Despite the wealth of evidence suggesting a protective role of lifestyle factors on Huntington's disease (HD) onset and progression, their association with mortality has not been extensively studied. The aim of this study was to examine whether lifestyle factors such as caffeine and alcohol consumption, smoking, physical activity level, and Mediterranean diet (MeDi) adherence are associated with mortality among a Spanish cohort of patients with HD with a 9-year surveillance period.
MethodsThis national study was performed using a nested, observational, longitudinal design. We included subjects diagnosed with HD who participated in the European Huntington's disease network and Enrol-HD studies. Date of death and baseline lifestyle factor information, demographics, disease severity assessed by the Unified Huntington's Disease Rating Scale (UHDRS), Problem Behaviours Assessment, total functional capacity (TFC) scores, and comorbidities were collected. Adjusted Cox proportional hazards models were conducted to determine the association of lifestyle factors with mortality.
ResultsWe included 87 patients (52 females) with a mean age of 48.62±14.43 years and CAG repeats of 43.76±5.92. Sixteen deaths were recorded. After correcting for multiple comparisons, deceased patients had higher UHDRS scores at baseline and lower caffeine consumption than live patients. In multivariate Cox regression models, after adjusting for age, CAG repeats, and TFC, mortality risk was associated with lower caffeine consumption (HR 0.13, 95% CI 0.04, 0.45).
ConclusionsThis national-based study suggests that increased caffeine consumption is associated with decreased HD mortality. Our results may help guide clinicians in counselling lifestyle practices for decreasing mortality in HD.
A pesar de la evidencia que existe sobre la importancia de los factores de estilo de vida con el inicio y la progresión de la enfermedad de Huntington (EH), su asociación con la mortalidad no ha sido ampliamente estudiada. El objetivo de este estudio es estudiar la asociación de factores de estilo de vida como el consumo de cafeína y alcohol, el tabaquismo, el nivel de actividad física y la adherencia a la dieta mediterránea, con la mortalidad en una cohorte española de pacientes con EH con un periodo de seguimiento nueve años.
MétodosEste estudio nacional, observacional, longitudinal se realizó mediante un diseño anidado. Se incluyeron sujetos diagnosticados con EH, participantes en la red Enroll europea de la enfermedad de Huntington. Se recogieron la fecha de fallecimiento y la información basal sobre los factores de estilo de vida, datos sociodemográficos, la gravedad de la EH con la Escala Unificada de la EH (UHDRS), evaluación de problemas conductuales (PBA), Capacidad Funcional Total (TFC) y las comorbilidades. Se realizaron análisis comparativos y de supervivencia con modelos de regresión de Cox ajustados.
ResultadosSe incluyeron 87 pacientes con EH (52 mujeres) con una edad media de 48,62±14,43 años, y un número medio de repeticiones CAG de 43,76±5,92. Se registraron 16 fallecimientos. En análisis comparativos ajustados por comparaciones múltiples, los pacientes fallecidos tenían puntuaciones basales UHDRS mayores, y un consumo menor de cafeína comparado con los pacientes vivos. En los modelos multivariantes de regresión de Cox, tras ajustar por edad, repeticiones CAG y TFC, el riesgo de mortalidad se asoció con un menor consumo de cafeína (HR 0,13; IC 95%: 0,04; 0,45).
ConclusionesEste estudio nacional sugiere que un mayor consumo de cafeína se asocia con una menor mortalidad en la EH. Nuestros resultados pueden ayudar a orientar a los profesionales sanitarios en el asesoramiento de una mejora de prácticas de estilo de vida para la EH asociadas con menor mortalidad.
Two types of risk factors contribute to the development and progression of neurodegenerative disorders: nonmodifiable risk factors, including genetic susceptibility, and modifiable risk factors, including a wide variety of health and lifestyle-related factors.1,2 Overall, various studies have shown that chronic diseases, including neurodegenerative diseases, may be prevented or delayed by adopting a healthier diet and lifestyle.3 Specifically, in Huntington's disease (HD), lifestyle factors have been reported to influence the onset and rate of neurological and clinical deterioration.4 In this regard, significant associations have been reported between physical inactivity, smoking, higher caffeine and alcohol consumption, and reduced dairy consumption with earlier onset of motor signs in HD.5
Malnutrition and low body mass index (BMI) have also been correlated with the higher development of dementia and mortality in neurodegenerative diseases.6 Furthermore, caffeine, moderate alcohol consumption, and physical activity are protective against mortality and progression in neurodegenerative diseases such as Parkinson's disease (PD).2 For HD, two independent longitudinal investigations have shown that greater cognitive reserve, education, and BMI are associated with a reduced rate of grey matter volume loss in striatal brain structures and slower deterioration of clinical decline in individuals with premanifest HD.7
However, despite the wealth of evidence suggesting an association between lifestyle factors and HD onset and the rate of clinical deterioration, their association with mortality has not been extensively studied. This study aimed to determine whether Mediterranean diet (MeDi) adherence, caffeine, moderate alcohol consumption, smoking, and physical activity are associated with mortality rates among a Spanish sample of patients with HD monitored over nine years.
Materials and methodsDesign, sample characteristics, and proceduresThis study was performed using a nested, observational, longitudinal, national design covering nine years (January 1st, 2012–December 31st, 2020). This study was approved by the Ethics Committee of Complejo Universitario de Burgos Hospital. Written informed consent was obtained from all participants. The original study aimed to determine the factors associated with Mediterranean diet (MeDi) adherence in a national cohort of subjects diagnosed with HD. We enrolled HD subjects from the European Huntington's Disease Network (EHDN) Registry study and Enrol-HD, a prospective, multicentre, observational Registry Study in a Global Huntington's Disease Cohort.8–11 More details of the characteristics of the HD sample and procedures have been described elsewhere.9 Briefly, the original cohort included information from 98 HD gene carrier participants with ≥36 CAG repeats in the HTT gene from 12 centres in Spain recruited between 2012 and 2013.
In this study, we included HD subjects from the original cohort followed yearly with complete HD clinical characteristics and lifestyle data, including dietary information, physical activity, caffeine, alcohol, and tobacco use (Supplemental Figure 1),9 following Strobe guidelines. Mortality information was obtained using the Enrol-HD registry.
Clinical and sociodemographic data of Huntington's diseaseClinical and sociodemographic information was obtained from the original study at one visit,9 including age at the onset of HD, severity of motor, cognitive, and behavioural symptoms, sex, ethnicity, CAG repeat length, comorbidity, severity of dysphagia, level of physical activity, and other nutritional and lifestyle factors.
All HD participants were evaluated by certified movement disorder neurologists at baseline using a standardized HD assessment tool, the Unified Huntington Disease Rating Scale (UHDRS) battery,12 including the motor subscale (UHDRSm) with high scores denoting greater impairment and the cognitive (UHDRS-Cog) with high scores denoting better performance. Participants defined as premanifest HD mutation carriers were those rated with a UHDRS motor score <5.13 Disease severity was assessed using the total functional capacity (TFC),12 with higher scores indicating more intact functioning. The severity of psychiatric symptoms was assessed using the Problem Behaviours Assessment (PBA), with higher scores indicating greater severity.14 Caregiver burden was obtained using the Caregiver Burden Inventory (CBI),15 with higher scores indicating higher caregiver burden and quality of life using the Short Form-36 Health Survey (SF-36), with higher scores indicating higher quality of life.16 Comorbidity information was collected using the Cumulative Illness Rating Scale-Geriatric (CIRS-G), with higher scores indicating higher severity.17
Baseline predictors of survival. Lifestyle factorsA 3-day dietary record assessed dietary intake and adherence to the traditional MeDi by a trained nutritionist at baseline.18 In all cases, oral nutritional supplements or vitamin and mineral supplements were considered. Food groups, macro- and micronutrients, current caffeine consumption (caffeinated coffee, caffeinated tea, and other caffeinated beverages), and calorie intake information were analyzed using the software Alimentación y Salud, version 2.0. We computed the MeDi adherence according to the Trichopoulou study.19 Briefly, sex-specific medians of food group intake were calculated. For beneficial components, such as cereals, vegetables, fruits, fish, legumes, and the ratio of monounsaturated/saturated fatty acids (MUFA/SFA), 1 point was attributed if consumption was at or above the sex-specific median value. For components presumed to be detrimental, such as meats and dairy products, 1 point was given if consumption was below the sex-specific median value. The MeDi adherence was generated for each participant by adding the food categories’ scores (ranging from 0–9); values 0–3 were considered low adherence, and values 4–9 were considered moderate/high adherence.
Tobacco status was based on whether the patient was currently smoking (yes vs. no). For alcohol intake, we stratified the sample based on mild–moderate alcohol consumption (>0 to <30g/day) and non-mild–moderate alcohol consumption (either 0g/day or ≥30g/day). We classified alcohol consumption dichotomously because of the skewed distribution of alcohol in our population (48.2% reporting no alcohol intake, 43.7% reporting less than 30g/day [mild-to-moderate intake], and 8.1% reporting ≥30g/day [heavy intake]).20,21 We used the International WHO standards for BMI classification: BMI ≥18.5 to <25.0kg/m2 (normal), BMI <18.5kg/m2 (underweight), BMI ≥25.0 to <30.0kg/m2 overweight, and BMI ≥30.0 obesity.22 The level of physical activity was assessed using the GPAQ developed by the World Health Organization (WHO),23 which comprises 19 questions, and physical activity is computed in terms of high, moderate, and low levels of physical activity, primarily validated in European populations.
CovariatesA list of potential baseline prognostic factors for mortality was generated from the literature. The prespecified factors were age; CAG repeats; gender; years of education; UHDRSm, UHDRS-Cog, and PBA scores; current tobacco use (yes vs. no); overall comorbidity burden, presence of cardiovascular risk factors (indirectly established by the intake of antihypertensive and lipid-lowering drugs, antidiabetic, anticoagulants, and antiplatelet drugs); and the use of pharmacological and nonpharmacological treatments (yes vs. no), including physical, occupational and speech therapies, and psychological intervention.
Mortality outcomesVital status, year, and cause of death information were obtained from the Enrol death report form. Trained raters completed these forms, and data were often gathered from patients’ families. Although it is optional to include autopsy information in the Enrol death report form, this information was not available for our cases.
Statistical analysisQuantitative data are expressed as the mean (standard deviation) or median [interquartile range (IQR)], based on the normal distribution of the data (Kolmogorov–Smirnov test), and qualitative data are expressed as frequencies (percentages). Comparisons were performed using U-Mann–Whitney or T tests based on the normal distribution for numerical variables and the chi-square test for categorical variables. The statistical analysis was carried out with IBM-SPSS version 28. All tests were two-sided with a significance level of α=0.05. A Bonferroni correction was applied for multiple comparison tests.
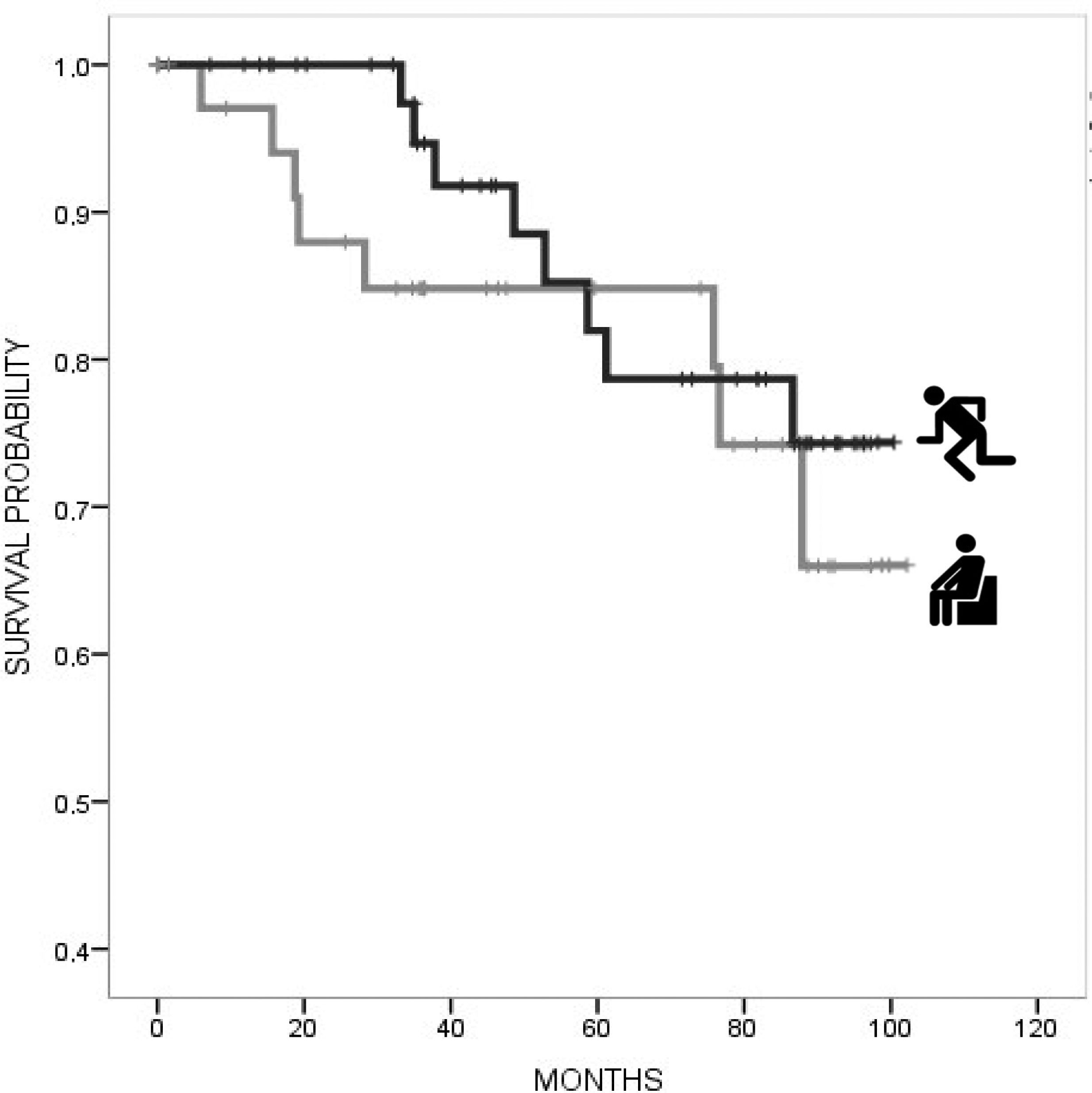
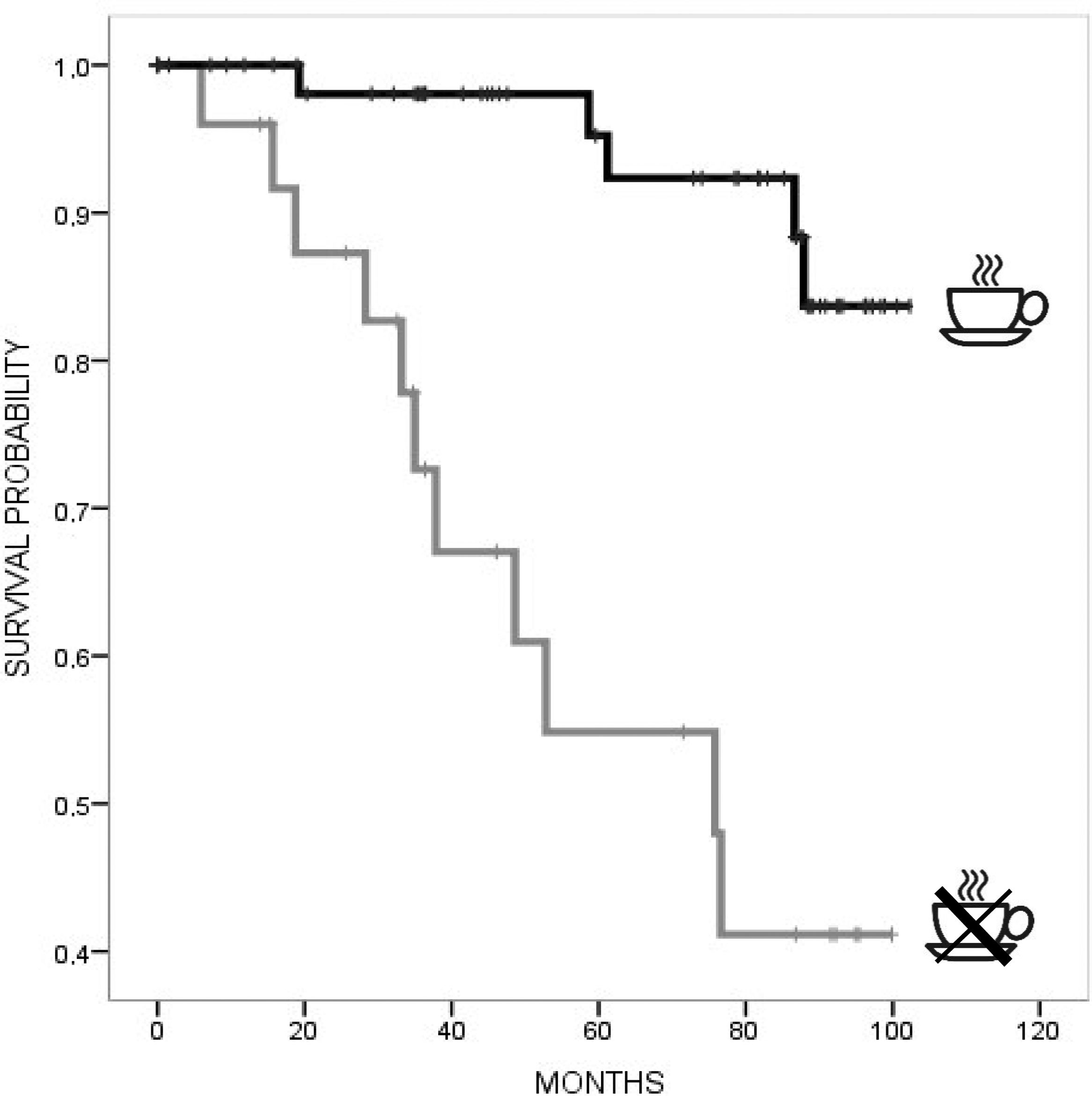
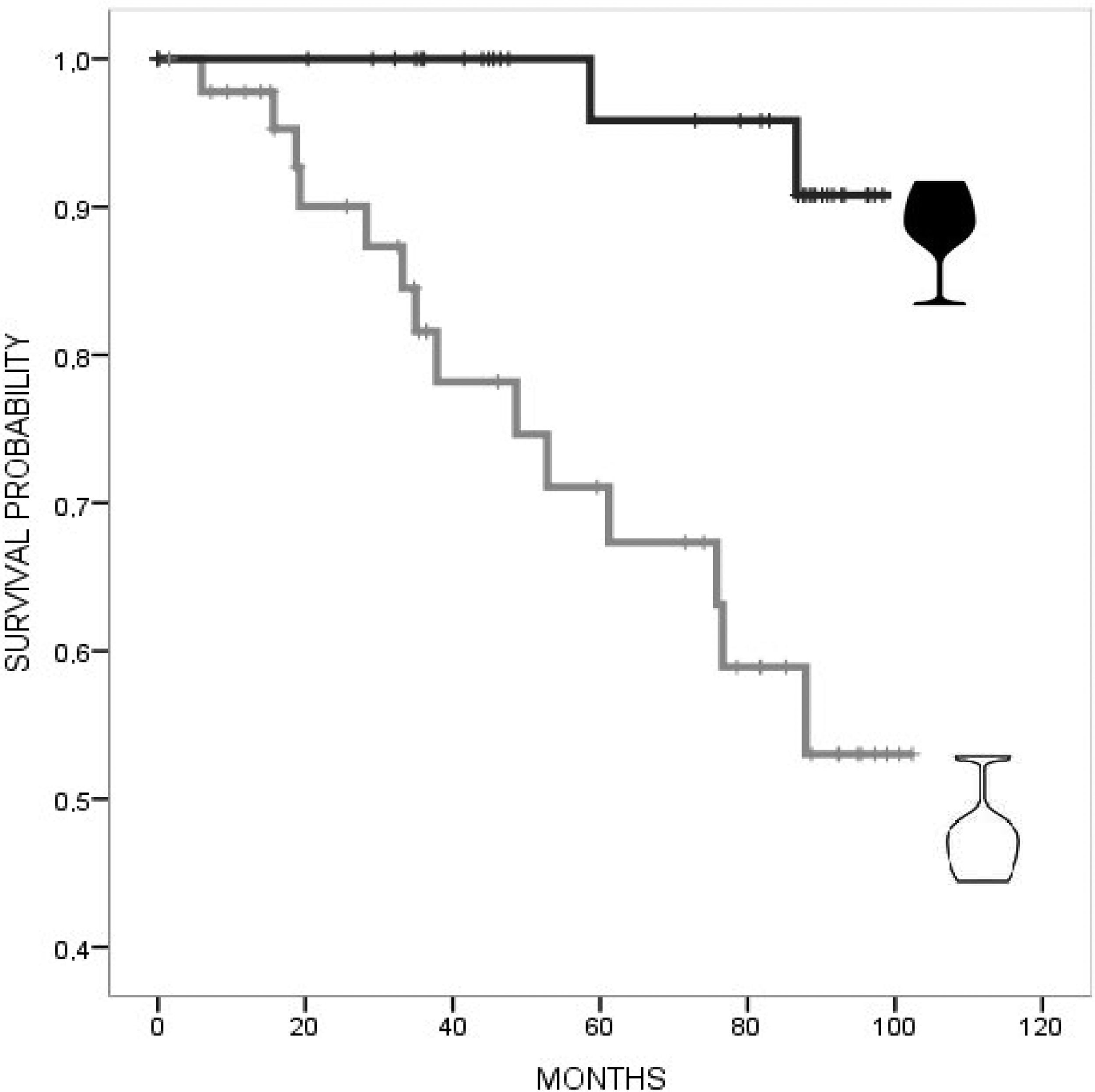
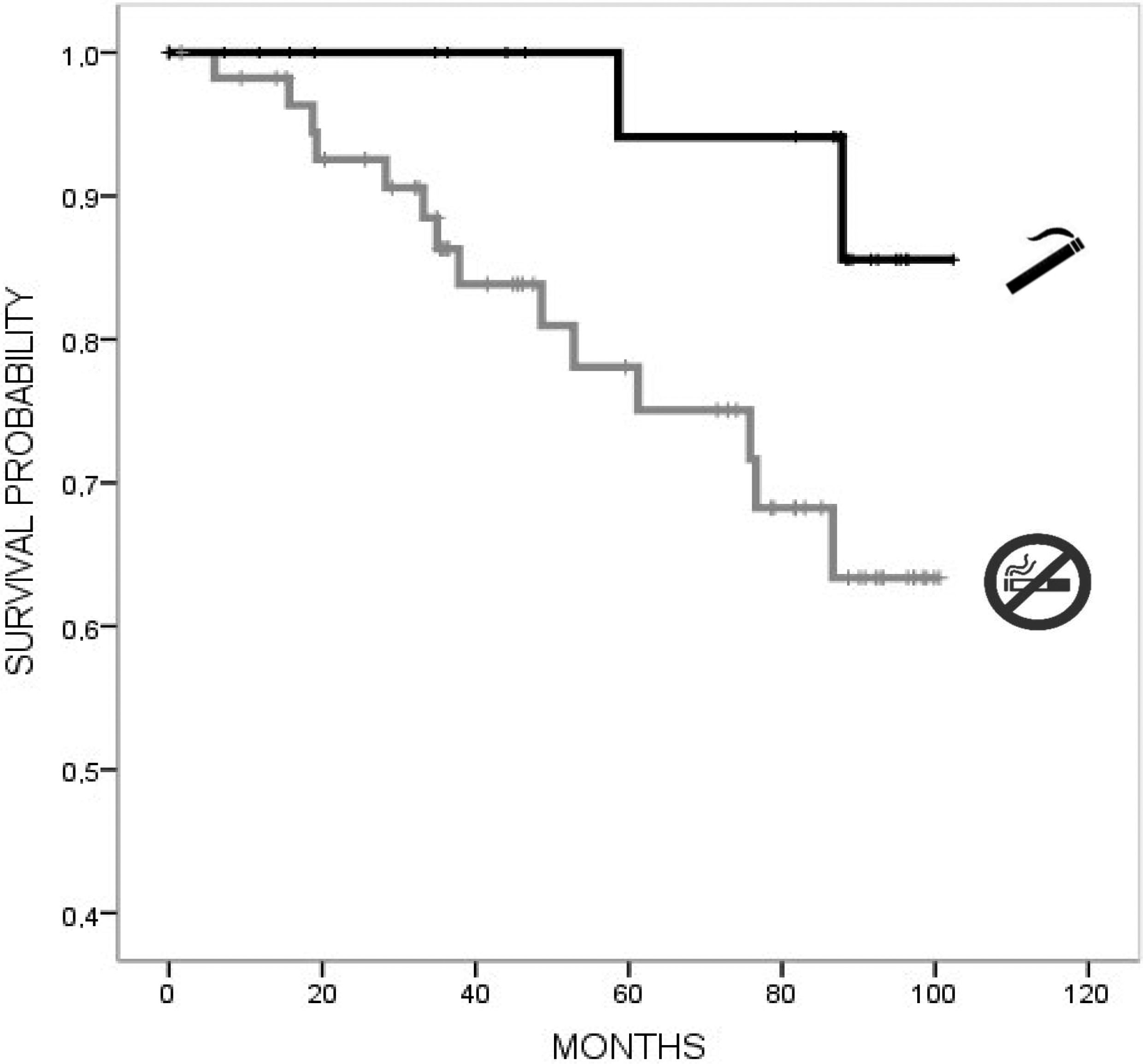
For individuals who were not identified as deceased, follow-up was censored when they were last known to be alive. The Kaplan–Meier method using death as the endpoint was used to construct survival plots for each lifestyle factor and estimate the survival time rates. We analyzed the relationship between each lifestyle factor (MeDi adherence, caffeine and alcohol consumption, physical activity, and smoking history) and time from study enrolment until death adjusted for age, years of education, CAG repeat length, and TFC using Cox proportional hazards regression models providing hazard ratios (HRs) (all sets from StataCorp LLC. v15.1). The time variable was the years from the date of the baseline evaluation (2012–2013) to either or to the date of death in participants who had died before December 31st, 2020. The goodness of fit measures of the Cox regression models were analyzed using Harrell's C-index.24
ResultsEighty-seven out of 98 HD patients with lifestyle data at baseline were included (88.77%), 52 females (59.80%), 35 males (40.20%), with a mean age of 48.62±14.43 years old, CAG repeats of 43.76±5.92, and mean follow-up of 4.73±2.77 years. Forty-five patients (51.7%) were lost to follow-up before December 31st, 2020. Sixteen deaths were recorded, ten females (62.5%) and 6 males (37.5%), with a mean age at baseline of 52.69±17.11 years and mean CAG repeats of 46.44±6.06. Only in 6 deaths, the cause was known (37.5%), including pneumonia in 5 subjects (31.2%) and cerebrovascular disease in 1 subject (0.06%).
In terms of lifestyle factors at baseline, 27 patients (31%) were smokers, 38 (43.70%) had mild–moderate alcohol consumption, 61 (70.10%) had caffeine consumption with a median caffeine intake of 7.68mg (0.00;62.84), 48 (55.20%) adhered to the MeDi, and 47 (54%) had moderate/high physical activity (Table 1). Education was mildly correlated with physical activity level (rs=0.29) and inversely correlated with PBA scores (rs=−0.33). After correcting for multiple comparisons (p=0.002), deceased HD patients had higher UHDRSm scores at baseline, lower caffeine consumption, and a trend for being older with lower UHDRS-Cog, TFC, and PBA scores, nonmoderate alcohol consumption, and low MeDi adherence compared to living patients (Table 1).
Risk factors associated with mortality.
| Baseline characteristics | MortalityN=16 | Non-mortalityN=71 | p value | Unadjusted hazard ratio, 95% CIp value |
|---|---|---|---|---|
| Age, years | 52.69±17.11(16) | 47.70±13.73(71) | 0.21 | 1.02 (0.98, 1.05)0.19 |
| Gender, male (%) | 6 (37.50)(16) | 29 (40.80)(71) | 0.53 | 0.77 (0.28, 2.13)0.62 |
| CAG repeat length | 46.44±6.06(16) | 43.15±5.76(69) | 0.04 | 1.09 (1.002;1.19)0.04 |
| Education, years | 13.13±5.23(16) | 11.54±3.47(70) | 0.27 | 1.03 (0.92;1.15)0.53 |
| Married (%) | 6 (37.5)(16) | 42 (59.2)(71) | 0.64 | 0.84 (0.59;1.18)0.32 |
| Follow-up, years | 3.58 (1.79, 6.01)(16) | 6.07 (2.71, 7.57)(71) | <0.0001 | _ |
| Baseline BMI | 21.97+3.56(16) | 25.07±4.87(71) | 0.01 | 0.86 (0.74;0.99)0.03 |
| Kilocalories intake | 2180.51±597.69(16) | 2208.94±605.53(71) | 0.86 | 1.0 (0.99, 1.001)0.69 |
| Smoking, yes (%) | 14 (87.5)(16) | 13 (18.2)(71) | 0.02 | 0.19 (0.04, 0.83)0.02 |
| Alcohol (>0 to <30g/d), yes (%) | 2 (12.5)(16) | 36 (50.7)(71) | 0.005 | 0.12 (0.02, 0.53)0.005 |
| Caffeine consumption, yes (%) | 5 (31.30)(16) | 56 (78.90)(71) | <0.0001 | 0.14 (0.05, 0.41)<0.0001 |
| MeDi score | 3.00±0.88(16) | 4.00±1.65(71) | 0.06 | 0.77 (0.54;1.09)0.14 |
| MeDi adherence, yes (%) | 6 (37.5)(16) | 42 (59.2)(71) | 0.34 | 0.45 (0.16;1.25)0.13 |
| High–moderate PA, yes (%) | 8 (50.0)(16) | 39 (54.9)(71) | 0.78 | 0.68 (0.25, 1.82)0.44, |
| CIRS-G score | 1.00 (0.00;2.00)(16) | 2.00 (1.00;2.00)(71) | 0.54 | 0.94 (0.72;1.25)0.71 |
| HD manifest, yes (%) | 13 (92.8)(14) | 47 (73.4)(64) | 0.16 | 2.90 (0.38, 22.23)0.30 |
| UHDRSm | 59.50 (39.50, 75.25)(14) | 27.50 (0.00, 48.50)(64) | 0.002 | 1.05 (1.02;1.07)0.001 |
| UHDRS-Cog | 104.00 (29.50, 149.50)(9) | 186.50 (112.25, 369.00)(48) | 0.01 | 0.98 (0.96;0.99)0.01 |
| TFC | 5.00 (2.00;7.00)(15) | 10.50 (5.25;13.00)(64) | 0.005 | 0.76 (0.66;0.88)<0.0001 |
| PBA | 6.00 (4.00;10.50)(8) | 12.90 (4.50;27.00)(32) | 0.006 | 0.92 (0.83;1.02)0.14 |
| SF-36 | 106.40±28.91(3) | 128.30±22.39(22) | 0.35 | 0.97 (0.93, 1.01)0.24 |
| Nutritional supplements, yes (%) | 4 (28.5)(14) | 6 (24.0)(25) | 1.00 | 0.78 (0.22, 2.75)0.70 |
| Antidopaminergics, yes (%) | 2 (20.0)(10) | 13 (52.0)(25) | 0.32 | 2.14 (0.47, 9.59)0.31 |
| Antidepressants, yes (%) | 8 (50.0)(16) | 13 (61.9)(21) | 0.39 | 0.54 (0.18;1.58)0.26 |
| Nonpharmacological therapies (%) | 1 (0.06)(16) | 1 (15.3)(26) | 0.63 | 0.43 (0.05;3.32)0.42 |
Abbreviations: HD: Huntington's disease; MeDi: Mediterranean diet; BMI: body mass index; CIRS-G: Cumulative Illness Rating Scale-Geriatric; UHDRS: Unified Huntington's Disease Rating Scale (m=motor subscale, Cog=cognitive subscale); TFC: total functional capacity; PBA: Problem Behaviour Assessment; SF-36: Short Form-36 Health Survey; PA: physical activity.
Overall, HD patients with high–moderate MeDi adherence were more likely to have better QoL. Subjects with mild–moderate alcohol consumption were more likely to have greater education, caffeine intake, physical activity, and UHDRS-Cog scores and lower UHDRSm scores. Subjects with high–moderate physical activity were more likely to have greater education, mild–moderate alcohol consumption, lower comorbidity and UHDRSm, and higher TFC scores. Subjects with current caffeine intake were more likely to have mild–moderate alcohol consumption, higher TFC, and lower UHDRSm scores. Subjects with current smoking status were more likely to be younger with lower UHDRSm and greater TFC scores (Supplemental Tables 1–5).
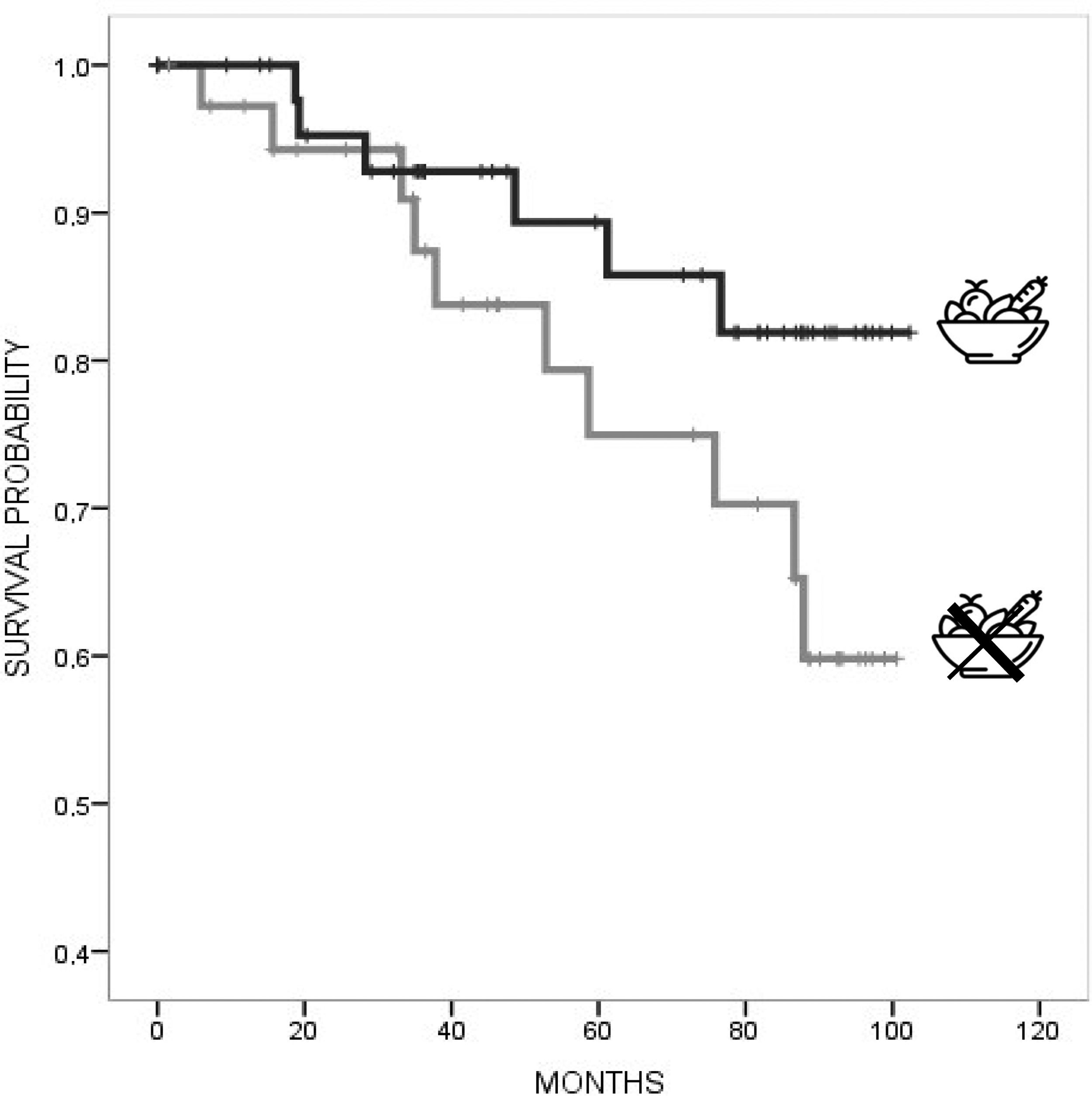
In univariate Cox regression analysis, a lower risk for mortality was associated with higher BMI (HR 0.86, 95% CI 0.74, 0.99), mild–moderate alcohol consumption (HR 0.12, 95% CI 0.002, 0.53), caffeine consumption (HR 0.14, 95% CI 0.05, 0.41), and lower TFC (HR 0.76, 95% CI 0.66, 0.88), with a trend for high–moderate MeDi adherence (HR 0.77, 95% CI 0.54, 1.09). Instead, a higher risk for mortality was associated with a higher CAG repeat length (HR 1.09, 95% CI 1.002, 1.09). In multivariate Cox regression models adjusted for age, TFC, education, and CAG repeats, a lower risk for mortality was associated with caffeine consumption (HR 0.13, 95% CI 0.04–0.45, p=0.001) (Table 2). Kaplan–Meier curves (Figs. 1 and 2) and log-rank testing revealed poorer survival among the patients with no current caffeine (p<0.001) or alcohol consumption (p=0.001). Survival curves were not modified with MeDi adherence, higher-moderate physical activity or smoking (Figs. 3–5).
Lifestyle factors associated with mortality.
| N=73 | Hazard ratio, 95% confidence intervals | p value |
|---|---|---|
| Age | 1.06 (1.02;1.10) | 0.004 |
| CAG repeats | 1.16 (1.04;1.31) | 0.01 |
| TFC | 0.93 (0.80;1.07) | 0.31 |
| Education, years | 1.10 (0.96;1.26) | 0.14 |
| Alcohol, yes | 0.29 (0.04;2.13) | 0.22 |
| Smoking, yes | 0.50 (0.09;2.75) | 0.42 |
| Mediterranean diet adherence* | 0.86 (0.22;3.43) | 0.84 |
| Physical activity** | 1.13 (0.20;6.26) | 0.88 |
| Caffeine consumption, yes | 0.13 (0.04;0.45) | 0.001 |
The following variables were included in this model.
In Europe, the average survival of individuals with HD is 35 years after the onset of symptoms, at a mean age of 58 years, most frequently from pneumonia and other infections.25 To our knowledge, this is the first study investigating modifiable lifestyles and the effects of nongenetic factors on HD mortality. Our national study found that increased caffeine consumption might protect against mortality, in agreement with previous findings in different populations.26 Caffeine (1,3,7-trimethylzanthine) is the most widely used psychostimulant in Western countries, and it is found in coffee, tea, energy drinks, several soft drinks, and cocoa.27 Although controversial, the most recent evidence indicates that caffeine exerts dose-dependent effects, and the implicated pathways of caffeine include the antagonism of adenosine receptors (A1R, A2AR, A2BR, and A3R), inhibition of phosphodiesterase, and activation of ryanodine receptors.28 Supporting this evidence, few nonrandomized trials have observed the benefit of caffeine in ADCY-5-related dyskinesias.29 In addition, caffeine protects against low-density lipoprotein oxidation and reduces oxidative DNA damage and glutamate-induced toxicity.28
For dietary patterns, the MeDi consists of high consumption of plant-based foods (fruits, vegetables, nuts, and legumes), fish, and extra virgin olive oil, which is the main source of MUFAs, and low to moderate intake of ethanol (wine) and low intake of red meat, poultry, and dairy products.9 Previous analytical and experimental studies have shown a relationship between MeDi and reductions in neurodegenerative disease risk and overall mortality, including cardiovascular and cancer mortality.30 However, we did not observe any association between MeDi adherence and mortality, in contrast to other neurodegenerative diseases, such as Alzheimer's disease, PD and other populations.31 Interestingly, HD patients with moderate–high MeDi adherence had a better quality of life and lower psychiatric burden, in agreement with other diseases.9,32 Although still controversial, other authors have suggested that avoiding pro-inflammatory foods in favour of an anti-inflammatory diet might contribute to preventing the incidence of depression and depressive symptoms.33
The association between the level of physical activity and HD mortality has not been widely studied. Previous population-based studies have indicated that middle-aged and older adults, including those with cardiovascular disease and cancer, can gain substantial longevity benefits by becoming more physically active, irrespective of past physical levels and established risk factors.34 Although we did not find any association between physical activity information and HD mortality, in other HD studies, physical activity seems to improve motor function, gait speed, balance, and a range of physical and social benefits in patient-reported outcomes.35 However, variability in the mode of intervention and outcome measures limits the interpretability of these results, and further research using wearable data about the frequency and intensity of physical activity is needed.35
Previous literature has shown that after controlling for CAG repeat length, an earlier onset of HD has been associated with lifetime smoking and caffeinated soda and not with alcohol consumption.36,37 The exact mechanisms by which smoking can modify the age at onset in HD remain largely unknown, but hypothetical mechanisms implicate increases in extracellular dopamine concentrations in the caudate and accumbens.37 Interestingly, we found a trend for lower mortality in smokers compared to nonsmokers in contrast to PD, where smokeless was inversely associated with mortality.38
For alcohol, several meta-analyses have found that low-volume alcohol consumption has no net mortality benefit compared with lifetime abstention or occasional drinking in the general population.39 In both cases, since alcohol consumption and smoking are likely associated with a better functional state, interpreting these data in HD is challenging. Finally, although we observed an association between education and moderate–high physical activity, lower severity of psychiatric symptoms, and alcohol consumption, education did not significantly impact HD mortality. In contrast, in other diseased populations, high levels of education and socioeconomic status have been associated with decreased mortality.40
This study has several limitations that must be considered when interpreting our findings, including the lack of information regarding the cause of death in 62.5% of the sample and the interpretability of the caffeine benefit due to the possible variability in caffeine doses and intervals in this population. We recognize that the main limitation is the lack of follow-up of patients with advanced HD, who are therefore at risk for mortality. Some of these issues would be solved, at least in part, by the introduction of external electronic death certificates in HD databases. Lifestyle factor information was collected once, with limited information on smoking (pack-year exposure) and alcohol consumption. We cannot rule out lifestyle behaviour modifications with the progression of HD or underreporting/misreporting related to cognitive dysfunction or recall bias in HD participants/caregivers. In addition, the noted associations between the studied lifestyle factors and mortality are based on a relatively small sample size with a small number of death events and a relatively short follow-up. More importantly, our observations could result from selection bias due to the inclusion of an ambulatory, hospital-based HD sample with access to specialized HD centres or other beneficial aspects, such as a favourable socioeconomic status.
This study has shown similar dropout rates for longitudinal studies, including other neurodegenerative diseases.41 Although various modifiable factors and strategies to minimize dropouts have been reported, they likely remain an important issue for observational studies and registries. However, despite these notable limitations, confidence in our findings is strengthened by the following factors. First, we investigated the association of multiple lifestyle factors with mortality in HD for the first time using previously widely validated instruments in epidemiologic studies. Second, measures for multiple potential mortality risk factors were carefully recorded and adjusted in multivariate regression analyses. Third, we included high-quality data obtained by HD-certified neurologists with frequent monitoring carried out locally at each site and centrally by trained monitors.10
Future directions: Traditionally, people have been advised to reduce their coffee, tea, and caffeine intake because it may be harmful to physical health. This national-based study suggests that increased caffeine consumption is associated with decreased HD mortality. Our results may help guide clinicians in counselling lifestyle practices that can improve mortality in genetic diseases such as HD. However, further studies, including those with larger HD sample populations with more extended surveillance periods, are needed to confirm our results.
Ethical approvalInformed patient consent was obtained for this study. This study was approved by the Institutional Review Board at Hospital Universitario Burgos, approval number CEIC 1198.
FundingThis work was partially supported by the European Huntington Disease Network (EHDN, Seed Fund 338) and by Nutricia and Advanced Medical Nutrition Fresenius-Kabi Pharmaceuticals. Data were analyzed independently by the funding sources.
Conflict of interestEC, GG, LA have consulting fees for UCB, Allergan, and Abbvie, the rest of authors report no disclosures.
We are grateful to all participants, Concha Fidalgo Rivero, for their time and efforts and Enrol-HD registry and European HD network.
Dolores Martínez, María Antonia Ramos (Hospital Virgen del Camino, Pamplona); Bárbara Vives Pastor (Hospital Universitario Son Espases, Mallorca); Raquel Pérez, Alex Sarradei, Javier Ruperti, Eris Zuñiga (Centro de Rehabilitación El Hayedo, Madrid); Juan Antonio Burguera, Francisco Casterá (Hospital La Fé, Valencia); Misericordia Floriach Robert, Jesús M. Ruiz (Hospital Mare de Déu de la Mercé, Barcelona); Jesús Pérez, Saúl Martínez-Horta (Hospital de la Santa Creu I San Pau, Barcelona), Esteban Muñoz (Hospital Clinic, Barcelona), Carmen Durán, Patrocinio García (Hospital Infanta Cristina, Badajoz), José Manuel García, Carolina Méndez (Hospital Virgen Macarena, Sevilla); María Fuensanta Noguera (Hospital Virgen de la Arrixaca, Murcia), María Angeles Acera, Juan Carlos Gómez Esteban, Koldo Berganzo González (Hospital Cruces, Bilbao).