Stereoelectroencephalography (SEEG) is a technique for preoperative evaluation of patients with difficult-to-localise refractory focal epilepsy (DLRFE), enabling the study of deep cortical structures. The procedure, which is increasingly used in international epilepsy centres, has not been fully developed in Spain. We describe our experience with SEEG in the preoperative evaluation of DLRFE.
Material and methodsIn the last 8 years, 71 patients with DLRFE were evaluated with SEEG in our epilepsy centre. We prospectively analysed our results in terms of localisation of the epileptogenic zone (EZ), surgical outcomes, and complications associated with the procedure.
ResultsThe median age of the sample was 30 years (range, 4-59 years); 27 patients (38%) were women. Forty-five patients (63.4%) showed no abnormalities on brain MR images. A total of 627 electrodes were implanted (median, 9 electrodes per patient; range, 1-17), and 50% of implantations were multilobar. The EZ was identified in 64 patients (90.1%), and was extratemporal or temporal plus in 66% of the cases. Follow-up was over one year in 55 of the 61 patients undergoing surgery: in the last year of follow-up, 58.2% were seizure-free (Engel Epilepsy Surgery Outcome Scale class I) and 76.4% had good outcomes (Engel I-II). Three patients (4.2%) presented brain haemorrhages.
ConclusionSEEG enables localisation of the EZ in patients in whom this was previously impossible, offering better surgical outcomes than other invasive techniques while having a relatively low rate of complications.
La estereoelectroencefalografía (E-EEG) es una técnica de evaluación prequirúrgica en pacientes con epilepsia focal refractaria de difícil localización (EFRDL) que permite explorar con electrodos profundos regiones cerebrales de difícil acceso y la profundidad de la corteza. Esta técnica, en auge en centros internacionales, apenas se ha desarrollado en España. Describimos nuestra experiencia con la E-EEG en la evaluación de pacientes con EFRDL.
Material y métodosEn los últimos 8 años, 71 pacientes con EFRDL fueron evaluados con E-EEG en nuestro centro. Analizamos prospectivamente los resultados obtenidos en la localización, los resultados quirúrgicos y las complicaciones asociadas a la técnica.
ResultadosLa mediana de edad fue de 30 años (rango 4-59 años), 27 pacientes eran mujeres (38%). La RM cerebral fue negativa en 45 pacientes (63,4%). Se implantaron 627 electrodos (mediana de 9 electrodos por paciente, rango 1-17), con un 50% de implantaciones multilobares. En 64 (90,1%) pacientes se localizó la zona epileptógena (ZE), siendo extratemporal o temporal plus en el 66% de los casos. En 55 pacientes de los 61 intervenidos el seguimiento fue superior al año: en el último año de seguimiento 32/55 pacientes (58,2%) estaban libres de crisis (Engel I) siendo los resultados favorables (Engel I-II) en el 76,4% de las intervenciones. Tres pacientes (4,2%) presentaron una hemorragia cerebral.
ConclusiónLa E-EEG permite localizar la ZE en pacientes en quienes anteriormente no era posible, ofreciendo unos resultados quirúrgicos superiores a otras técnicas invasivas y una tasa de complicaciones relativamente baja.
Pharmacological treatment fails to control epileptic seizures in approximately 30% of cases1,2; in these patients, surgical treatment is effective if the epileptogenic zone (EZ) can be localised and resected. To date, epilepsy surgery has been considered to be more effective when brain MRI studies identify a clear, delimited epileptogenic lesion whose localisation is consistent with seizure characteristics and the findings of EEG studies and other complementary tests. In patients meeting these conditions, seizures are controlled in more than 70% of cases.3,4 However, recent series of patients in whom brain MRI does not reveal a well-defined lesion found surgery to be effective after stereoelectroencephalography (SEEG) studies employing depth electrodes.5–8 This situation is referred to as difficult-to-localise refractory focal epilepsy (DLRFE). Improvements in the localisation of the EZ in patients with DLRFE are of great clinical relevance, as these account for 25% to 43% of patients with refractory focal epilepsy.9–12 Uncontrolled epilepsy in this population has a considerable negative impact on quality of life, and is associated with high rates of depression and sudden death.13
The aim of the present study is to describe our experience with SEEG in the preoperative assessment of children, adolescents, and adults with DLRFE attended within the Epilepsy Programme at Hospital Ruber Internacional, and to compare our results against those of other centres. In particular, we describe the capacity to localise the EZ, surgical outcomes, and the safety of the procedure.
Material and methodsBetween September 2010 and June 2018, 71 patients with DLRFE underwent assessment with SEEG at our centre. We prospectively collected data on the characteristics of electrode implantation, complications of SEEG, and surgical outcomes.
Non-invasive preoperative assessment included the following procedures: 1) prolonged video EEG with recording of seizures; 2) neuropsychological study; and 3) brain MRI study (3T scanner). Fifty-nine patients underwent brain MRI/PET studies and 16 underwent magnetoelectroencephalography and MRI. Brain MRI results were considered negative when the neuroradiologist (JAL) and the remaining members of the team were unable to identify an epileptogenic lesion during the multidisciplinary session.
Selection criteria for stereoelectroencephalographySEEG was indicated for all patients with DLRFE in whom the initial noninvasive assessment did not enable us to establish a hypothesis on the localisation of the EZ, or if the working hypothesis was not sufficiently solid for us to proceed directly to surgery.14–16 The criteria for indication of SEEG were as follows: a) inconsistency between MRI findings and seizure characteristics; b) extensive lesions on the brain MRI scan that could not be resected due to eloquent cortex involvement; c) suspected overlap and/or involvement of eloquent areas in the EZ; d) need to study deep cortical areas not evaluable with other techniques; or e) negative MRI results.
Method of electrode implantationElectrodes were implanted by the same neurosurgeon (RMA) in all cases, targeting the lesion identified on MRI (where available), structures suspected of participating in seizure onset, propagation pathways, and, in some cases, the symptomatogenic zone.
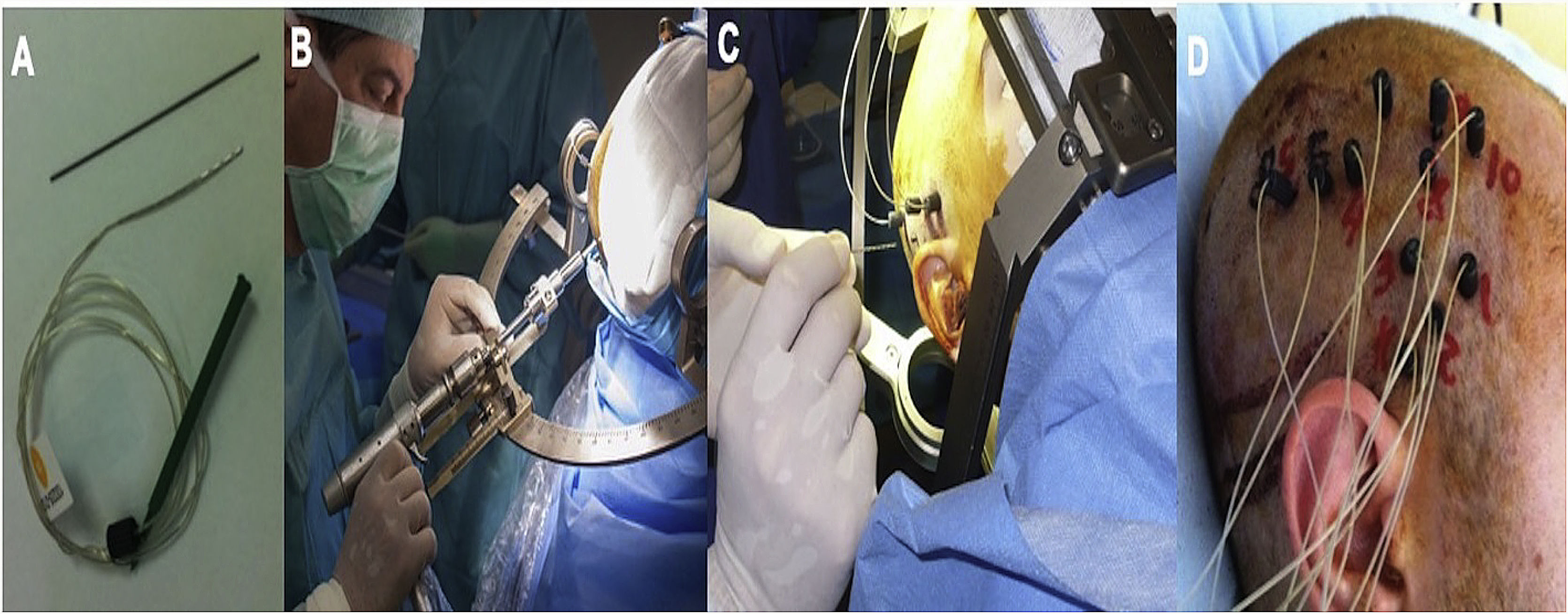
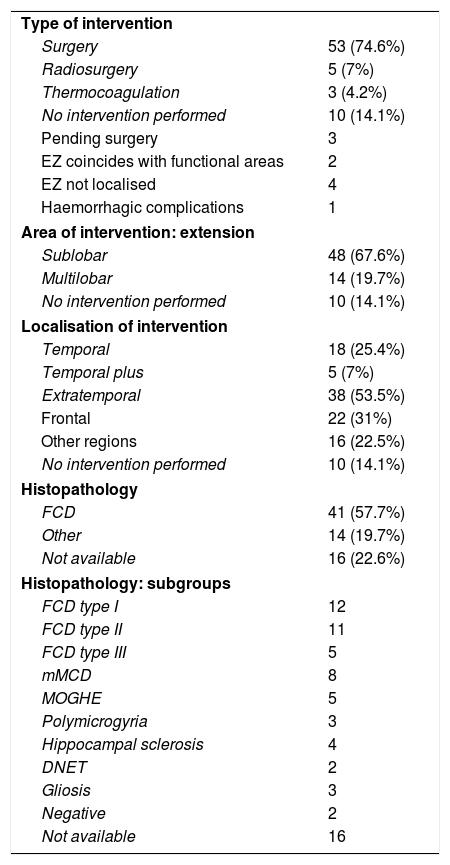
We implanted Microdeep® electrodes (Dixi Medical, Chaudefontaine, France) with multiple contacts (Fig. 1). The trajectory of each electrode was planned using a specific program (Steronauta®) developed by our team (GR and WC), applied to each patient’s baseline brain MRI study. On the day of electrode implantation, we performed an additional MRI study under stereotactic conditions (Leksell frame, Elekta®, Stockholm, Sweden), obtaining a 3D T1-weighted sequence with a double dose of gadolinium. In a subsequent step, the previously planned trajectories were transferred to this new sequence and reviewed individually to avoid collision with blood vessels. From September 2013, a 3D Toft model was also included; and from 2015, we added a selective CT angiography study (developed by GR and WC) that enables visualisation of the vessels surrounding each electrode in the cerebral area it traverses, excluding vessels beyond its trajectory. This maximises the number of possible trajectories and improves the safety of the procedure. After implantation, a multiplanar CT scan was performed and fused with the MRI sequence to verify the trajectory of each electrode and rule out any complications. Patients were admitted to the video EEG unit the same day they underwent electrode implantation. A control brain MRI was performed before removal of the electrodes and a CT study was performed after.
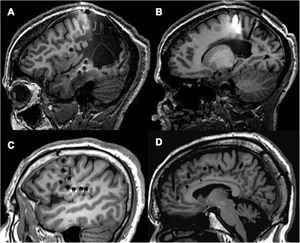
Example of depth electrode implantation in SEEG. A) The procedure uses 0.8 mm electrodes. A mechanical pencil lead is shown for comparison. B) Electrodes are implanted individually, guided by coordinates established in surgical planning, translated to a Leksell stereotactic frame. C) After a guide has been used to establish the intracranial trajectory, the electrode is inserted with millimetre precision. D) The final image shows the completed implantation in a patient with suspected right peri-insular epilepsy.
Surgical planning took into account the findings of the non-invasive assessment and the SEEG study, considering the structures where seizure onset occurred (ictal onset zone), the areas with greatest epileptiform activity and pathological slowing (irritative zone), and proximity to potential eloquent areas and risk of neurological deficits. The ictal onset zone was defined according to ictal patterns described in the literature.17,18 Patients eligible for surgery underwent the procedure a month after electrode implantation; all surgeries were performed by the same surgeons (JGA and JP).
Surgical outcomesThe resected tissue was fixed in formaldehyde and studied by optical microscopy using haematoxylin and eosin staining and other immunohistochemical techniques (NeuN, Map2). Histopathological assessment was performed by a single pathologist (IB), who established a diagnosis according to the current criteria.19–21
Surgical outcomes were recorded prospectively through patient interviews at a series of follow-up consultations. The analysis of surgical outcomes only includes data from patients who were followed up for at least one year. Outcomes are classified according to the Engel classification,22 with class I-II representing favourable surgical outcomes and class III-IV indicating unsatisfactory outcomes. Engel classes were established based on seizure frequency during the last year of follow-up, compared against baseline. All complications occurring in the first 30 days after SEEG were considered to be procedure-related.
Statistical analysisStatistical analysis was conducted using the R software (version 3.1.2), and included descriptive analyses and univariate and multivariate association tests. The univariate association analysis included the Wilcoxon rank sum test, the t test, the chi-square test, and the Fisher exact test. Logistic regression included the variables with P < .02 in the univariate tests and was adjusted for age, sex, and disease duration. Values of P < .05 were considered statistically significant.
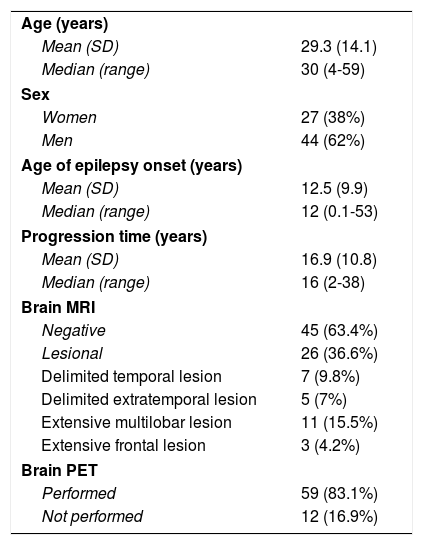
ResultsDemographic characteristicsDuring the study period (8 years), SEEG was performed in 71 patients with DLRFE (27 women, 38%) (Table 1), with a median age of 30 years (range, 4-59). The sample included 16 patients aged ≤ 18 years (median, 8.5 years; range, 4-18). Median age of epilepsy onset was 12 years (range, 0.1-53), and median disease progression time when the SEEG study was performed was 16 years (range, 2-38). At the time of assessment, 62 patients (87%) presented complex partial seizures, and 31 (42%) presented secondarily generalised tonic-clonic seizures. Nine patients presented other types of focal motor seizures (hypermotor, asymmetric tonic, and akinetic seizures).
Clinical and demographic characteristics (n = 71).
| Age (years) | |
| Mean (SD) | 29.3 (14.1) |
| Median (range) | 30 (4-59) |
| Sex | |
| Women | 27 (38%) |
| Men | 44 (62%) |
| Age of epilepsy onset (years) | |
| Mean (SD) | 12.5 (9.9) |
| Median (range) | 12 (0.1-53) |
| Progression time (years) | |
| Mean (SD) | 16.9 (10.8) |
| Median (range) | 16 (2-38) |
| Brain MRI | |
| Negative | 45 (63.4%) |
| Lesional | 26 (36.6%) |
| Delimited temporal lesion | 7 (9.8%) |
| Delimited extratemporal lesion | 5 (7%) |
| Extensive multilobar lesion | 11 (15.5%) |
| Extensive frontal lesion | 3 (4.2%) |
| Brain PET | |
| Performed | 59 (83.1%) |
| Not performed | 12 (16.9%) |
Brain MRI findings were negative in 45 cases (63.4%) and revealed a lesion in 26 patients (36.6%); lesions were delimited in 12 cases and extensive in 14 (Table 1). Brain PET scans were performed in 59 patients (83.1%), including all MRI-negative patients and 14 patients with MRI lesions. No significant differences were observed in MRI results (lesional vs non-lesional) or demographic variables. MRI-negative patients more frequently underwent PET studies (likelihood ratio; P < .001).
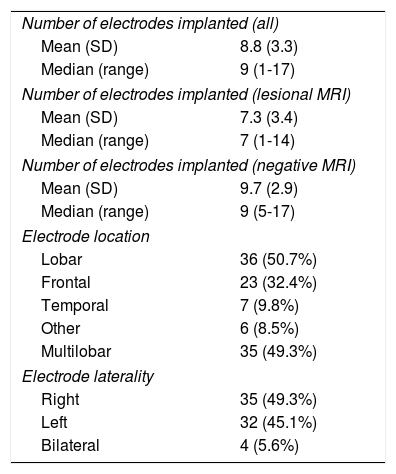
Electrode implantation characteristics and localisation of the epileptogenic zoneMedian duration of hospitalisation was 5 days. A total of 627 electrodes were implanted (median, 9 per patient; range, 1-17) (Table 2). Fewer electrodes were implanted in patients aged 18 years and younger (median, 7; range, 5-14). In 2 patients, depth electrodes were combined with subdural grid electrodes. More electrodes were implanted in MRI-negative patients than in those with lesional MRI findings (t [69] = 3.06; P = .03). Electrode implantation was lobar and multilobar in equal measure (50.7% vs 49.3%), with frontal regions most frequently being studied (64%). No significant differences were observed in laterality. Electrodes were only implanted bilaterally in 4 patients. One patient was studied twice, with 2 separate admissions. The number of electrodes implanted per patient decreased over the study period, with a median of 9.9 in the first 4 years and 6.8 in the last 3. Cortical electrical stimulation was performed in 70 patients. Before removal of the electrodes, 27 patients underwent thermocoagulation guided by the findings of the preoperative assessment (this technique was introduced at our centre in 2012).
SEEG study characteristics (n = 71).
| Number of electrodes implanted (all) | |
| Mean (SD) | 8.8 (3.3) |
| Median (range) | 9 (1-17) |
| Number of electrodes implanted (lesional MRI) | |
| Mean (SD) | 7.3 (3.4) |
| Median (range) | 7 (1-14) |
| Number of electrodes implanted (negative MRI) | |
| Mean (SD) | 9.7 (2.9) |
| Median (range) | 9 (5-17) |
| Electrode location | |
| Lobar | 36 (50.7%) |
| Frontal | 23 (32.4%) |
| Temporal | 7 (9.8%) |
| Other | 6 (8.5%) |
| Multilobar | 35 (49.3%) |
| Electrode laterality | |
| Right | 35 (49.3%) |
| Left | 32 (45.1%) |
| Bilateral | 4 (5.6%) |
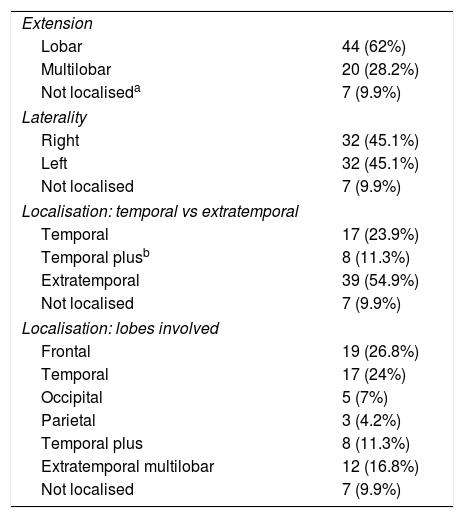
SEEG localised the EZ in 64 patients (90.1%) (Table 3). The EZ was lobar in 62% of patients and multilobar in 28.2%. Localisation was exclusively temporal in 23.9% of patients, temporal plus (temporal lobe + another contiguous lobe) in 11.3%, and extratemporal in 54.9%; no statistically significant differences were found between MRI-negative patients and those with lesional MRI findings. We were unable to localise the EZ in 7 cases.
Epileptogenic zone characteristics (n = 71).
| Extension | |
| Lobar | 44 (62%) |
| Multilobar | 20 (28.2%) |
| Not localiseda | 7 (9.9%) |
| Laterality | |
| Right | 32 (45.1%) |
| Left | 32 (45.1%) |
| Not localised | 7 (9.9%) |
| Localisation: temporal vs extratemporal | |
| Temporal | 17 (23.9%) |
| Temporal plusb | 8 (11.3%) |
| Extratemporal | 39 (54.9%) |
| Not localised | 7 (9.9%) |
| Localisation: lobes involved | |
| Frontal | 19 (26.8%) |
| Temporal | 17 (24%) |
| Occipital | 5 (7%) |
| Parietal | 3 (4.2%) |
| Temporal plus | 8 (11.3%) |
| Extratemporal multilobar | 12 (16.8%) |
| Not localised | 7 (9.9%) |
The type of surgery was determined by the localisation of the EZ with relation to potential eloquent areas, lesion extension, accessibility, and response to thermocoagulation, in patients who underwent this technique. After this assessment, some type of intervention was indicated for 61 patients (85.8%): 53 (74.6%) underwent conventional surgery and 5 (7%) underwent gamma-knife radiosurgery (Table 4). Three patients (4.2%) responded to thermocoagulation and did not need another procedure. Sublobar resection was the most frequent procedure (48 patients; 67.6%), and the most frequent localisation was extratemporal (38 patients; 53.5%). Frontal resection was the most frequent sublobar intervention (31%). The median time from epilepsy onset to surgery was 16 years (range, 2-38). Ten patients (14.1%) did not undergo surgery.
Surgical (n = 71) and histopathological (n = 55) characteristics.
| Type of intervention | |
| Surgery | 53 (74.6%) |
| Radiosurgery | 5 (7%) |
| Thermocoagulation | 3 (4.2%) |
| No intervention performed | 10 (14.1%) |
| Pending surgery | 3 |
| EZ coincides with functional areas | 2 |
| EZ not localised | 4 |
| Haemorrhagic complications | 1 |
| Area of intervention: extension | |
| Sublobar | 48 (67.6%) |
| Multilobar | 14 (19.7%) |
| No intervention performed | 10 (14.1%) |
| Localisation of intervention | |
| Temporal | 18 (25.4%) |
| Temporal plus | 5 (7%) |
| Extratemporal | 38 (53.5%) |
| Frontal | 22 (31%) |
| Other regions | 16 (22.5%) |
| No intervention performed | 10 (14.1%) |
| Histopathology | |
| FCD | 41 (57.7%) |
| Other | 14 (19.7%) |
| Not available | 16 (22.6%) |
| Histopathology: subgroups | |
| FCD type I | 12 |
| FCD type II | 11 |
| FCD type III | 5 |
| mMCD | 8 |
| MOGHE | 5 |
| Polymicrogyria | 3 |
| Hippocampal sclerosis | 4 |
| DNET | 2 |
| Gliosis | 3 |
| Negative | 2 |
| Not available | 16 |
DNET: dysembryoplastic neuroepithelial tumour; EZ: epileptogenic zone; FCD: frontal cortical dysplasia; mMCD: mild malformation of cortical development; MOGHE: mMCD with oligodendroglial hyperplasia.
Histopathological studies were performed in 55 cases, with different subtypes of focal cortical dysplasia (FCD) being the most frequent diagnosis (Table 4). Negative MRI results were more common in patients with FCD than among those with other histopathological findings (chi-square [2] = 9.19; P = .01).
Surgical outcomesFifty-five of the 61 patients undergoing surgery were followed up for longer than one year (Table 5). Median age in this patient group was 29 years (range, 4-59) and mean follow-up time was 4.8 years (range, 1-7.8). Thirty-four of these patients (62%) presented negative brain MRI results, and 32 (58%) underwent extratemporal resection. In the first 2 years of follow-up (in which the number of patients was greater), 35/55 patients (63.6%) were seizure-free at one year (Engel class I); 30 remained at Engel class I at 2 years (63.8%; 30/47 patients). In the final year of follow-up, 32 patients remained seizure-free (Engel class I, 58.2%), with 76.4% of patients presenting favourable outcomes (Engel class I or II).
Surgical outcomes in patients with follow-up longer than one year (n = 55).
| Total | Engel class I | Not Engel class I | P | Adjusted P | |
|---|---|---|---|---|---|
| Total | 55 | 32 (58.2%) | 23 (41.8%) | ||
| Sex | .079 | .120 | |||
| Women | 19 | 8 (42.1%) | 11 (57.9%) | ||
| Men | 36 | 24 (66.7%) | 12 (33.3%) | ||
| Age | .522 | – | |||
| Median (Q1-Q3) | 29 (4-59) | 29.5 (6-59) | 25 (4-48) | ||
| Age of epilepsy onset | .305 | – | |||
| Median (Q1-Q3) | 12 (3.5-16) | 14 (5.5-18.2) | 12 (2.3-15.5) | ||
| Disease duration | .966 | – | |||
| Median (Q1-Q3) | 16 (7.5-23.5) | 16 (6.8-23.2) | 17 (8.5-24) | ||
| MRI | .118 | .184 | |||
| Lesional | 21 | 15 (71.4%) | 6 (28.6%) | ||
| Negative | 34 | 17 (50%) | 17 (50%) | ||
| Number of electrodes | |||||
| Median (Q1-Q3) | 9 (1-15) | 8.5 (1-14) | 9 (3-15) | .211 | – |
| Intervention | .494 | – | |||
| Lobar | 45 | 25 (55.6%) | 20 (44.4%) | ||
| Multilobar | 10 | 7 (70%) | 3 (30%) | ||
| Intervention | .351 | – | |||
| Temporal | 18 | 12 (66.7%) | 6 (33.3%) | ||
| Temporal plus | 5 | 4 (80%) | 1 (20%) | ||
| Extratemporal | 32 | 16 (50%) | 16 (50%) | ||
| Histopathology | .434 | – | |||
| FCD | 38 | 23 (60.5%) | 15 (39.5%) | ||
| FCD type II | 10 | 9 (90%) | 1 (10%) | ||
| Other FCD types | 28 | 14 (50%) | 14 (50%) | ||
| Other lesions | 13 | 8 (61.5%) | 5 (38.5%) | ||
| Not available | 4 | 1 (25%) | 3 (75%) |
FCD: focal cortical dysplasia; MRI: magnetic resonance imaging; P: univariate analysis (see text); adjusted P: logistic regression adjusted for age, sex, and disease duration; Q1-Q3: quartile 1-quartile 3.
While no variable was significantly associated with surgical outcomes (Table 5), we did observe some trends. In particular, the best surgical outcomes were observed among patients with lesional MRI findings (71.4% at Engel class I) and undergoing temporal resection (66.7% at Engel class I). However, 50% of the patients undergoing extratemporal resection were seizure-free in the last year of follow-up, with 72% presenting favourable outcomes (Engel class I or II). These results were slightly lower in patients who also presented negative MRI results (45% at Engel class I, 70% at Engel class I or II). Thirteen of the 16 patients aged ≤ 18 years who underwent surgery were followed up for longer than one year; 7 of these (53.8%) were seizure free, and another presented a reduction in seizure frequency greater than 90% (Engel class II).
Most patients with FCD type II presented good surgical outcomes (90% at Engel class I), although the differences between groups were not statistically significant (Fisher exact test; P = .434), even when comparing between patients with FCD type II, other types of FCD, and other pathologies (Fisher exact test; P = .087) (Table 5).
Complications related with stereoelectroencephalographyThree patients (4.2%) presented brain haemorrhage, requiring immediate removal of the electrodes and surgical treatment. All haemorrhages occurred before the introduction of the selective CT angiography technique, with no further haemorrhages occurring after 2015. Haemorrhage affected the left frontal lobe in 2 cases and the right temporal lobe in the remaining patient. One patient developed right hemiparesis, with partial recovery during follow-up, and another presented left homonymous hemianopsia. Although none of these patients underwent a second SEEG study, 2 underwent surgery several months later, based on information from the preoperative assessment (surgical outcomes included in the analysis); larger areas were resected than would have been the case if surgical planning were based on SEEG findings. One patient developed transient monoparesis following thermocoagulation.
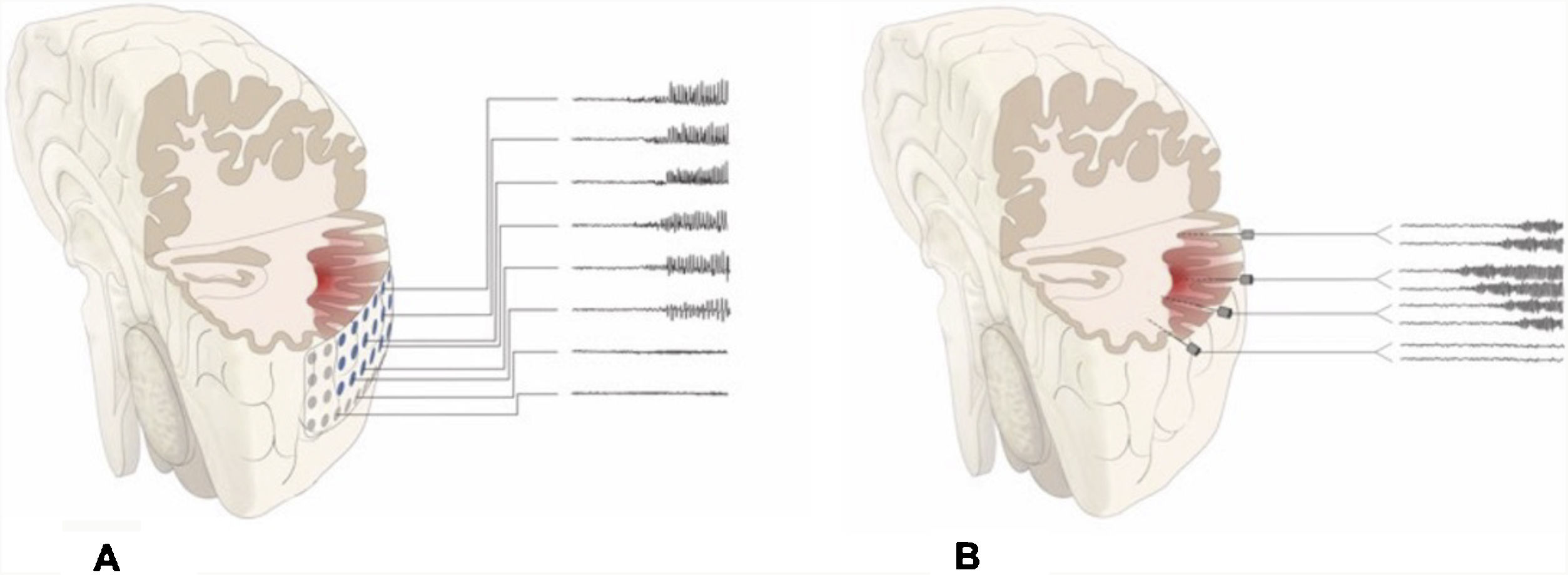
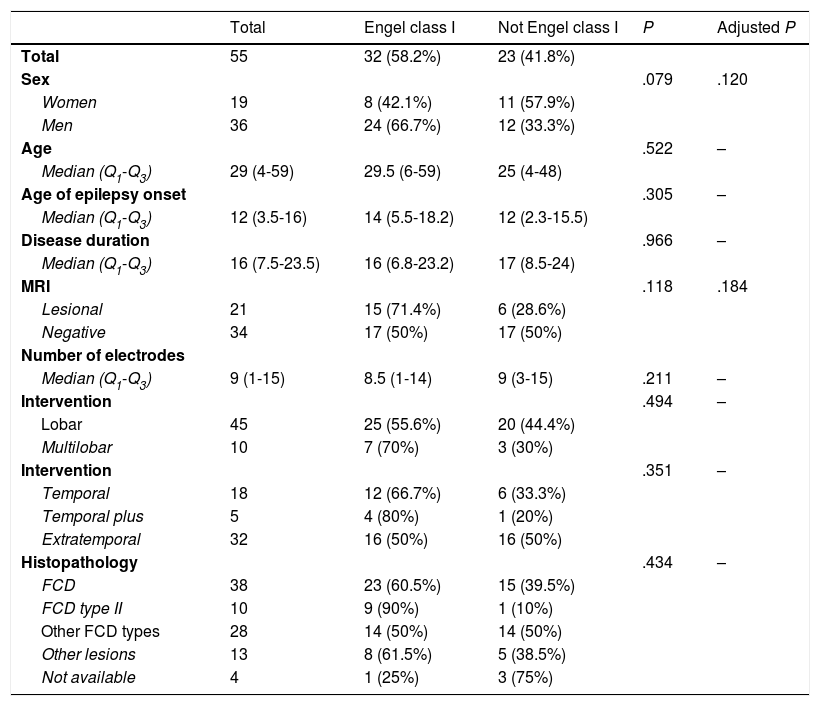
DiscussionAlthough patients presenting no MRI alterations or with extensive lesions that cannot be fully resected have not traditionally been considered eligible for epilepsy surgery, the growing body of knowledge on seizure semiology and EEG patterns and recent advances in functional imaging techniques have made effective surgical treatment a possibility for many of these patients.23–27 The introduction of SEEG has also contributed to the improvement in surgical outcomes. This technique, mainly developed in France and northern Italy,5,7,15 offers greater precision in localising the EZ than subdural electrodes, whose use was widespread between 1990 and 2010 at the majority of American and European epilepsy surgery centres (Fig. 2).12,28–30
Schematic representation of the onset and propagation of a seizure with intracranial electrodes. A) Seizure recorded with subdural electrodes, which explore the gyral crown and are unable to record activity at deeper levels. Recordings from such a study may give a false localisation, with onset of the discharge being registered simultaneously by several contacts on the subdural grid. B) Seizure recorded with depth electrodes (SEEG). If the working hypothesis for electrode implantation was correct, we may localise seizure onset in a very specific cortical area (in this example, deep within the gyrus), from which activity propagates to other electrodes in a clearly progressive ictal pattern.
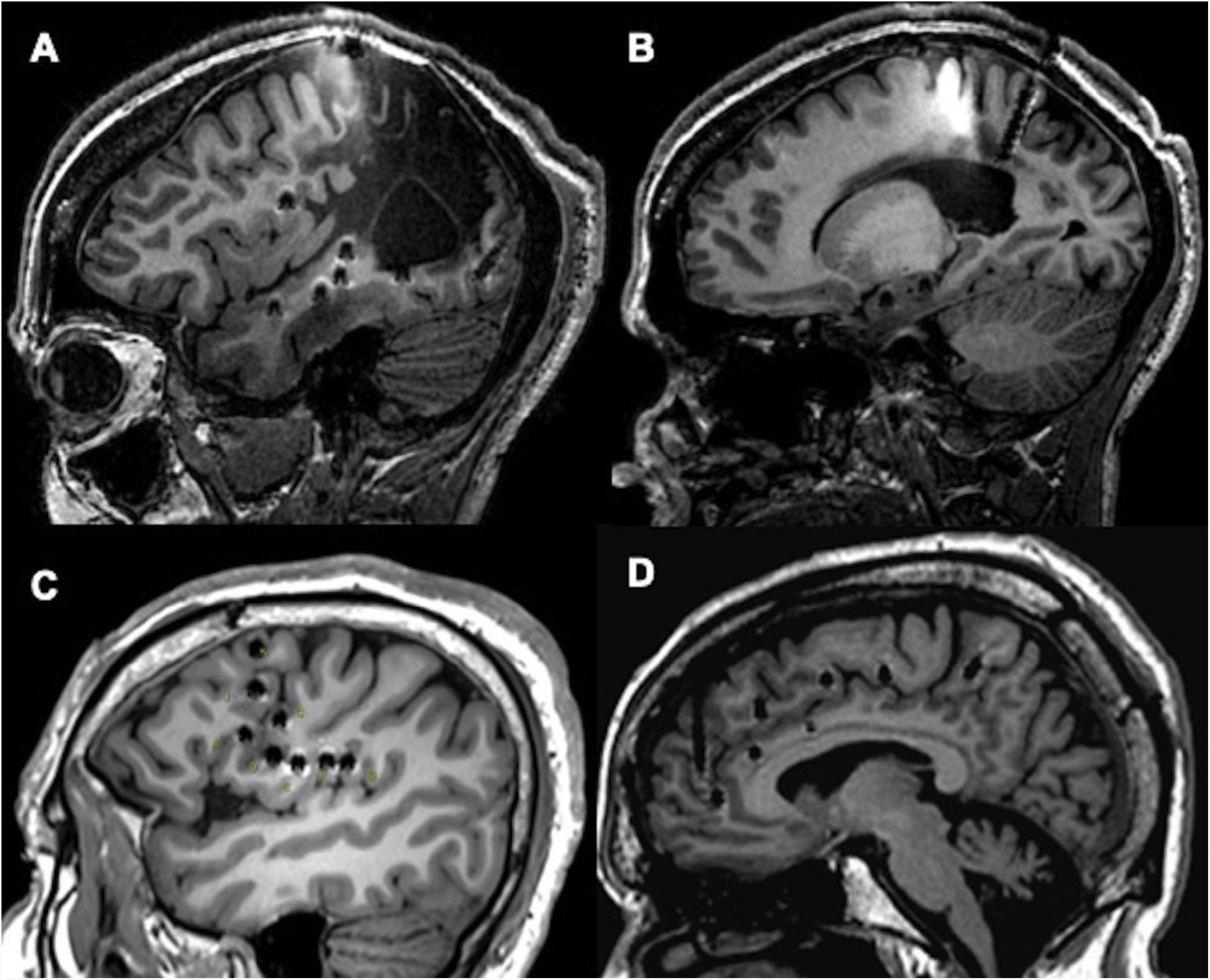
In this study, we share our experience with SEEG as a diagnostic technique in patients with DLRFE. Although we included a heterogeneous sample of consecutive patients assessed over the last 8 years, all patients had difficult-to-localise EZs, and the sample reflects everyday practice at our centre. The complexity of preoperative assessment findings in this series is supported by the fact that 63% of patients were MRI-negative, and EZ localisation was extratemporal or temporal plus in 66% of cases; these factors are frequently associated with poor surgical outcomes (Fig. 3).9,31 Our data are comparable with those reported in recent studies from other European and American centres,5–8 where SEEG is the gold-standard technique for patients with DLRFE. Unlike other series including patients undergoing brain MRI studies with different characteristics (for instance, with a 1.5T scanner),5,16 all our patients underwent brain MRI with a specific protocol, in a 3T scanner. Compared with 1.5T scanners, this reduces the likelihood of certain subtle epileptogenic lesions being overlooked.
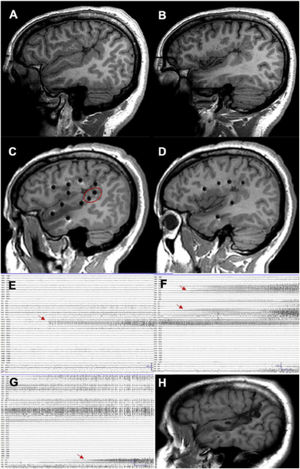
Examples of different approaches, showing how SEEG enables exploration of extratemporal areas that are difficult to access with such other techniques as subdural electrodes. A and B) Exploration of neocortical and medial regions in a patient with an extensive porencephalic lesion. C) Exploration of perirolandic and insular regions in an MRI-negative patient. D) Exploration of frontal and medial areas with an orthogonal and oblique approach.
We were able to localise the EZ in 90% of patients; this result is similar to those reported in the most recent series of patients undergoing SEEG,5,8 and considerably better than those reported in older studies using subdural electrodes, in which surgery could not be indicated following removal of the electrodes in up to 30% to 40% of cases.29,30 The mean number of electrodes implanted was approximately 9, and was slightly higher among patients with negative brain MRI findings. Compared to other centres, where the mean number of electrodes implanted ranges from 11 to 13,5,16 we were more conservative and rarely needed to perform bilateral implantation. The differences between our results and those of other centres may be explained by the development in recent years of more advanced neuroimaging techniques, which are also used at our centre; as a result, the non-invasive preoperative assessment enables us to target a smaller area for exploration with depth electrodes.24–27 Furthermore, as our implantation technique enables us to perform both orthogonal and oblique approaches, a single electrode can explore brain regions that would require several electrodes if orthogonal approaches were followed. As a result, we reduced the number of electrodes implanted in the latter years of the study period.
Type of interventionWhile electrode implantation was multilobar in nearly 50% of the patients explored, SEEG detected a sublobar EZ in 62% of cases; localisation was most frequently extratemporal or temporal plus (66%). With the data from both SEEG and the preoperative assessment, we were able to resect relatively small volumes of tissue in most cases (Fig. 4). These data reflect the complexity of assessment of these patients, and everyday practice at most surgical centres.5,23 For instance, a recent European study reported how in recent years, the increased number of procedures performed was explained by extratemporal resections and MRI-negative patients.32
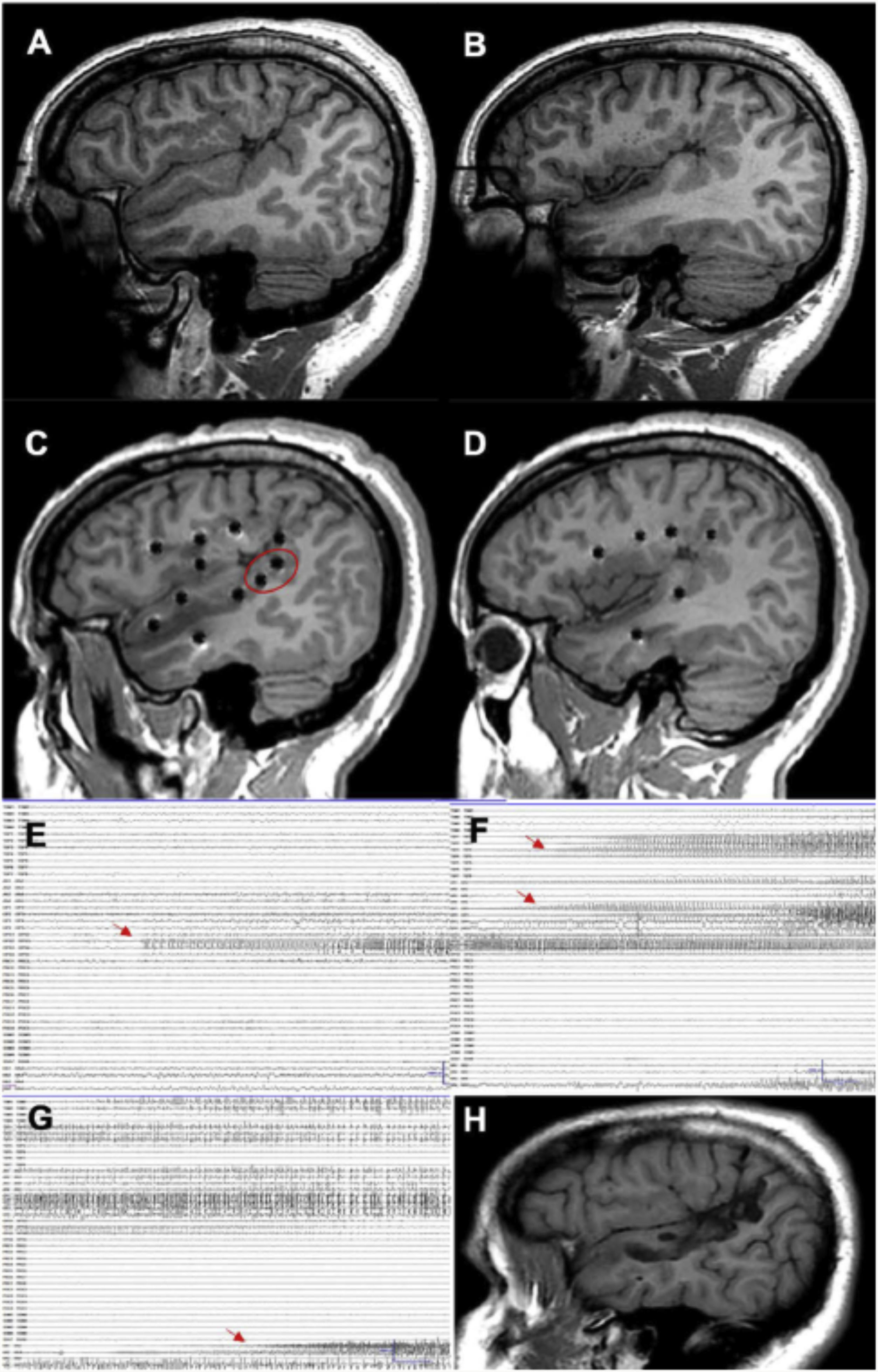
A patient with focal epilepsy associated with extensive right perisylvian polymicrogyria. A and B) Sagittal T1-weighted sequence showing the extension of the malformation, with involvement of deep areas of the insula and operculum. C and D) Extensive implantation of depth electrodes enabling exploration of the cortex on different planes, including the insula. E) Seizure onset occurred in the posterior part of the superior temporal gyrus (circle in C). F) Progression to the temporal cortex and posterior insula. G) Progression to the hippocampus. H) Sagittal T1-weighted sequence showing how resection limited to the superior temporal gyrus was able to control this patient’s epilepsy, despite the extensive lesion.
Thermocoagulation was performed in 27 patients; while this technique is usually used for palliative treatment,33,34 3 of our patients achieved lasting improvements that prevented the subsequent need for surgery. While we still lack long-term follow-up data on a sufficiently large number of patients, it is possible that transient improvements following thermocoagulation may be predictive of favourable surgical outcomes. Furthermore, 5 of our patients underwent gamma-knife radiosurgery. We opted to use this technique due to the surgical risk associated with a possible corticectomy in the region where the EZ was located.
Surgical outcomesIn the final year of follow-up, approximately 76% of patients undergoing surgery presented considerable improvements, with nearly 60% remaining seizure-free. While our sample and follow-up data are not fully comparable with those of other studies, our outcomes are similar to those of the most recent series, which report seizure freedom in 56% to 62% of patients with DLRFE and assessed with SEEG.5,7,8 Outcomes in the first 2 years after surgery, for which the most data are available, are consistent with the more optimistic results from the literature5: 80% of patients presented a marked improvement, with 63% being seizure-free 2 years after surgery. While children and adolescents constituted a smaller group, outcomes were favourable in 54% of patients undergoing surgery in this group.
In our series, such variables as histopathological findings, location of the resected area, and brain MRI findings were not significantly associated with more favourable surgical outcomes. These findings may be explained by the homogeneity of our series in terms of the complexity of EZ localisation: we included patients with extensive lesions, in whom localising the EZ can be as complex as in MRI-negative patients. However, given the limited number of patients in each subgroup, we cannot rule out the possibility that this is an effect of the sample size. Despite this, we did identify a trend toward a significant association between favourable outcomes and type of intervention. The best outcomes were observed in patients with temporal epilepsy, especially those with MRI lesions; this was the smallest patient group in our series, as SEEG is generally not needed in these patients. Historically, the outcomes of epilepsy surgery have been less favourable in patients with extratemporal epilepsy, particularly in MRI-negative patients.9,10,29–32,35 In our series, half of patients with extratemporal epilepsy were seizure-free in the final year of follow-up; this percentage was slightly lower among MRI-negative patients. These figures are more optimistic than those obtained in a meta-analysis and a series of previous studies, in which fewer than 40% of patients were seizure-free,29,35,36 and are more similar to the results of patients undergoing SEEG examination at other centres.5,8
The most frequent histopathological diagnosis was FCD, which was detected in the majority of MRI-negative patients (Fig. 5). In this group, poorly delimited FCD was most frequent; these lesions are often associated with normal MRI findings and more extensive cortical involvement, factors associated with poorer surgical outcomes.9,12,29,35 Therefore, seizure recurrence in some patients in the latter years of follow-up may be explained by the difficulty of fully delimiting the EZ with SEEG and other techniques.
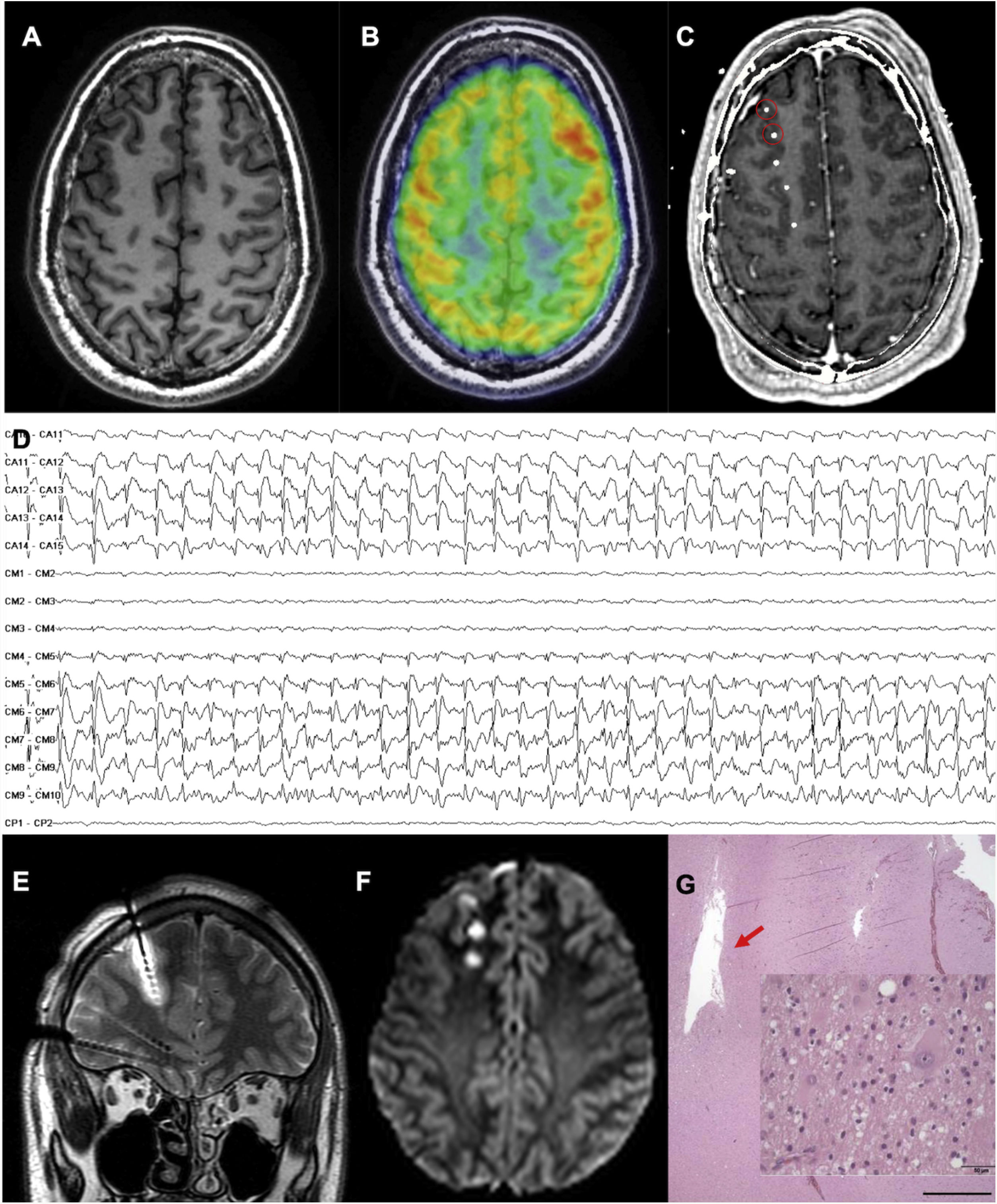
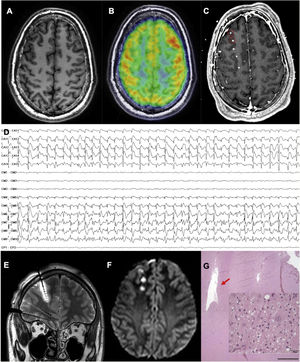
An MRI-negative patient with frontal lobe epilepsy, in whom the epileptogenic zone was localised and thermocoagulation performed. A) Axial T1-weighted sequence with normal gyral pattern. B) Fused brain MRI and PET study showing an unclear area of hypometabolism in the right frontopolar region. C) Fused brain MRI and CT study after electrode implantation, exploring this region (not all electrodes are shown). D) Interictal recording showing a periodic spike pattern in the first 2 electrodes, suggestive of cortical dysplasia type IIb. E) Coronal T2-weighted sequence showing oedema due to thermocoagulation around the previously described electrode. F) Axial DWI sequence showing the lesions caused by thermocoagulation after removal of the electrodes. G) The patient finally underwent surgery. This histopathological image (haematoxylin and eosin staining) shows the cavity caused by thermocoagulation. The magnified view (also haematoxylin and eosin staining) displays dysmorphic and globoid cells, confirming the diagnosis of focal cortical dysplasia type IIb.
The rate of complications in our series (haemorrhages in 4% of patients), in which SEEG included a series of modifications over the original method,14,15 is similar to those observed in other studies8,14–16 and lower than those described in patients studied with subdural electrodes.37,38 Unlike other European and American centres, electrode implantation at our centre did not use digital subtraction angiography to visualise the vascular anatomy. Instead, and similarly to other centres,39 we designed a specific program that shows the individual trajectory of each electrode in relation to the vessels detected in a brain MRI study with a double dose of gadolinium and a vascular sequence (3D Toft model). Since 2015, we have developed a specific technique, selective CT angiography, which has replaced digital subtraction angiography and enables specific angiographic visualisation of the brain region surrounding the trajectory of an individual electrode; no complications have occurred since the implementation of this technique.
ConclusionsSEEG enables assessment of complex cases of DLRFE, offering better surgical outcomes than previous invasive techniques, with a relatively low rate of complications. When properly planned on the basis of robust preoperative assessment, the technique enables us to treat patients not traditionally considered to be eligible for epilepsy surgery, and improves the localisation of the EZ. The published series, including our own, show similar outcomes at centres with experience using the technique.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are hugely grateful to the nursing team at the video EEG unit for their dedication.
Please cite this article as: Toledano R, Martínez-Alvarez R, Jiménez-Huete A, García-Morales I, Aledo-Serrano Á, Cabrera W, et al. Estereoelectroencefalografía en la evaluación prequirúrgica de epilepsias focales refractarias: experiencia de un centro de epilepsia. Neurología. 2022;37:334–345.