of care | 83.8 | 21.6 |
| No. 10: A medical and surgical unit of reference must include 2 neurologists, 2 neurosurgeons, and 2 neurophysiologists | 78.3 | 35.1 |
| No consensus reached in either the 1st or the 2nd round | ||
Most surveys aimed at establishing consensus on epilepsy-related issues, referring equally to those employing the Delphi method11–19 and those that do not,10–13 have focused on experts’ opinions regarding pharmacological treatment and general management of the epileptic patient in both childhood and adulthood. In our survey, which made use of the Delphi method with 2 rounds of responses, we aimed to gather experts’ opinions about the ILAE's definition of DRE and recommended actions and referral protocols that should be implemented for DRE patients in Spain. The experts’ opinions on the ILAE's definition of DRE, levels of care, and patient referral were fairly uniform, based on the fact that consensus established to a greater or lesser extent for 13 of the 14 items.
Questions regarding the concept of DREThe ILAE's definition of DRE provides a more standardised criteria to indicate when an epileptic patient should be considered as having DRE. Before the definition was proposed, opinions varied considerably, and the ILAE crafted its definition with a view to improving care for DRE patients and facilitating clinical research.6 As we did not know to what extent doctors in our area had accepted the definition, our survey included 3 questions on that topic.
The first and second items on the RATE-España consensus questioned whether or not the ILAE's definition of DRE was necessary from a clinical and scientific point of view, and discussed the usefulness and applicability of the ILAE's definition. Experts reached a high degree of consensus on both DR1 and DR2, which corroborates the high level of agreement among the experts on different aspects of epilepsy who worked together to formulate the definition, and also confirms the definition's usefulness for purposes of research and clinical management of patients. These results highlight the need to adopt an active attitude with DRE patients and not insist on a diagnosis that may be erroneous or on therapeutic approaches that may not be the most appropriate; the goal must be to provide patients with all available means of controlling their epileptic seizures. It is highly significant that 100% of the experts agree completely or somewhat with the statement that the ILAE's definition is useful and applicable.
The third item mentions the possibility of administering another drug in addition to the 2 drugs that are required for a diagnosis of DRE before resorting to more aggressive measures, such as surgery. During DR2, experts also reached a consensus regarding the appropriateness of proposing treatment with an additional drug to patients who may opt for elective surgery. The definition of DRE is given as the failure of 2 different drug treatments to control seizures because results from numerous studies have shown that if 2 different drugs fail to provide seizure control, control will rarely be achieved through treatment with a new drug, whether it is taken in monotherapy or combination therapy. Limiting drugs to 2 also prevents the patient evaluation process from being unnecessarily time-consuming.20–22 On the other hand, it has been shown that the administration of up to 6 drugs over time has resulted in effective seizure control in some patients who did not respond to the initial treatment.23 It is therefore advisable to try an additional drug before resorting to other non-pharmacological types of treatment. Some authors even propose delaying surgery in patients receiving treatment until they have been tested with a series of at least 6 drugs.24 Nevertheless, according to the ILAE and the results of our own consensus, all DRE patients should be studied in a CEU as early as possible so as to reach a diagnosis and plan the most appropriate treatment.
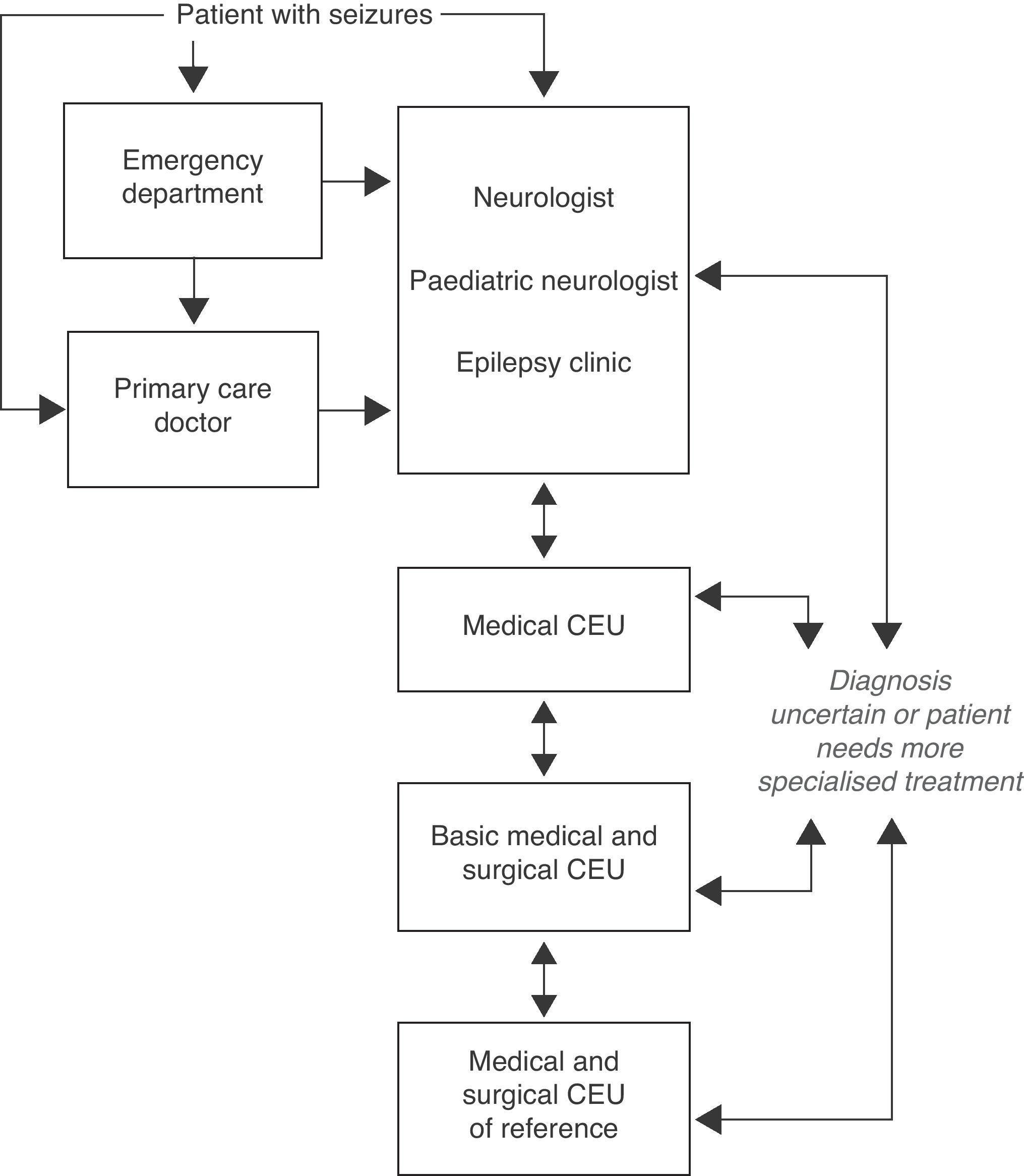
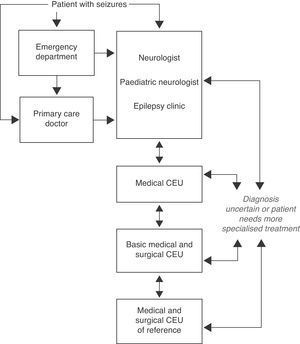
Questions related to levels of care and referral protocols for DRE patientsFig. 3 shows the protocol for referring patients with seizures to another level of care as proposed by our consensus document (Consenso RATE-España).7
Algorithm for levels of care in epilepsy (extract from Ref. 7).
Items 4 and 5 of the survey refer to the evaluation and monitoring of DRE patients in a CEU. Experts expressed a high degree of consensus on both items, and this result confirms the importance of prolonged video-EEG monitoring which provides critical data in the diagnostic study of epilepsy, and the urgent need for video-EEG monitoring in DRE patients. This result is also confirmed by question 6 (“epilepsy units that do not provide surgical treatment are justified”), in which experts reached the highest degree of consensus. They highlighted the current relevance of prolonged video-EEG monitoring in epilepsy management, regardless of whether or not the patient is a candidate for surgery. They also reached a high degree of consensus on item 7, regarding the importance of having advanced centres specialised in the surgical treatment of epilepsy.
Item 8 deals with the figure of the epileptologist. Experts reached a consensus on the suggested definition, with 91.9% of the participants in the RATE-España consensus expressing complete or some agreement. Our study highlights the need for epilepsy specialists, whether or not their experience surpasses the number of years suggested here.7,25 Moreover, epileptologists should be included in the various neurology departments in addition to forming part of the specialised CEU so as to better promote and foster consultations with epilepsy specialists.
Items 9 and 10 refer to a document describing the system by which the Inter-regional Council of Spain's National Health System accredits medical and surgical CEUs of reference.26 Experts reached a moderate degree of consensus, with a certain trend towards agreement, on both items. Those with dissenting opinions mainly disagreed with the need for 2 staff neurophysiologists to provide care for DRE patients, and with the volume of 15 to 20 surgeries per year (this number may be too low to guarantee doctors’ experience, good care, and a reasonable level of efficiency). Although the absolute number of surgical procedures is not usually specified in other studies, experts stress the importance of having a volume of more than 50 cases of video-EEG monitoring every year, and of need for ample experience among medical specialists (epileptologists and neurosurgeons).7,27
Items 11 to 14 raise questions about the need to refer a patient to a specialised epilepsy clinic, and the criteria for doing so. The results we obtained justify the presence of such epilepsy clinics which assess patients, provide treatment according to the most appropriate and updated criteria, provide proper follow-up, and determine which cases should be referred to a CEU for prolonged video-EEG monitoring. Experts reached a high degree of consensus on questions about referring DRE patients or patients with unresolved diagnostic questions to an epilepsy clinic. There was less consensus regarding the course of action to adopt when patients did not respond to treatment with an initial antiepileptic drug. The only question for which no consensus was reached was number 11, asking if all patients with epileptic seizures should be referred to a specialised epilepsy clinic for evaluation and monitoring.
Lack of consensus on question 11 could be explained by the fact that experts come from a variety of different medical centres, ranging from hospitals with long-standing CEUs that offer a full range of services to others in which specialised epilepsy clinics are just starting to appear. The authors of this study believe that patients requiring specialised care for epilepsy are the ones with an uncertain diagnosis or those whose seizures are not brought under sufficient control at an early stage. However, this depends on the type of medical centre, whether or not a specialised epilepsy clinic is available, and the number of specialised clinics compared to the number of patients requiring care. On the other hand, although experts reached a consensus on question number 5 regarding following up on DRE patients from a CEU, we also believe that after video-EEG monitoring and assignment of a precise diagnosis, every patient with DRE may be monitored by a CEU or by a specialised epilepsy clinic.
Conclusions- 1.
The consensus of the panel of experts was that the ILAE's definition of DRE is necessary from a clinical and scientific point of view. Moreover, the definition put forth by the ILAE was considered useful and applicable, with experts stating that it lends clarity to a concept that was previously poorly defined.
- 2.
Experts agreed that some DRE patients who may opt for elective surgery should try 1 more drug in addition to the 2 drugs required for diagnosis before proceeding with surgery.
- 3.
Experts also agreed that all DRE patients need to be assessed and monitored in a CEU, justified the existence of CEUs that do not offer surgical treatment, and approved of categorising medical and surgical CEUs as either basic units or units of reference.
- 4.
Experts reached a moderate consensus on resources allotted to medical and surgical CEUs of reference and on the minimum number of surgeries required in order to guarantee proper care, according to the proposal by the Council of Spain's National Health System.
- 5.
Experts agreed on the importance of the figure of the epileptologist and expressed support for specific epilepsy clinics to which doctors can refer DRE patients, patients with uncertain diagnoses, and those who do not respond to the initial treatment.
- 6.
The panel did not reach a consensus regarding whether or not patients who suffered an epileptic seizure should be referred to a specific epilepsy clinic for evaluation and monitoring.
The study was funded by GlaxoSmithKline S.A. (GSK), which enabled us to carry out the questionnaire using the Delphi method. GSK did not participate in the design of the draft or final study, selection of questions, data analysis process, or in the elaboration of this manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank GlaxoSmithKline S.A. (GSK) for their unconditional funding and administrative support, which were necessary for the completion of this project; Salutis Research S.L. for the administrative and methodology assistance we required in order to use the Delphi method; and all the participants, without whose cooperation the RATE-España consensus could not have been completed.
| Elena Alarcón Martínez |
| Montserrat Asensio Asensio |
| Clara Cabeza Álvarez |
| Rafael Camino León |
| Dulce María Campos Blanco |
| Francisco Cañadillas Hidalgo |
| Carlos Casas Fernández |
| Nuria García Barragán |
| Juan José García Peñas |
| Antonio Gil-Nagel Rein |
| Juan Gómez Alonso |
| Ana Gómez Caicoya |
| Apolinar Gómez Díaz Castroverde |
| José Luis Herranz Fernández |
| Vicente Ibáñez Mora |
| Javier López González |
| Jesús Macarrón Vicente |
| Ainhoa Marinas Alejo |
| Antonio Martínez Bermejo |
| Carlos Martínez Quesada |
| Eduardo Martínez Salcedo |
| José Ángel Mauri Llerda |
| Jaime Parra Gómez |
| Pilar de la Peña Mayor |
| María Ángeles Pérez Jiménez |
| Juan José Poza Aldea |
| Álex Quílez Martínez |
| Julio Ramos Lizana |
| Juan Rodríguez Uranga |
| Jesús Ruiz Giménez |
| Rosa Ana Saiz Díaz |
| Javier Salas Puig |
| Juan Carlos Sánchez Álvarez |
| Rocío Sánchez Carpintero |
| Jerónimo Sancho Rieger |
| Francesc Sanmartí Vilaplana |
| Pedro Serrano Castro |
| David Sopelana Garay |
| Rafael Toledano Delgado |
| Manuel Toledo Argany |
| Francisco Villalobos Chávez |
Please cite this article as: Sánchez-Álvarez JC, et al. Consenso de las Recomendaciones de Actuación diagnóstica y Terapéutica sobre Epilepsia resistente a fármacos antiepilépticos en España (Consenso RATE-España). Neurología. 2012;27:481–90.
Representing the group of experts who participated in Consenso RATE-España: Alarcón Martínez E., Asensio Asensio M., Cabeza Álvarez C., Camino León R., Campos Blanco D, Cañadillas Hidalgo F., Casas Fernández C., García Barragán N., García Peñas J.J., Gil-Nagel Rein A., Gómez Alonso J., Gómez Caicoya A., Gómez Díaz Castroverde A., Herranz Fernández J.L., Ibáñez Mora V., López González J., Macarrón Vicente J., Marinas Alejo A., Martínez Bermejo A., Martínez Quesada C., Martínez Salcedo E., Mauri Llerda J.A., Parra Gómez J., Peña Mayor P., Pérez Jiménez M.A., Poza Aldea J.J., Quílez Martínez A., Ramos Lizana J., Rodríguez Uranga J., Ruiz Giménez J., Sáiz Díaz R.A., Salas Puig J., Sánchez Álvarez J.C., Sánchez Carpintero R., Sancho Rieger J., Sanmartí Vilaplana F., Serrano Castro P.J., Sopelana Garay D., Toledano Delgado R., Toledo Argany M., Villalobos Chávez F.