In the current population, strokes are one of the most important causes of morbidity and mortality, to which new risk factors are increasingly being attributed. Of late, there is an increased interest in the relationship between sleep disorders and strokes as regards risk and prognosis.
DevelopmentThis article presents the changes in sleep architecture and brain activity in stroke patients, as well as the interaction between stroke and sleep disorders, including those which may also influence the outcome and recovery from strokes. The different treatments discussed in the literature are also reviewed, as correct treatment of such sleep disorders may not only improve quality of life and reduce after-effects, but can also increase life expectancy.
ConclusionsSleep disorders are becoming increasingly associated with stroke. In addition to being a risk factor, they can also interfere in the outcome and recovery of stroke patients.
This article aims to present an exhaustive and current review on strokes and their relationship with sleep alterations and sleep disorders.
Los ictus son una causa importante de morbimortalidad en la población actual. Cada vez, a los ictus, se les han ido atribuyendo nuevos factores de riesgo. Últimamente está aumentando el interés de los trastornos del sueño y su influencia tanto como factor de riesgo y pronóstico en los ictus.
DesarrolloEn este trabajo se exponen los cambios de la arquitectura del sueño y de la actividad cerebral en los pacientes con ictus, además de la interacción existente entre los ictus y los diferentes trastornos del sueño, así como los resultados de estas interacciones que modifican el transcurso de la enfermedad. Se enumeran los posibles tratamientos descritos hasta la actualidad, ya que un correcto tratamiento de estos trastornos del sueño no sólo puede mejorar la calidad de vida y disminuir las secuelas, sino mejorar las expectativas de vida de estos pacientes.
ConclusionesLos trastornos del sueño se están consolidando como una entidad asociada a los ictus, que en ocasiones puede ser un factor precursor, pero que también puede interferir en la evolución y en la recuperación posterior del ictus. Con este artículo pretendemos realizar una revisión exhaustiva de lo que se ha descrito hasta la actualidad en relación con los ictus, con el sueño y las alteraciones del mismo.
Stroke, which has an annual incidence rate of 2–18 per 1000 inhabitants, is the second most frequent cause of death in the world, and the leading cause of disability in the adult population.2 Furthermore, strokes have a 13%–14% risk of recurrence during the first year, and a risk of 6% in subsequent years.3
For these reasons, we must both understand and be able to manage the different risk factors that may promote first-ever strokes and stroke recurrence. The main risk factors for stroke which are currently confirmed are atrial fibrillation, age above 65 years, high blood pressure, heart disease, asymptomatic carotid stenosis, history of transient ischaemic attacks, alcohol abuse, smoking, diabetes mellitus, and hypercholesterolaemia. However, these risk factors are able to explain only half of all stroke cases. New risk factors have been proposed in recent years, including inflammatory markers, infections, homocysteine, and sleep-disordered breathing (SDB).
Over the last 15 years, we have observed increasing interest in the relationship between sleep disorders and stroke. Recent studies have shown that sleep disorders (SDs) such as SDB, insomnia, hypersomnia, parasomnia, circadian rhythm disorders, and periodic limb movement disorder may play an important role in the prognosis for neurological and psychiatric functions, and therefore in the prognosis of stroke as well.
SDB includes some of the most thoroughly studied types of SDs because of their association with cardiovascular4–6 and cerebrovascular diseases.7–10 Furthermore, SDB increases the risk of stroke incidence, recurrence, and mortality.11,12
The purpose of this study is to present an exhaustive review of alterations in sleep architecture and the different SDs described to date which may cause or result from stroke; the impact of such disorders on prognosis; and treatment options.
Stroke and its impact on sleep architectureSleep architecture is defined as the structure and pattern of the different sleep stages experienced by the subject. Sleep is divided into NREM (N1, N2, N3) and REM sleep; these types of sleep alternate throughout the night in 90-minute cycles.13
Lesions affecting the central nervous system, whether focal or diffuse, may disturb sleep structure and patterns.
Both interpreting brain activity on an electroencephalogram (EEG) and reading sleep phases on a polysomnogram (PSG) are difficult to accomplish with stroke patients. This being the case, we must be aware of the signal alterations that may be present in order to reduce interpretation errors and not mistake slower cerebral activity or pathological signs secondary to vascular lesions for the activity and/or physiological signs of sleep, and vice versa.
It is therefore crucial to assess such changes in activity correctly, considering that some studies confirm that changes in brain activity and sleep activity during a stroke are linked to poorer patient prognosis.14–17
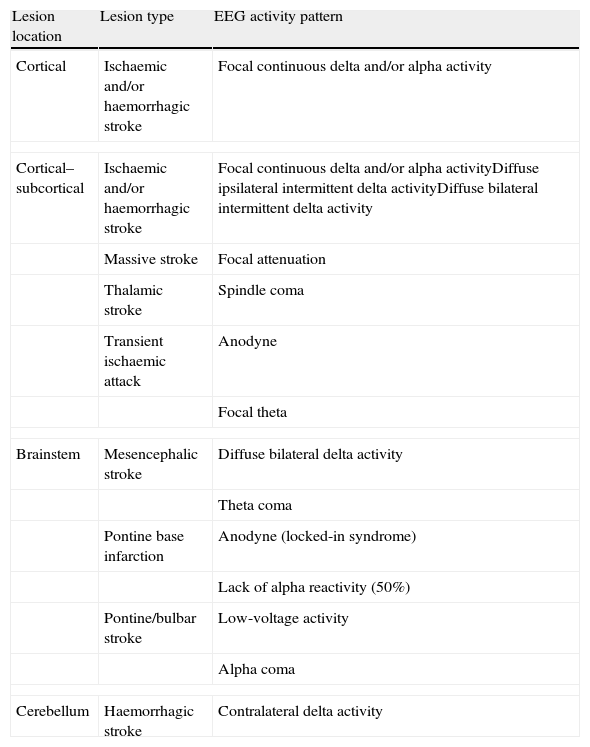
Changes in brain activityStrokes show different EEG patterns depending on their location. Cortical/subcortical strokes typically present focal neuronal dysfunction with focal delta activity (1–4Hz) which may be accompanied by more rapid alpha activity (Table 1).18,19 Subcortical strokes may display diffuse neuronal dysfunctions with intermittent bursts of ipsilateral or bilateral delta waves.16 Thalamic and brainstem infarcts may present pathological EEG patterns (alpha coma, spindle coma, theta coma), while small infarcts or those located in the internal capsule may show unremarkable EEG readings or slight focal theta activity.16 In contrast, massive acute infarcts present a pattern of focal attenuation of cerebral activity with no associated delta activity.20
Acute vascular lesions and their electroencephalographic correlations.
| Lesion location | Lesion type | EEG activity pattern |
| Cortical | Ischaemic and/or haemorrhagic stroke | Focal continuous delta and/or alpha activity |
| Cortical–subcortical | Ischaemic and/or haemorrhagic stroke | Focal continuous delta and/or alpha activityDiffuse ipsilateral intermittent delta activityDiffuse bilateral intermittent delta activity |
| Massive stroke | Focal attenuation | |
| Thalamic stroke | Spindle coma | |
| Transient ischaemic attack | Anodyne | |
| Focal theta | ||
| Brainstem | Mesencephalic stroke | Diffuse bilateral delta activity |
| Theta coma | ||
| Pontine base infarction | Anodyne (locked-in syndrome) | |
| Lack of alpha reactivity (50%) | ||
| Pontine/bulbar stroke | Low-voltage activity | |
| Alpha coma | ||
| Cerebellum | Haemorrhagic stroke | Contralateral delta activity |
We should not lose sight of the fact that strokes are dynamic processes that may progress favourably or unfavourably. There may also be complications such as stroke recurrence (34%), epileptic seizure (1.8%–15%), cerebral oedema, intracerebral haemorrhage, and adverse effects from treatments (10%). When such complications are present, the EEG will reveal significant changes in cerebral activity.16 These changes, which affect both the morphology and frequency of the EEG signal, have been linked to altered cerebral blood flow and the extension of brain damage.14
In 70%–80% of cases of vascular epileptic attacks, onset occurs within 24hours after the stroke. Between 2% and 9% of stroke patients are reported as having convulsive status epilepticus, with non-convulsive status epilepticus presenting in 27%.16
Preservation of baseline brain activity and lack of delta waves, presence of theta and beta waves, and intermittent bursts of theta–delta waves after stroke all indicate a good prognosis. In contrast, an increase in delta activity, a decrease in alpha or beta activity in the affected hemisphere, and slower brain activity are associated with a poorer long-term prognosis.14–17
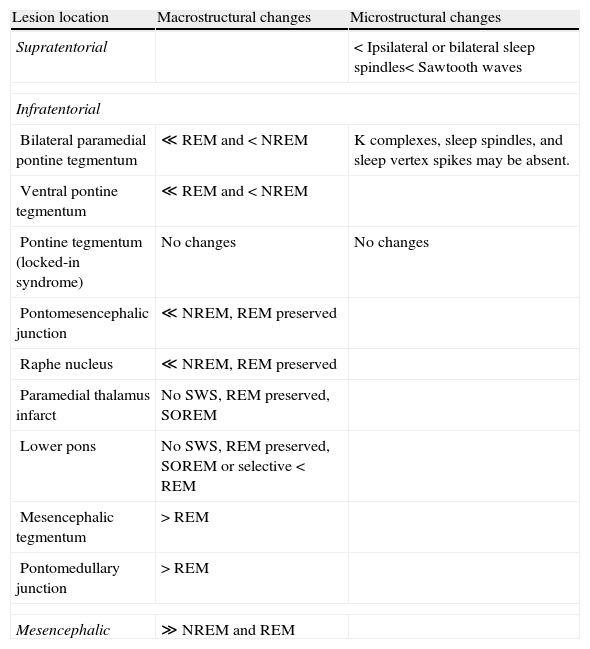
Changes in sleep macrostructure and microstructureLesions caused by a stroke may change sleep architecture (Table 2).
Changes to sleep macrostructure and microstructure broken down by stroke location.
| Lesion location | Macrostructural changes | Microstructural changes |
| Supratentorial | < Ipsilateral or bilateral sleep spindles< Sawtooth waves | |
| Infratentorial | ||
| Bilateral paramedial pontine tegmentum | ≪ REM and < NREM | K complexes, sleep spindles, and sleep vertex spikes may be absent. |
| Ventral pontine tegmentum | ≪ REM and < NREM | |
| Pontine tegmentum (locked-in syndrome) | No changes | No changes |
| Pontomesencephalic junction | ≪ NREM, REM preserved | |
| Raphe nucleus | ≪ NREM, REM preserved | |
| Paramedial thalamus infarct | No SWS, REM preserved, SOREM | |
| Lower pons | No SWS, REM preserved, SOREM or selective < REM | |
| Mesencephalic tegmentum | > REM | |
| Pontomedullary junction | > REM | |
| Mesencephalic | ≫ NREM and REM | |
NREM: non-REM sleep; SOREM: REM sleep at sleep onset; SWS: slow wave sleep; TST: total sleep time; >: more; <: less.
Supratentorial strokes have been linked to decreases in NREM sleep and total sleep time, and to lower sleep efficiency.21 There may be an ipsilateral or bilateral decrease in sleep spindles following a hemispheric stroke,22–25 but these changes do not always occur.22,26–28 Sleep spindles, K complexes, and slow waves indicative of slow-wave sleep are partially suppressed in cases of bilateral paramedian thalamic stroke, with a sustained increase of stage N1 sleep. This indicates inability to be completely awake.29–31
A temporary reduction in REM sleep may be observed in the first days following a supratentorial stroke. This phenomenon may be permanent in cases of hemispheric stroke with a poor prognosis.24,27,32,33 The sawtooth waves typical of REM sleep may be reduced after a hemispheric stroke.27
Occipital strokes associated with cortical blindness have been linked to a decrease in REM sleep.34
Changes in sleep architecture following a stroke are not indicative of the stroke's location. However, some studies suggest that right-sided strokes decrease REM and REM density, while left-sided strokes decrease NREM stages.32
Infratentorial strokes have been linked to decreases in NREM sleep and in REM sleep most of all. Lesions near the pontine tegmentum (locked-in syndrome) and unilateral lesions in this region do not affect sleep architecture.35,36 On occasions, only REM or only NREM sleep may be affected. Strokes in the pontomesencephalic junction and the raphe nucleus may diminish the amount of NREM sleep while not affecting the amount of REM sleep.37 Strokes in the paramedian thalamus and the lower pons have been linked to lack of slow-wave sleep with preserved REM sleep and to onset of REM at the beginning of the sleep cycle. Nevertheless, strokes in the lower pons may selectively reduce REM sleep.35,36,38–41 Strokes in the mesencephalic tegmentum and pontomedullary junction may lead to an increase in REM sleep. Mesencephalic infarcts may lead to increases in both NREM and REM sleep.42
There are reports that absence of REM sleep following an infratentorial stroke may continue for years, without any signs of cognitive or behavioural changes.43
Some studies state that infratentorial strokes may also change the microstructure of NREM sleep by eliminating sleep spindles, K complexes, and/or sleep vertex spikes.35,36,40
Other studies observe that stroke patients with an associated SDB have an increased percentage of N1 stage sleep in their total sleep time, a higher frequency of micro-arousals, and decreased sleep efficiency. Treatment with CPAP reverts these changes.44,45
Some of the changes in sleep architecture which have been related to unfavourable prognosis in stroke patients include low sleep efficiency, increase in intrasleep wakefulness, decrease in stage N2 duration, decrease in sleep spindles and K complexes, and increase in slow, deep sleep.22,23,27 Broken sleep has also been linked to increased risk of stroke.11
The poor functional prognosis for these patients could be explained by the role sleep plays in neuronal plasticity and protein synthesis, and by its protective function that involves reducing neurotoxic activity and metabolic demand.46
Stroke and its relationship with sleep disordersThe sleep–wake cycle and the different phases of sleep are regulated by the complex interaction of multiple mechanisms located in the brainstem, hypothalamus, preoptic area, and the thalamus.42 It is therefore logical to raise the possibility of focal encephalic lesions resulting in SDs.
A large percentage of stroke patients (20%–63%) experience SDs, which may manifest as hypersomnia, insomnia, parasomnias, circadian rhythm disorders, periodic limb movement disorder, and/or respiratory abnormalities during sleep.47
Some studies have suggested that daytime drowsiness with prolonged sleep periods may be an independent risk factor for stroke.48,49 It has also been suggested that the presence of SD in stroke patients may be linked to a poorer prognosis and increased mortality.47
We must not forget that in patients with acute stroke, SDs may be caused by external factors during hospitalisation or by the damage inherent to the stroke. External factors include noise, light, monitoring, anxiety, depression, and complications of illness, such as heart problems, infections, epileptic seizures, fever, and the patient's medications, which may affect sleep quality.
Stroke and hypersomniaHypersomnia is defined as increased daytime drowsiness and/or an increase in the amount of sleep needed daily. Hypersomnia prevalence among stroke patients ranges from 1.1% to 27%.50,51
Most forms of central hypersomnia secondary to ictus are caused by a decrease in the activation threshold due to impairment of the ascending reticular system (ARS). Lesions tending to cause marked hypersomnia include bilateral thalamic lesions, thalamo-mesencephalic lesions, upper pons lesions, and lesions to the medial pontomedullary region, that is, in the area where RAS fibres are concentrated. In cortical and subcortical lesions, except those in the thalamus, the activating system is affected to a lesser degree since RAS projections are more scattered, unless a very extensive lesion compresses the upper brainstem due to oedema. During sleep, cerebral micro-arousals seem to be more frequent in patients with medial lesions, while more lateral lesions tend to elicit motor micro-arousals.52 Areas which may occasionally cause hypersomnia include the striatum, the pontine tegmentum, the medulla, and the cerebral hemispheres. Lesions in the cerebral hemispheres only elicit hypersomnia if they are very large, and more typically if they affect the left hemisphere more than the right and the anterior region more than the posterior region.22
The most severe cases of hypersomnia secondary to stroke are caused by paramedian thalamic infarcts. Patients tend to present sudden-onset stupor while demonstrating normal responses to stimuli, associated with attention and memory deficits. While hypersomnia may improve in the 12 months following the infarct, cognitive deficits may persist.31,53
Using 24-hour PSG monitoring, doctors have detected hypersomnias in patients with thalamic, hypothalamic, mesencephalic, and pontine infarcts.31,41
The effects of stroke may also resemble entities linked to CNS hypersomnia, such as narcolepsy with cataplexy and Kleine-Levin syndrome. Narcolepsy has been described in lesions caused by cerebral hypoxia and in bilateral diencephalic infarcts.54,55 Kleine-Levin syndrome (hypersomnia with hyperphagia) has been described in patients with multiple cerebral infarcts.56
Although stroke is closely linked to SDB, we must not forget that hypersomnia may be secondary to the respiratory problem and not to the CNS lesion itself.51,57–59 However, patients suffering both stroke and SDB have milder cases of hypersomnia than do patients with SDB only.60
It is very important to distinguish between hypersomnia, a simple increase in the need for sleep, drowsiness due to encephalopathy (degree of arousal), and fatigue. Fatigue is a physical sensation of tiredness, lack of energy, and marked drowsiness, accompanied by a normal or less-than-normal amount of sleep. A study found fatigue in 46% of stroke patients. Although hypersomnia may improve after the first few months, fatigue persists in the chronic phase.61 Linking fatigue to stroke is extremely difficult, since symptoms may coincide with those of mood disorders, neurological sequelae, and neuropsychological sequelae secondary to ictus, and even SDs.
Post-ictal treatment of hypersomnia is often ineffective. Some patients with thalamic infarcts have improved under amphetamine, modafinil, methylphenidate, or levodopa treatment; others with mesencephalic stroke have improved with modafinil treatment.62 Two authors have observed favourable outcomes in acute stroke rehabilitation and degree of arousal following treatment with methylphenidate or levodopa over 3 weeks.63,64
Patients with associated symptoms of depression have improved with stimulant antidepressants.
Stroke and insomniaInsomnia is defined as difficulty initiating and/or maintaining sleep and/or early waking the next day. Its physical and psychological sequelae include fatigue, impaired concentration, and irritability.
Insomnia is a common complaint among stroke patients, affecting between 20% and 56% of the total.50,65,66 One study by Leppavuori et al.50 observed that 56% of all stroke patients complained of insomnia; of these patients, 37% met the criteria for insomnia defined by the DSM-IV. They also observed that 38.6% already had insomnia prior to the stroke; 18.1% reported it as a new symptom following stroke.
Evidence is sufficient to confirm that in 50% of stroke patients, insomnia is a result of SDB, whether obstructive, central, or due to hypoventilation.10,59,67–69
Strokes that present with insomnia are classified as thalamic and brainstem (thalamo-mesencephalic, pontomesencephalic, and pontine tegmentum). They cause inversion of the sleep–wake cycle with insomnia, night-time agitation, and daytime hypersomnia.47
Insomnia frequently occurs during the acute phases of stroke, whether it is caused by external factors associated with hospitalisation or by complications of the disease itself, monitoring, or use of medications.
Treating insomnia in stroke patients should include, from the very beginning, fostering simple habits such as isolating the patient from noise and light during the night and/or increasing exposure to daylight. This will create a favourable environment for regulating the sleep–wake cycle.
Benzodiazepines, zolpidem, or sedative antidepressants may be used as pharmacological treatment, but their side effects (sedation and neurocognitive alteration) can worsen neurological symptoms.70
Stroke and parasomniaParasomnia is mainly defined as motor or sensory activity that occurs during sleep, whether during the REM or NREM phases.
Cases of tegmental pontine stroke have been described in association with behaviour disorders during REM sleep (RBD).71,72
Lesions in the pontine tegmentum, midbrain, or paramedial thalamus may trigger visual hallucinations, especially at nightfall or at sleep onset.47
Strokes in the thalamus and in the temporal, parietal, and occipital lobes may lead to increased dreaming and nightmares and/or a syndrome of dream-reality confusion.73
RBD may be treated with clonazepam (0.5–2mg) an hour before the patient goes to bed.47
Stroke and periodic limb movement disorder during sleepPeriodic limb movement (PLM) during sleep may increase or decrease after a unilateral ictus and persist after a spinal cord stroke.74,75
Lee et al.76 described de novo restless leg syndrome (RLS) as being present in 12% of all stroke patients. RLS has mainly been reported in cases of pontine, thalamic, basal ganglia, and corona radiata lesions. Bilateral symptoms are reported by two-thirds of patients with RLS after a stroke; a third report symptoms on the side contralateral to the stroke.
RLS and PLM associated with stroke may be treated with dopaminergic agonists. It is important to recall that treatment with antidepressants, neuroleptics, metoclopramide, and lithium may aggravate RLS and PLM.47
Stroke and circadian rhythmCircadian or biological rhythms are oscillations of biological variables that follow regular time intervals. Circadian rhythm sleep disorders are characterised by producing inability to sleep owing to an imbalance between the brain mechanism controlling the person's circadian rhythm for sleep and the desired or necessary sleep–wake schedule which is normally followed in that person's environment.
During sleep, NREM and REM sleep repeat cyclically throughout the night. In NREM sleep, sympathetic activity decreases while parasympathetic activity becomes predominant. This results in decreases in heart rate, cardiac output, blood pressure, and respiratory rate. The nocturnal drop in blood pressure is a normal phenomenon, and changes in it are associated with an increased risk of stroke.77 REM sleep is characterised by the variability of both the parasympathetic and sympathetic nervous systems with phase oscillations and spikes that deliver a net increase in parasympathetic tone and lower the influence of the sympathetic nervous system. During REM sleep, heart rate and blood pressure are variable, while cortical and spinal blood flow increases.
Catecholamine levels in plasma increase from 6 am until the afternoon. During the same period, heart rate and blood pressure increase. Fibrinolytic activity slows down in the morning,78 while platelet aggregability increases.79 These phenomena have been linked to increased vascular morbidity during the morning hours.
Strokes, myocardial infarctions, and sudden death tend to occur in the morning (between 6 am and 12 pm), especially in the first hour after waking. There are no circadian rhythm differences between first-ever events and recurring events.
Circadian variations in stroke onset timing show a curve with 2 peaks during the day (6 am to 12 pm and 6 pm to 7 pm), but the first peak is by far the most typical.80–82 The probability of a stroke occurring upon awakening rather than during the rest of the day, broken down by stroke subtype, is 66% for ischaemic strokes and 34% for haemorrhagic strokes.83
Most ischaemic strokes occur upon waking and they are typically embolic, but between 20% and 40% of ischaemic strokes occur during the night, especially at onset of sleep. These strokes are more commonly atherothrombotic and lacunar.82–86
Haemorrhagic strokes rarely occur at night (10%) and are the most common in the morning.85,86
From the above, we can deduce that sleep may provide protection from stroke, and that strokes may be triggered by waking. At the same time, sleep favours ischaemic infarcts and protects us from haemorrhagic infarcts. One explanation may be that blood pressure tends to decrease by 10%–20% during sleep. When a person wakes, blood pressure rises abruptly and is accompanied by increased sympathetic activity. Low pressure during the night may explain what causes the ischaemic strokes,87 while increased pressure upon waking could explain haemorrhagic strokes and why so few occur during sleep.80
Another study links nocturnal infarcts to respiratory changes during sleep.88 Patients with obesity and/or SDB may be more prone to stroke during sleep since they do not benefit from sleep's protective effects.1 The study states that sleep may therefore be active in the aetiopathogenesis of a sizeable subgroup of strokes.
The cause of the second spike in the evening is unclear and rarely addressed. Some studies have linked it to afternoon naps,80 while others show little evidence to support this. The variance can probably be explained by cultural and social differences between countries.89,90
In addition to being associated with changes in circadian rhythm, strokes can also alter the circadian rhythms themselves. Examples include infarcts that affect the secretion of growth hormone and melatonin and right insular infarcts. The latter may change autonomic circadian variations and contribute to an increase in post-infarct morbidity and mortality.42
One study suggests that circadian rhythm changes in the acute phase of stroke are associated with poorer prognosis.91 Other studies suggest that circadian rhythm alterations in blood pressure predict stroke severity and prognosis, and that they are not associated with SDB severity.83,92
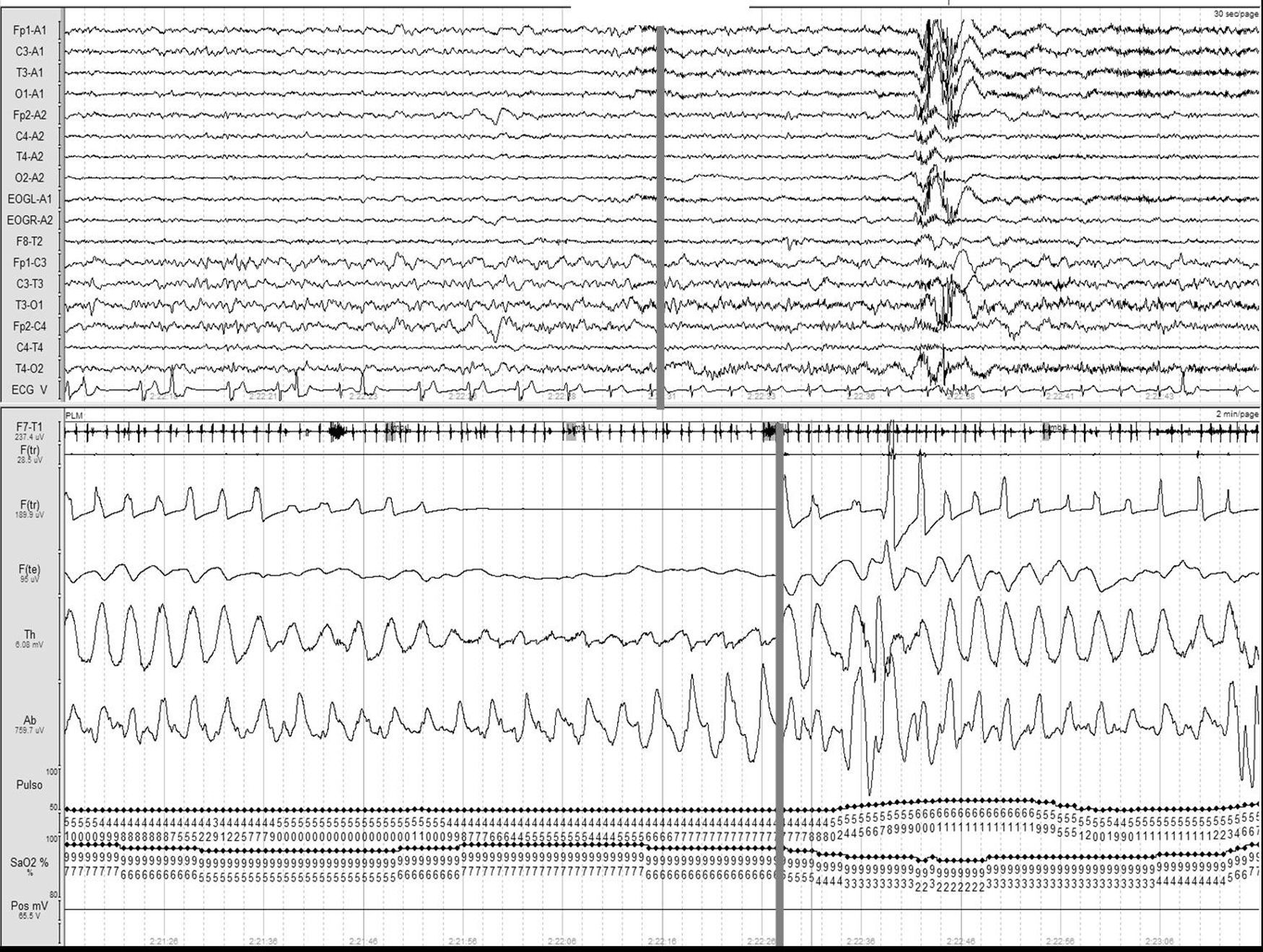
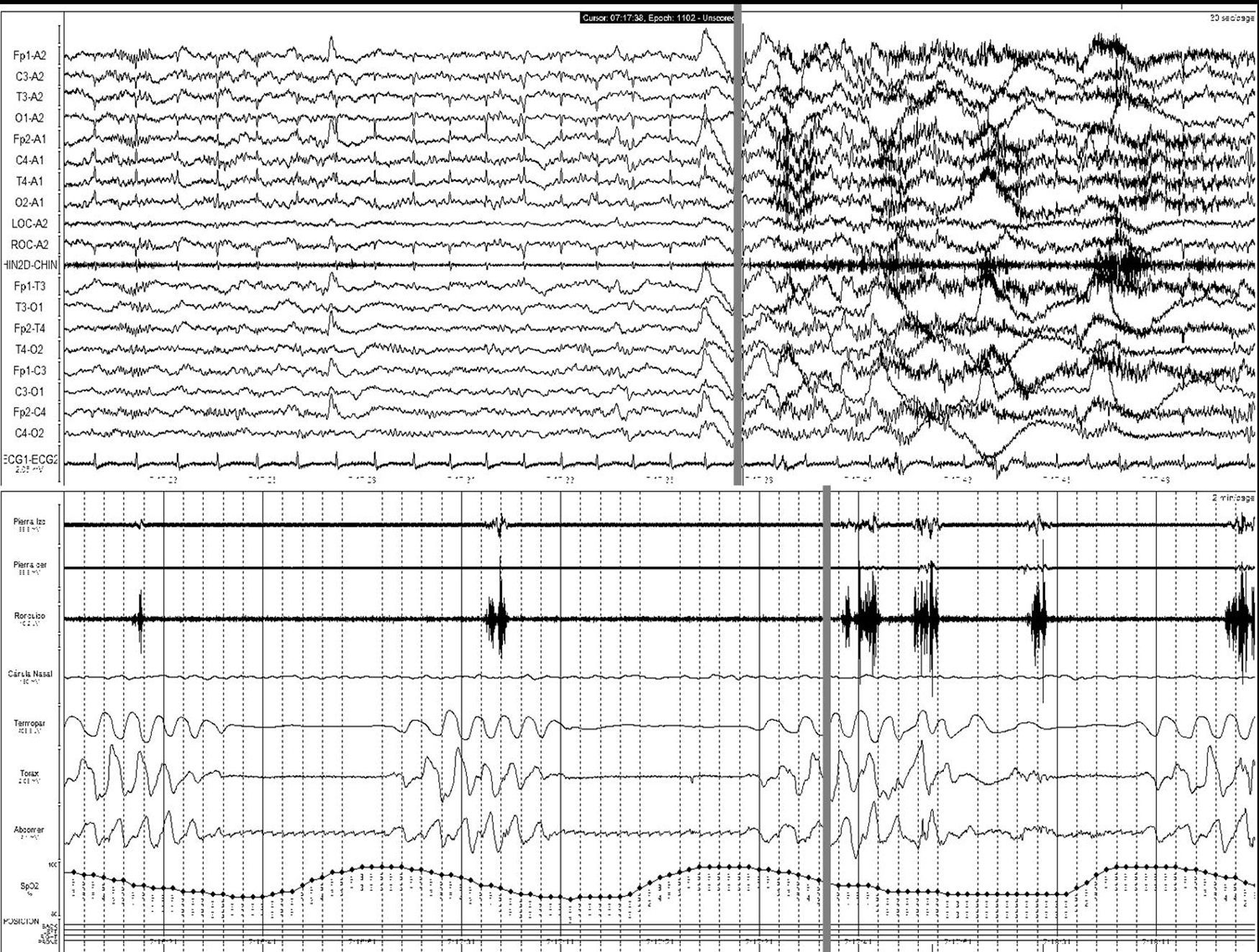
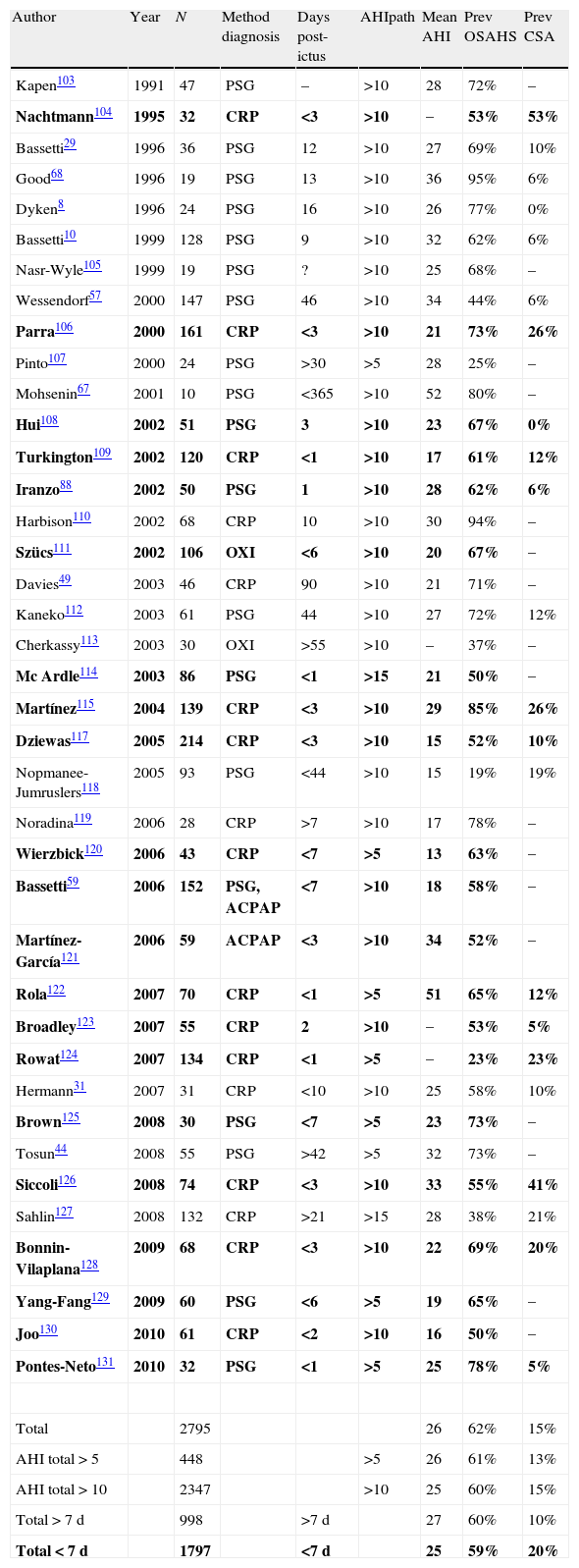
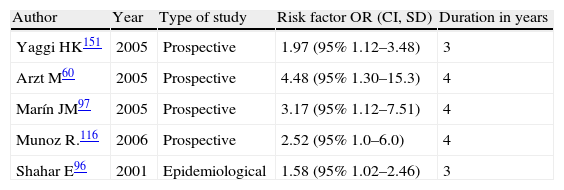
Stroke and sleep-disordered breathingSDBs currently include obstructive sleep apnoea/hypopnea syndrome (OSAHS) (Fig. 1), central sleep apnoea (CNA) (Fig. 2), and sleep-related hypoventilation/hypoxaemic syndromes (SRH). SDBs are proven risk factors for cardiovascular diseases5,6,9,10,93–98 in addition to increasing patient morbidity and mortality.8,59,68,99–102
Obstructive apnoea. Polysomnogram taken within 24hours of stroke. Patient was admitted with intracerebral haemorrhage in the basal ganglia and revealed to have frequent obstructive apnoea episodes. The grey line links the respiratory event with the onset of a micro-arousal on the EEG trace.
Central apnoea. Polysomnogram taken within 24hours of stroke. Patient was admitted with haemorrhage in the basal ganglia and revealed to have frequent central apnoea episodes. The grey line links the respiratory event with the onset of a micro-arousal on the EEG trace (the snoring signal contains an artefact caused by movement of the patient's legs).
Studies completed to date have shown a prevalence of SDBs of 62% among patients who have suffered a stroke (Table 3).8,10,29,31,44,49,57,59,67,68,88,103–131 This suggests that SDB is likely to be a factor that triggers CVA. We must not overlook the fact that strokes may also cause SDB.95,124,132,133
Studies published to date on stroke and its relationship with sleep-disordered breathing.
| Author | Year | N | Method diagnosis | Days post-ictus | AHIpath | Mean AHI | Prev OSAHS | Prev CSA |
| Kapen103 | 1991 | 47 | PSG | – | >10 | 28 | 72% | – |
| Nachtmann104 | 1995 | 32 | CRP | <3 | >10 | – | 53% | 53% |
| Bassetti29 | 1996 | 36 | PSG | 12 | >10 | 27 | 69% | 10% |
| Good68 | 1996 | 19 | PSG | 13 | >10 | 36 | 95% | 6% |
| Dyken8 | 1996 | 24 | PSG | 16 | >10 | 26 | 77% | 0% |
| Bassetti10 | 1999 | 128 | PSG | 9 | >10 | 32 | 62% | 6% |
| Nasr-Wyle105 | 1999 | 19 | PSG | ? | >10 | 25 | 68% | – |
| Wessendorf57 | 2000 | 147 | PSG | 46 | >10 | 34 | 44% | 6% |
| Parra106 | 2000 | 161 | CRP | <3 | >10 | 21 | 73% | 26% |
| Pinto107 | 2000 | 24 | PSG | >30 | >5 | 28 | 25% | – |
| Mohsenin67 | 2001 | 10 | PSG | <365 | >10 | 52 | 80% | – |
| Hui108 | 2002 | 51 | PSG | 3 | >10 | 23 | 67% | 0% |
| Turkington109 | 2002 | 120 | CRP | <1 | >10 | 17 | 61% | 12% |
| Iranzo88 | 2002 | 50 | PSG | 1 | >10 | 28 | 62% | 6% |
| Harbison110 | 2002 | 68 | CRP | 10 | >10 | 30 | 94% | – |
| Szücs111 | 2002 | 106 | OXI | <6 | >10 | 20 | 67% | – |
| Davies49 | 2003 | 46 | CRP | 90 | >10 | 21 | 71% | – |
| Kaneko112 | 2003 | 61 | PSG | 44 | >10 | 27 | 72% | 12% |
| Cherkassy113 | 2003 | 30 | OXI | >55 | >10 | – | 37% | – |
| Mc Ardle114 | 2003 | 86 | PSG | <1 | >15 | 21 | 50% | – |
| Martínez115 | 2004 | 139 | CRP | <3 | >10 | 29 | 85% | 26% |
| Dziewas117 | 2005 | 214 | CRP | <3 | >10 | 15 | 52% | 10% |
| Nopmanee-Jumruslers118 | 2005 | 93 | PSG | <44 | >10 | 15 | 19% | 19% |
| Noradina119 | 2006 | 28 | CRP | >7 | >10 | 17 | 78% | – |
| Wierzbick120 | 2006 | 43 | CRP | <7 | >5 | 13 | 63% | – |
| Bassetti59 | 2006 | 152 | PSG, ACPAP | <7 | >10 | 18 | 58% | – |
| Martínez-García121 | 2006 | 59 | ACPAP | <3 | >10 | 34 | 52% | – |
| Rola122 | 2007 | 70 | CRP | <1 | >5 | 51 | 65% | 12% |
| Broadley123 | 2007 | 55 | CRP | 2 | >10 | – | 53% | 5% |
| Rowat124 | 2007 | 134 | CRP | <1 | >5 | – | 23% | 23% |
| Hermann31 | 2007 | 31 | CRP | <10 | >10 | 25 | 58% | 10% |
| Brown125 | 2008 | 30 | PSG | <7 | >5 | 23 | 73% | – |
| Tosun44 | 2008 | 55 | PSG | >42 | >5 | 32 | 73% | – |
| Siccoli126 | 2008 | 74 | CRP | <3 | >10 | 33 | 55% | 41% |
| Sahlin127 | 2008 | 132 | CRP | >21 | >15 | 28 | 38% | 21% |
| Bonnin-Vilaplana128 | 2009 | 68 | CRP | <3 | >10 | 22 | 69% | 20% |
| Yang-Fang129 | 2009 | 60 | PSG | <6 | >5 | 19 | 65% | – |
| Joo130 | 2010 | 61 | CRP | <2 | >10 | 16 | 50% | – |
| Pontes-Neto131 | 2010 | 32 | PSG | <1 | >5 | 25 | 78% | 5% |
| Total | 2795 | 26 | 62% | 15% | ||||
| AHI total>5 | 448 | >5 | 26 | 61% | 13% | |||
| AHI total>10 | 2347 | >10 | 25 | 60% | 15% | |||
| Total>7 d | 998 | >7d | 27 | 60% | 10% | |||
| Total<7 d | 1797 | <7d | 25 | 59% | 20% |
ACPAP: AutoCPAP; AHI: apnoea–hypopnoea index; mean: mean patient AHI: Diag Meth: diagnostic method; N: number of cases; OXI: oximetry; path.: cut-off value for a pathological result; CRP: cardiorespiratory polygraph; Prev: prevalence; OSAHS: obstructive sleep apnoea–hypopnoea syndrome; PSG: night time polysomnography; CSA: central sleep apnoea; total<7d: result for mean of all articles on acute stroke patients in which studies were performed during the first 7 days (in bold); total>7d: result for mean of all articles on stroke patients in which additional studies were completed after the first 7 days; total AHI>10: result for mean scores on AHI greater than 10; total AHI>5: result for mean scores on AHI greater than 5; total: result for mean of all articles reviewed.
This table shows that of all CVA patients recorded, 62% presented sleep apnoea–hypopnea syndrome (predominantly obstructive) and a mean AHI of 26. We found no differences between results from different studies whether using AHI>5 or AHI>10 as a cut-off point. If we use records that only include patients within 1 week of CVA, we can see that the percentage of patients and AHI total remain almost the same, while the percentage of central respiratory events increases.
Snoring, daytime drowsiness, and SDB have been described collectively as an independent risk factor for suffering a stroke; in turn, they are associated with higher morbidity and mortality in stroke patients.127,134,135
Studies performed to date confirm that there is a direct relationship between OSAHS, daytime drowsiness,136,137 and body mass index (BMI) in the general population.138,139 However, recent studies limited to stroke patients have shown no such relationship between OSAHS severity and daytime drowsiness or BMI.51,57–59 Only 1 study reported a link between daytime drowsiness and risk of stroke among stroke patients (OR=3.1; 95% CI=1.6–6.1).49 This means that disease-predicting factors in the general population are not as useful for detecting OSAHS in stroke patients.
A number of case–control studies7,69,140–143 and a prospective study in women144 have shown snoring to be a risk factor for stroke, but other studies show contradictory results.49,145,146 The high prevalence of snoring in the community points to an independent but weak risk factor for stroke.147
All these studies present the same limitation: it is difficult to know if there is a link between stroke and snoring itself, or between stroke and the OSAHS with which snoring is associated. In any case, the link between snoring and stroke is particularly obvious in the presence of symptoms suggesting OSAHS (confirmed apnoea, hypersomnia, and obesity).7,143,146
Numerous studies have suggested that OSAHS is an independent risk factor for stroke.7,98,127,148–150 An epidemiological study96 and 4 prospective studies60,97,116,151 all reported a higher incidence rate of stroke with an AHI (apnoea/hypopnoea index) within the pathological range (Table 4). While these studies do not allow us to conclude that OSAHS is an independent risk factor for stroke, they strongly suggest that this is the case.
Risk factors for stroke in patients with sleep apnoea–hypopnea syndrome.
| Author | Year | Type of study | Risk factor OR (CI, SD) | Duration in years |
| Yaggi HK151 | 2005 | Prospective | 1.97 (95% 1.12–3.48) | 3 |
| Arzt M60 | 2005 | Prospective | 4.48 (95% 1.30–15.3) | 4 |
| Marín JM97 | 2005 | Prospective | 3.17 (95% 1.12–7.51) | 4 |
| Munoz R.116 | 2006 | Prospective | 2.52 (95% 1.0–6.0) | 4 |
| Shahar E96 | 2001 | Epidemiological | 1.58 (95% 1.02–2.46) | 3 |
SD: standard deviation; CI: confidence interval; OR: Odds ratio.
A recent project by the Sleep Heart Health Study,152 a prospective study spanning 8 years, discovered a direct relationship between OSAHS severity and risk of stroke. It found that men with moderate to severe OSAHS had 3 times the risk of suffering a stroke, and that the risk of stroke rose by 6% for every unit on the AHI scale between 5 and 25. No such increases in risk of stroke were observed among women unless AHI scores reached 25 or higher.
In addition, other studies correlate OSAHS severity to increased prevalence of lacunar infarcts,10,150 while another study by McArdle et al.114 never identified such an association.
The mechanism explaining why OSAHS is a risk factor for ictus is unknown and likely to involve multiple factors. The following 3 premises were proposed: (a) the increase in intrathoracic pressure occurring during apnoea diminishes blood flow to the brain. Repeated incidents may elicit ischaemic changes, especially in watershed locations.153–155 (b) OSAHS may increase the risk of stroke because these patients have a prevalence of high blood pressure,5 heart disease, patent foramen ovale,156 changes in endothelial function, atherogenesis, prothrombotic changes, proinflammatory changes, and increased platelet aggregation42,157–159; and (c) OSAHS may trigger the stroke event.88
In its most recent protocol for stroke risk factor prevention, the National Institute of Health (NIH)160 lists OSAHS among the less-studied risk factors. It recognises its prevalence, indicates its relationship with hypertension as an independent stroke factor, and recommends identifying patients at a high risk for OSAHS. This way, they may be treated properly and reduce their risk of first-ever stroke.
Respiratory changes and sleep-disordered breathing during the acute phase of strokeAnomalous respiratory patterns are often found during the acute phase of stroke, whether they are caused by the location of the lesion itself161 or by SDB existing prior to the stroke.133 These respiratory patterns are more frequent in patients with impaired consciousness or severe deficiencies, during sleep, and in patients with medullary infarcts.161 Whether they are located in the hemispheres or the brainstem, ictal lesions that affect muscles of the respiratory system (upper respiratory system, intercostal muscles, diaphragm) may result in respiratory changes. Stroke in the frontal lobe, basal ganglia, or internal capsule may cause respiratory apraxia. Brainstem strokes may result in a number of different respiratory patterns, such as neurogenic hyperventilation (mesencephalon or pons), apneustic respiration (inferior medial posterior area of the pons), ataxic (Biot) respiration, central apnoea syndrome and Ondine's curse (medulla), and hypoventilation (medulla and upper medulla). Medullary strokes at the level of C1 may impair both voluntary respiration (posterior lesion) and automatic respiration (anterior lesion).42,133 Many of these respiratory patterns have no prognostic significance except for tachypnoea with low CO2, which is associated with poor prognosis.
We must not forget that patients in the acute phase of stroke must be intubated when an open airway cannot be guaranteed or when the patient presents lung oedema or status epilepticus.162 Intubation tends to be more common in haemorrhagic stroke than in ischaemic stroke, and it has been linked to increased morbidity and mortality in patients.163
These respiratory patterns described in the acute phase of stroke appear infrequently in the studies completed to date. This is explained by the fact that such studies have examined patients with a certain degree of alertness and who are not haemodynamically unstable. Furthermore, evaluating sleep may be impossible in patients with severe central nervous system lesions. Brain activity observed in these cases would be typical of encephalopathy, which would prevent us from distinguishing between wakefulness and sleep, or between different sleep stages. This in turn would complicate our assessment of respiratory changes during sleep.
Studies performed during the acute phase of stroke confirm that patients frequently present respiratory changes; mean prevalence is 59% (Table 3 and Fig. 1). OSAHS is the predominant respiratory disorder in this phase and maintains the same level of prevalence throughout the sub-acute phase.
SHS has a prevalence of 20% in the acute phase of stroke, but this percentage decreases to 10% in the sub-acute phase following stroke (10%) (Fig. 2 and Table 3). SHS mainly presents during NREM sleep and its traits tend to disappear during the REM phase. It is detected during wakefulness in only a small minority of stroke patients.104 Researchers have traditionally described Cheyne–Stokes respiration as being associated with the following: infarctions presenting as bihemispheric lesions, unilateral lesions, or brainstem lesions124,132; heart failure; and severely decreased level of consciousness. However, these other entities are not necessarily present.133 Parra et al.106 conclude that central respiratory disorders are uncommon in transient ischaemic attacks (TIAs) and more frequent in cerebral infarctions, especially haemorrhagic infarctions.
Bonnin-Vilaplana et al.128 found that 69% of TIA patients presented respiratory changes (49% obstructive; 20% central) with an OR of 3.17 (95% CI: 1.02–9.79). Harbison et al.110 determined that cases of TIA have a higher apnoea–hypopnoea index than other cases of infarction do, and that OSAHS severity was not correlated with higher 3-year mortality rates.
The high prevalence and persistence of respiratory changes after stroke lead us to speculate that OSAHS may act as a trigger for ischaemic stroke.10,88,106,133
Most published studies exclude cases of haemorrhagic stroke. Only 3 studies include haemorrhagic stroke, and we find no significant differences in respiratory disorder prevalence between these studies and those examining ischaemic stroke.8,106,112 Nevertheless, Szucs et al.111 found that SDBs associated with haemorrhagic stroke during the subacute phase progressed better than those associated with ischaemic stroke. Pontes-Neto et al.131 found that OSAHS severity in haemorrhagic stroke was directly related to perihaematomal oedema.
There are a number of neuronal, haemodynamic, and inflammatory explanations of how SDBs can trigger stroke. During a respiratory event, the patient experiences decreased blood pressure, heart rate, cerebral blood flow, and oxygenation; when it ends, all 4 parameters increase abruptly.42,164–166 These factors result in a 10%–20% decrease in cerebral blood flow velocity during respiratory events.167 Repeated episodes may increase damage to the ischaemic penumbra zone or contribute to haemorrhage.
OSAHS has also been linked to prothrombotic and proinflammatory factors; alteration of the function of nitric oxide synthase; alteration of endothelin pathways; increase in coagulation factor VII; increased platelet aggregation; higher leptin levels; increase in glucose intolerance; higher levels of fibrinogen, C-reactive protein, cytokines, and cell adhesion molecules; and increase in oxidative stress.122,153,168–176
During prolonged apnoea episodes in patients with patent foramen ovale, paradoxical embolisation may also be a stroke mechanism.177
Stroke may also contribute to an increase in SDBs due to higher upper airway collapsibility secondary to the pharyngeal weakness that arises in 30%–50% of cases of acute stroke.109,121This weakness is linked to linear improvement of SDB symptoms once the swallowing function normalises.121
Although numerous studies have been completed to date, researchers have been unable to link SDB frequency, type, or severity to the location of the stroke. A single article by Bonnin-Vilaplana et al.128 states that TIAs in the internal capsule and pons of smokers show a significant association with SDB.
Sleep-disordered breathing as a prognostic factor in strokeSeveral different studies suggest that SDB affects prognosis in stroke. Snoring adversely affects outcomes in stroke patients.142,178 The presence of apnoea on the first night following a stroke is associated with early neurological deterioration,59,68,88,102,106,112,129 longer recovery times112 with higher long-term morbidity and mortality rates,8,100,109,122 and a greater risk of stroke recurrence.117,179 Parra et al106 determined that OSAHS in stroke patients is an independent prognostic factor for mortality, and that risk of mortality increases in proportion to the apnoea–hypopnoea index. However, another study of a 10-year follow-up period suggests that OSAHS does not predict higher risk of mortality in stroke patients.127
The condition of 12%–43% of stroke patients declined, and of these patients, 87% suffered deterioration in the first 48hours.106 The main causes of decline include hypotension, arrhythmias, and hypoxaemia. SDB may also cause deterioration, but this has not yet been proved.
As we mentioned before, OSAHS prevalence among patients in the subacute and chronic phases following a stroke is 60% (Table 3). In consequence, the patient's full potential for rehabilitation may be hampered by decreased motivation and cognitive capacity. Furthermore, patient survival may be compromised by an increased risk of stroke and death.8,106
Stroke prognosis and response to treatment for sleep-disordered breathingAt present, doctors strive for early detection of the risk factors of stroke so as to provide more effective treatment. In addition, we must not forget that stroke survivors are at an increased risk for recurrence, which increases their morbidity and mortality rates.
Some suggest that OSAHS is a modifiable independent risk factor for stroke which indicates of a higher risk of death or recurrence.113,180 The most cost-effective treatment for OSAHS has been shown to be CPAP.181
There are currently no randomised studies that show that treating OSAHS with CPAP decreases stroke incidence. Existing prospective studies provide incomplete and contradictory information.100,182 What has been shown, however, is that treating OSAHS decreases blood pressure,183 which is a known risk factor for stroke. It is therefore reasonable to believe that treating OSAHS could also reduce the risk of stroke. Wessendorf et al.45,184 showed that administering adequate antihypertensive treatment and CPAP to stroke patients with OSAHS was associated with a decrease in nocturnal blood pressure. They also indicated that treatment with CPAP decreased plasma fibrinogen in stroke patients.176 Other authors observe that patients whose OSAHS is treated with CPAP experience improved cerebral blood flow169 and associate CPAP with lower rates of recurrence of atrial fibrillation.146
Among stroke patients presenting associated OSAHS, CPAP treatment has been shown to decrease mortality over both the short-term59 and the long-term.185 It should be noted that compliance with CPAP treatment for SDBs in stroke patients is observed in 50%–70% of cases during the acute phase of stroke. Only half of these patients, however, comply with treatment on a long-term basis.45,59,100,186 There are only 2 studies in which CPAP treatment was administered in the first 24hours after the stroke. One reports poorer compliance rates than those described108 and treatment was not tolerated by patients in the other.110
Stroke patients’ compliance with CPAP may be affected by the lack of symptoms after the acute phase, low motivation, poor tolerance of CPAP, and neurological impairments such as dementia, delirium, aphasia, anosognosia, pseudobulbar palsy, and/or paresis.
The relationship between OSAHS and the patient's sleeping posture is now well-established.187,188 The dorsal decubitus position is associated with an increase in upper airway collapsibility189 and with increased apnoea frequency and duration.190 Changing posture, whether by adopting a lateral position191,192 or elevating the head of the bed,193 has been proposed as a second line of treatment for OSAHS in the general population.
A study by Brown et al.125 states that patients hospitalised for stroke during the acute phase have a greater tendency, due to their illness, to remain in a supine position while in hospital. Furthermore, stroke patients with associated OSAHS are greatly affected by the sleeping posture they adopt, and postural therapy may therefore be regarded as a treatment option.125
Patients with stroke and central respiratory disorders benefit from oxygen therapy.104 At present, adaptive servo-ventilation is being implemented as treatment for central respiratory disorders.194,195
Patients with central hypoventilation syndrome will benefit from mechanical ventilation and/or non-invasive mechanical ventilation.47
Treating SDB in patients in the sub-acute phase of stroke has been shown to effectively improve well-being, mood, and sleep without affecting how the patient recovers from the stroke.45,58,196 These findings are extremely relevant considering that key symptoms (difficulty concentrating, fatigue, and depression) have historically been associated with the side effects of stroke. In fact, they may be related to SDB. Other studies even show a link between these symptoms and a poor long-term prognosis for stroke.197
From these studies, we can conclude that CPAP is useful in treating stroke patients in both the acute and subacute phases. Further information is needed regarding how to measure and increase treatment compliance over the long term, determine when to start treatment, and decide which device is the most appropriate. Furthermore, it remains to be seen whether use of a well-adjusted ventilating devices in acute-phase stroke patients with associated SDB would improve oxygenation and cerebral blood flow, as this might decrease the area of the penumbra.
ConclusionStrokes damage the central nervous system and therefore often provoke changes in both brain activity and sleep architecture. They also favour the appearance of new sleep disorders and exacerbate any pre-existing disorders. Multiple factors may be at work in the aetiologies of sleep disorders associated with stroke, and these disorders may have a considerable impact on recovery. With this in mind, identifying underlying sleep disorders is crucial. Providing proper treatment may decrease both morbidity and mortality in these patients.
SDB is very prevalent among stroke patients, and we therefore recommend SDB screenings while the patient is hospitalised.102 It is especially likely to be found in elderly male patients with a history of snoring and observed apnoeic episodes, vascular risk factors, and strokes with night-time onset.10,106
Objectively diagnosing forms of SDB in stroke patients as early as possible is a good idea, but the patient's location and condition during the acute phase of stroke make PSG monitoring difficult to perform. The few studies that have been carried out recommend using simplified methods, but there are currently no protocols for the diagnostic method or recommendations on the utility of early diagnosis. Even if the patient's baseline condition cannot be restored, treatment may minimise neural damage and improve prognosis.
Despite the new treatment options available after a stroke, the most effective treatment is still prevention. Treating SDs, which are often underdiagnosed in stroke patients,11,12 provides a range of new treatment possibilities for stroke. Such possibilities are not without their clinical and technical challenges, however, and few studies cover this topic. In any case, exploring new opportunities will deliver better approaches to treating stroke, more favourable prognoses, and better quality of life for stroke patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferre A, et al. Los ictus y su relación con el sueño y los trastornos del sueño. Neurología. 2013;28:103–18.