Migraine attacks have a high impact on daily activities. There is limited research on the burden of migraine on sexual functioning.
ObjectiveTo determine the prevalence of sexual dysfunction in patients with migraine and its relationship with migraine features and comorbidities.
MethodThis is a cross-sectional study. We included migraine patients between 18 and 60 years-old from 8 Headache Clinics in Spain. We recorded demographic data and migraine features. Patients fulfilled a survey including comorbidities, Arizona Sexual Experiences Scale, Hospital Anxiety and Depression Scale and a questionnaire about migraine impact on sexual activity. A K-nearest neighbor supervised learning algorithm was used to identify differences between migraine patients with and without sexual dysfunction.
ResultsWe included 306 patients (85.6% women, mean age 42.3±11.1 years). A 41.8% of participants had sexual dysfunction. Sexual dysfunction was associated with being female (OR [95% CI]: 2.42 [1.17–5.00]; p<0.001), being older than 46.5 years (4.04 [2.48–6.59]; p<0.001), having chronic migraine (2.31 [1.41–3.77]; p=0.001), using preventive medication (2.45 [1.35–4.45]; p=0.004), analgesic overusing (3.51 [2.03–6.07]; p<0.001), menopause (4.18 [2.43–7.17]; p<0.001) and anxiety (2.90 [1.80–4.67]; p<0.001) and depression (6.14 [3.18–11.83]; p<0.001). However, only female gender, age, menopause and depression were the statistically significant variables selected in the model to classify migraine patients with or without sexual dysfunction (Accuracy [95% CI]: 0.75 (0.62–0.85), Kappa: 0.48, p=0.005).
ConclusionsSexual dysfunction is frequent in migraine patients visited in a headache clinic. However, migraine characteristics or use of preventive medication are not directly associated with sexual dysfunction. Instead, risk factors for sexual dysfunction were female gender, higher age, menopause and depression.
La migraña tiene un alto impacto en las actividades diarias, pero los datos sobre el impacto de la migraña en el funcionamiento sexual son limitados.
ObjetivoDeterminar la prevalencia de disfunción sexual en pacientes con migraña y su relación con las características y comorbilidades de la migraña.
MétodosEste es un estudio transversal. Se incluyeron pacientes con migraña de entre 18 y 60 años de ocho consultas de cefalea en España. Registramos datos demográficos y características de migraña. Los pacientes completaron una encuesta que incluía comorbilidades, la Escala de Experiencias Sexuales de Arizona, la Escala de Ansiedad y Depresión Hospitalaria y un cuestionario sobre el impacto de la migraña en la actividad sexual. Se usó un algoritmo de aprendizaje supervisado (k-nearest neighbors) para identificar diferencias entre pacientes con migraña, con y sin disfunción sexual.
ResultadosSe incluyeron 306 pacientes (85,6% mujeres, edad media 42,3 ± 11,1 años). El 41,8% de los participantes tenía disfunción sexual. La disfunción sexual se asoció con ser mujer (OR [95%]: 2,42 [1,17-5,00]; p < 0,001), tener más de 46,5 años (4,04 [2,48-6,59]; p < 0,001), tener migraña crónica (2,31 [1,41-3,77]; p = 0,001), uso de medicación preventiva (2,45 [1,35-4.45]; p = 0,004), uso excesivo de analgésicos (3,51 [2,03-6,07]; p < 0,001), menopausia (4,18 [2,43-7,17]; p < 0,001), ansiedad (2,90 [1,80-4,67]; p < 0,001) y depresión (6,14 [3,18-11,83]; p < 0,001). Sin embargo, solo el sexo femenino, la edad, la menopausia y la depresión fueron las variables estadísticamente significativas seleccionadas en el modelo para clasificar a los pacientes con migraña, con o sin disfunción sexual (precisión [IC 95%]: 0,75 (0,62-0,85), kappa: 0,48, p = 0,005).
ConclusionesLa disfunción sexual es frecuente en pacientes con migraña que son visitados en una consulta de cefalea. Sin embargo, las características de la migraña o el uso de medicamentos preventivos no están directamente asociados con la disfunción sexual. En cambio, el sexo femenino, mayor edad, menopausia y depresión son los factores de riesgo para la disfunción sexual en este grupo de pacientes.
Migraine is a prevalent neurological disorder defined by the presence of recurrent episodic headache attacks and neurological associated symptoms. Migraine is one of the ten leading causes of global disability especially in young adults.1 Prevalence and headache-related disability peaks between 30 and 50 years-old2 so, migraine clearly has an impact during the most demanding years of life on work, social and family activities.3,4 Among personal dynamics, sexual life is an important aspect that contributes to personal and relational quality of life.5
Sexual dysfunction refers to difficulties that occur during the sexual cycle that prevent the individual from experiencing satisfaction from sexual activity. Risk factors for sexual dysfunction in women and men include biological, psychological, and sociocultural factors.6 Sexual dysfunction is a common symptom present in many neurologic chronic disorders7–9 as well as in pain syndromes10,11 with multiple factors contributing directly or indirectly to its pathophysiology (i.e. primary nervous system injury, other symptoms as fatigue, pain, mood-related disorders or medication side effects).12
Patients with primary headache disorders such as migraine, could be at risk for sexual dysfunction as it is a chronic neurological and pain disease with relevant comorbidity with mood disorders and pharmacological treatments used have potential side effects on sexual function. Our hypothesis is that patients with more severe migraine phenotypes, meant by higher headache frequency, disability and comorbidities, have a higher prevalence of sexual dysfunction and an impact on sexual performance.
The aim of the MIGREX study was to determine the prevalence of sexual dysfunction in patients with migraine and its relationship with migraine features, treatment, comorbidities and perception of sexual impact.
Material and methodsThis is a multicenter cross-sectional study performed in 8 sites in Spain including 4 Specialized Headache Clinics and 4 General Neurology Outpatient Clinics. We included male and female patients between 18 and 60 years old, fulfilling criteria for episodic migraine or chronic migraine according to the 3rd edition of the International Classification of Headache Disorders13 during a first or follow-up visit. We excluded patients with other headaches and patients not willing to participate.
Headache-specialized neurologists recorded demographics and data on migraine diagnosis (episodic or chronic, presence of aura and medication overuse); headache frequency during the last 3 months which was classified into 5 categories (<5, 5–9, 10–14, 15–19, <20 headache days/month) and current acute and preventative treatment. After that, patients fulfilled an anonymous survey with information regarding current reproductive health situation (childbearing, perimenopausal or menopausal), use of hormonal contraception (yes/no), marital status (married, single, divorced, or widowed), sexual partner status (steady partner for more or less than 1 year, multiple partners or none) and medical and psychiatric comorbidities, including Hospital Anxiety and Depression Scale (HADs).14 HADs is a 14-item self-report screening scale that was originally developed to indicate the possible presence of anxiety and depression states in the setting of a medical non-psychiatric outpatient clinic with validated Spanish version.15 All participants completed 6 questions about subjective impact of migraine on their sexual performance using 5-point Liker scale and 3 more questions about possible treatment side effects. Sexual dysfunction was screened through Arizona Sexual Experiences Scale (ASEX). The ASEX is a brief, a relatively nonintrusive, five-item rating scale designed to assess the core elements of sexual function: sex drive, arousal, penile erection/vaginal lubrication, ability to reach orgasm and orgasmic satisfaction in both men and women.16 ASEX scale total scores range from 5 to 30, with the higher scores indicating more sexual dysfunction. Sexual dysfunction was defined as total ASEX score of >19, any item with a score of >5, or any three items with a score of >4.16 A Spanish version has been validated17 and widely used in clinical research as well as clinical trials to assess sexual functioning. Sexual dysfunction (yes/no) assessed with the ASEX scale was the dependent outcome of the present study and the rest were considered potential explanatory variables.
The study protocol had been approved by the Vall d’Hebron Ethics Committee (PR (AG)65/2018), and all participants provided informed written consent before data collection.
Statistical analysisThis is a primary data analysis. The sample size was calculated based on the previously estimated sexual dysfunction prevalence in Spanish adults.18,19 Assuming a prevalence of 28%,20 with a 5% significance level and a 90% power, a total of 309 subjects were estimated to be needed to participate in this study.21
An electronic clinical research data capture was created using REDCap® (Research Electronic Data Capture), a secure web application for building and managing online surveys and databases (https://projectredcap.org/software).22 There were no missing data. Descriptive and frequency statistics were obtained and comparisons made using the R software version (3.6.1) for Windows. Nominal (categorical) variables were reported as frequencies (percentages) and continuous variables were as mean±standard deviation. Normality assumption of quantitative variables was checked through visual methods (Q-Q plots) and normality tests (Kolmogorov–Smirnov test). Statistical significance in the comparisons with sexual dysfunction (ASEX) was assessed by the Person's chi-square when comparing with categorical variables except in the case of having an expected count less than 5 in more than 20% of cells in the contingency table, where Fisher's exact test was used (use of combined analgesics, presence of diabetes or cardiovascular disease and marital status). Odds-ratio (RR) is shown to estimate the strength of the association between study outcome and categorical variables. Sexual dysfunction was compared with independent t-test for continuous variables that followed a normal distribution (age), and the Mann–Whitney U test was used for the rest of continuous variables (HAD scale punctuations for anxiety and depression).
Only variables associated with statistical significance in the univariate analysis were considered in the classification stage. Feature selection methods can reduce the number of variables, which can avoid the redundant and highly correlated features (multicollinearity challenge). There are several feature selection methods; in this study, we used the method of Recursive Feature Elimination.23 A Random Forest (RF) algorithm is used to select the best combination of features for prediction in each iteration of the RFE.24 The k-Nearest-Neighbors (kNN) algorithm was as classifier because is less prone to be biased using nonparametric methods and it has been employed in neuromedical research.25–27 Thus, data were divided into two balanced subsets: training (80% initial, 246 samples) and testing (20% initial, 60 samples). Training and testing classifiers on the same data could cause model overfitting28; for that reason, we also applied a 10-fold cross-validation procedure with 5 repeats in order to validate the model computed. KNN model was computed in the training set and the accuracy, specificity, sensitivity, correct classification rate (CCR), kappa coefficient and receiver operating characteristic (ROC) curve were estimated in the testing set in order to quantify the performance of the classification model and identify the better subset of statistically significantly explanatory variables to classify migraine patients according to their sexual function. The ROC analysis was also carried out to identify the best discriminant cut-off point for the age in order to identify patients with or without sexual dysfunction; the optimal cut-off point was obtained using the maximum Youden Index value (sensitivity+specificity−1). The feature selection and classification stage were done with the caret package (v6.0-84). p-Values presented are for a two-tailed test and p-values <0.05 were considered statistically significant.
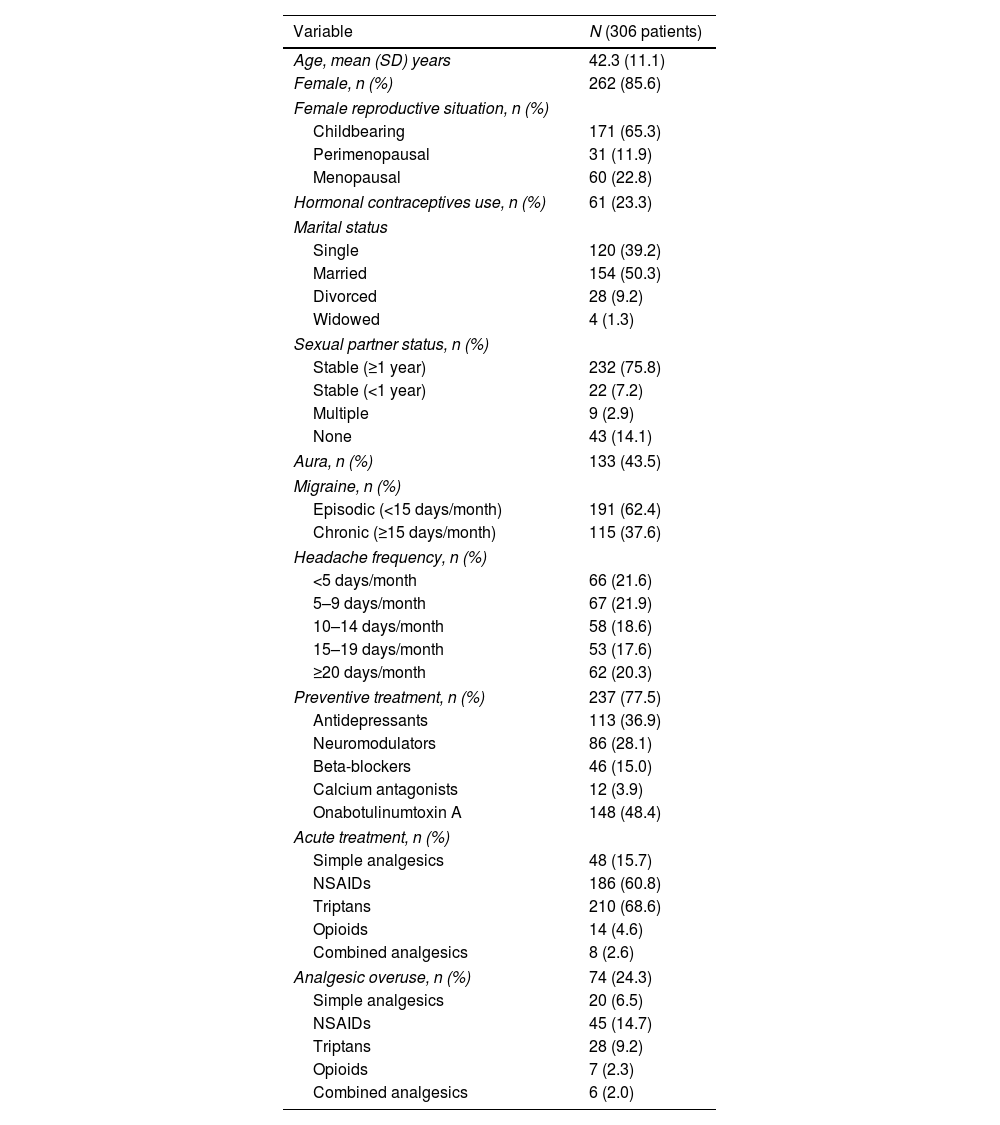
ResultsDemographic and migraine dataWe included 306 patients, 85.6% were women, mean age 42.3±11.1 years. Table 1 shows the sample demographics data. In regards to their marital status: 50.3% were married while 39.2% were single. A 75.8% had a steady partner (more than 1 year with same partner), 7.2% had new partner, 14.1% had no sexual partner and the 2.9% had occasional couples. Regarding their reproductive status, 65.3% of women included were in childbearing age. In relation to their migraine, 62.4% of patients were diagnosed with episodic migraine and 37.6% had chronic migraine; and at the moment of the evaluation, the 62.1% had less than 15 headache days/month (Table 1 shows current headache status for the last 3 months before evaluation). Regarding preventive treatment, 77.5% were on current preventive therapy. A 24.3% met criteria for analgesic overuse and the most frequently used medication for the acute treatment were non-steroidal anti-inflammatory drugs (NSAIDs) and triptans (see Table 1).
Demographic and migraine data.
| Variable | N (306 patients) |
|---|---|
| Age, mean (SD) years | 42.3 (11.1) |
| Female, n (%) | 262 (85.6) |
| Female reproductive situation, n (%) | |
| Childbearing | 171 (65.3) |
| Perimenopausal | 31 (11.9) |
| Menopausal | 60 (22.8) |
| Hormonal contraceptives use, n (%) | 61 (23.3) |
| Marital status | |
| Single | 120 (39.2) |
| Married | 154 (50.3) |
| Divorced | 28 (9.2) |
| Widowed | 4 (1.3) |
| Sexual partner status, n (%) | |
| Stable (≥1 year) | 232 (75.8) |
| Stable (<1 year) | 22 (7.2) |
| Multiple | 9 (2.9) |
| None | 43 (14.1) |
| Aura, n (%) | 133 (43.5) |
| Migraine, n (%) | |
| Episodic (<15 days/month) | 191 (62.4) |
| Chronic (≥15 days/month) | 115 (37.6) |
| Headache frequency, n (%) | |
| <5 days/month | 66 (21.6) |
| 5–9 days/month | 67 (21.9) |
| 10–14 days/month | 58 (18.6) |
| 15–19 days/month | 53 (17.6) |
| ≥20 days/month | 62 (20.3) |
| Preventive treatment, n (%) | 237 (77.5) |
| Antidepressants | 113 (36.9) |
| Neuromodulators | 86 (28.1) |
| Beta-blockers | 46 (15.0) |
| Calcium antagonists | 12 (3.9) |
| Onabotulinumtoxin A | 148 (48.4) |
| Acute treatment, n (%) | |
| Simple analgesics | 48 (15.7) |
| NSAIDs | 186 (60.8) |
| Triptans | 210 (68.6) |
| Opioids | 14 (4.6) |
| Combined analgesics | 8 (2.6) |
| Analgesic overuse, n (%) | 74 (24.3) |
| Simple analgesics | 20 (6.5) |
| NSAIDs | 45 (14.7) |
| Triptans | 28 (9.2) |
| Opioids | 7 (2.3) |
| Combined analgesics | 6 (2.0) |
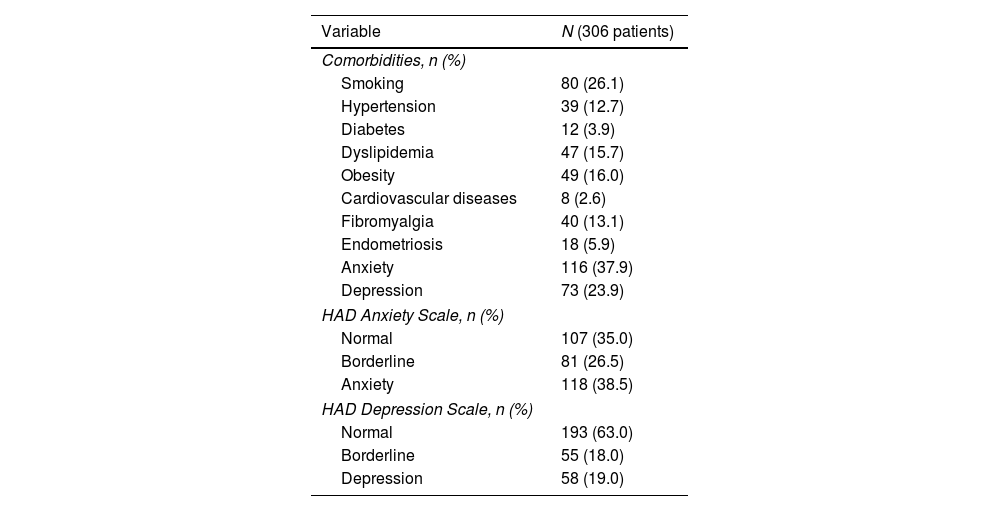
The most common comorbidities in our cohort were anxiety (37.9%), depression (23.9%) and obesity (16.0%). This was confirmed by the results of HAD scale with 38.5% abnormal scores for anxiety and 19.0% for depression (see Table 2).
Comorbidities prevalence.
| Variable | N (306 patients) |
|---|---|
| Comorbidities, n (%) | |
| Smoking | 80 (26.1) |
| Hypertension | 39 (12.7) |
| Diabetes | 12 (3.9) |
| Dyslipidemia | 47 (15.7) |
| Obesity | 49 (16.0) |
| Cardiovascular diseases | 8 (2.6) |
| Fibromyalgia | 40 (13.1) |
| Endometriosis | 18 (5.9) |
| Anxiety | 116 (37.9) |
| Depression | 73 (23.9) |
| HAD Anxiety Scale, n (%) | |
| Normal | 107 (35.0) |
| Borderline | 81 (26.5) |
| Anxiety | 118 (38.5) |
| HAD Depression Scale, n (%) | |
| Normal | 193 (63.0) |
| Borderline | 55 (18.0) |
| Depression | 58 (19.0) |
Forty one point eight percent of patients had sexual dysfunction according to ASEX scale. Regarding ASEX items, women had more abnormal scores in sex drive, arousal, and orgasmic dysfunction [sex drive (women: 27.8% vs. men: 11.1%, p=0.016); arousal (women: 18.8% vs. men: 4.4%, p=0.017); orgasm (women: 18.0% vs. men: 4.4%, p=0.022)]. When subjectively asked, 62.2% of migraine patients agreed or strongly agreed that suffering from migraine affected their sexual activity; while only a 7.0% who strongly disagreed. Subjective interference of migraine in sexual performance was not consistent with presence of sexual dysfunction according to ASEX scale (kappa coefficient=0.17).
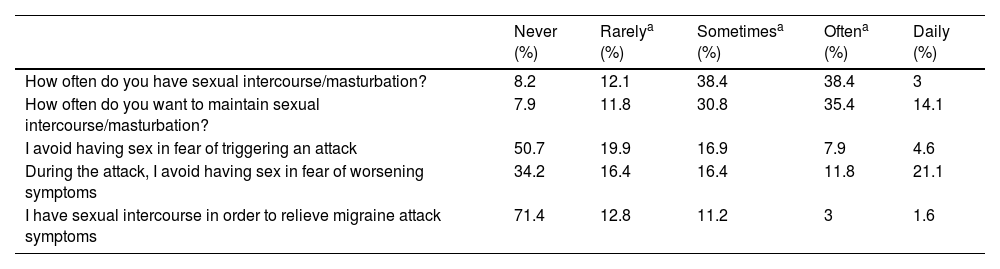
In regards to the presence of usual sexual activity, 20.3% of the cohort had sexual intercourse less than once a month, 76.8% had sex at least monthly. With respect to sexual desire, a 19.7% experienced desire less than once per month, 30.8% between once per month and less than twice a week, and more than twice a week for the 49.9% of the patients asked (see Table 3). Patients with sexual dysfunction had statistical significantly less often sexual intercourse or desire than patients without sexual dysfunction (p<0.05). Frequent sexual desire (more than 2/week) was statistically associated with demographic and migraine features as male gender (OR [95%]: 2.689 [1.342–5.386]; p=0.007), fertile female (2.564 [1.609–4.086]; p<0.001), younger age (<46.5 years: 0.249 [0.151–0.408]; p<0.001), episodic migraine patients (0.551 [0.344–0.881]; p=0.017), without medication overuse (0.534 [0.313–0.914]; p=0.030) and without depression (0.424 [0.232–0.775]; p=0.007).
Subjective impact of migraine on sexual life.
| Never (%) | Rarelya (%) | Sometimesa (%) | Oftena (%) | Daily (%) | |
|---|---|---|---|---|---|
| How often do you have sexual intercourse/masturbation? | 8.2 | 12.1 | 38.4 | 38.4 | 3 |
| How often do you want to maintain sexual intercourse/masturbation? | 7.9 | 11.8 | 30.8 | 35.4 | 14.1 |
| I avoid having sex in fear of triggering an attack | 50.7 | 19.9 | 16.9 | 7.9 | 4.6 |
| During the attack, I avoid having sex in fear of worsening symptoms | 34.2 | 16.4 | 16.4 | 11.8 | 21.1 |
| I have sexual intercourse in order to relieve migraine attack symptoms | 71.4 | 12.8 | 11.2 | 3 | 1.6 |
| Strongly disagree (%) | Disagree (%) | Neither agree nor disagree (%) | Agree (%) | Strongly agree (%) | |
|---|---|---|---|---|---|
| I consider that suffering from migraine affects my sexual performance | 7.6 | 9.2 | 21.2 | 28.0 | 34.2 |
A 49.3% of patients asked avoided having sexual intercourse for fear of triggering attacks and 34.2% never had sex during a migraine attack because they feared their attack to be worsen. Although, 28.0% had tried having sex in order to relieve the symptoms of a migraine attack.
In relation to the medications prescribed for migraine, 24.0% considered that migraine treatment interfered on sexual functioning, mainly those who had received preventive medication with antidepressants and neuromodulators. Furthermore, 8.2% thought that current treatment affected sexual functioning, nonetheless, 77% had never talked with their doctor or neurologist about the effects of migraine or its treatment on sexual performance.
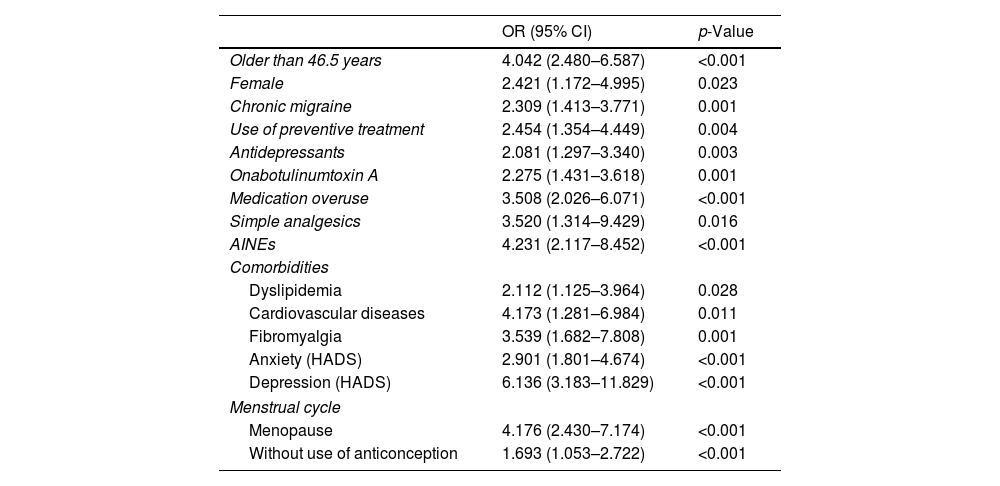
Factors associated with sexual dysfunctionThe presence of sexual dysfunction was significantly higher in the female gender (p=0.023), older age (p<0.001), menopause (p<0.001), or women not using contraceptives (p<0.001) and presence of comorbidities as dyslipidemia (p=0.028), cardiovascular disease (p=0.011), fibromyalgia (p=0.001), anxiety (p<0.001), and depression (p<0.001) (see Table 4). The ROC analysis for the age showed and AUC of 0.69 (95% CI: 0.63–0.75; p=0.001), suggesting that an age equal or greater than 46.5 years old predicted sexual dysfunction in our migraine population with a sensitivity of 0.57% and a specificity of 0.75%: 4.04 [2.48–6.59]; (p<0.001).
Odds-ratio (95% CI) estimated from the univariate analysis of the statistically significant variables associated to sexual dysfunction.
| OR (95% CI) | p-Value | |
|---|---|---|
| Older than 46.5 years | 4.042 (2.480–6.587) | <0.001 |
| Female | 2.421 (1.172–4.995) | 0.023 |
| Chronic migraine | 2.309 (1.413–3.771) | 0.001 |
| Use of preventive treatment | 2.454 (1.354–4.449) | 0.004 |
| Antidepressants | 2.081 (1.297–3.340) | 0.003 |
| Onabotulinumtoxin A | 2.275 (1.431–3.618) | 0.001 |
| Medication overuse | 3.508 (2.026–6.071) | <0.001 |
| Simple analgesics | 3.520 (1.314–9.429) | 0.016 |
| AINEs | 4.231 (2.117–8.452) | <0.001 |
| Comorbidities | ||
| Dyslipidemia | 2.112 (1.125–3.964) | 0.028 |
| Cardiovascular diseases | 4.173 (1.281–6.984) | 0.011 |
| Fibromyalgia | 3.539 (1.682–7.808) | 0.001 |
| Anxiety (HADS) | 2.901 (1.801–4.674) | <0.001 |
| Depression (HADS) | 6.136 (3.183–11.829) | <0.001 |
| Menstrual cycle | ||
| Menopause | 4.176 (2.430–7.174) | <0.001 |
| Without use of anticonception | 1.693 (1.053–2.722) | <0.001 |
OR=odds-ratio; CI=confidence interval; HADS=Hospital Anxiety and Depression Scale.
Regarding migraine characteristics, sexual dysfunction was statistically significantly associated with higher headache frequency including chronic migraine (p=0.001), use of preventive medication (p=0.003) and analgesic overuse (p<0.001) (see Table 4). There was also a statistically significant relationship between current headache frequency and sexual dysfunction, as the prevalence of sexual dysfunction in patients with less than 10 headache days/month was 28.9%, 42.2% in patients between 10 and 19 headache days per month and 28.9% in patients with 20 or more headache days/month. Sexual dysfunction was also more prevalent in the group of patients overusing simple analgesics (p=0.016) and NSAIDs (p<0.001). The classes of preventive medication more frequently associated with sexual dysfunction were antidepressants and Onabotulinumtoxin A (see Table 4). We did not find statistically significant differences in prevalence of sexual dysfunction for the different preventive medication classes when prescribed in monotherapy. Although, sexual dysfunction was more frequent in patients taking polytherapy (no preventives: 26.0%; monotherapy 45.0%; polytherapy 52.5%; p=0.001).
All variables associated with sexual dysfunction in the univariate analysis were considered in the classification stage. No statistically significant differences were found between both patient groups (training and testing) and the proportion of patients with sexual dysfunction. Several models in the RFE were computed attending to different combinations of the explanatory variables in training set and only 4 of them were selected as main features for distinguishing sexual functioning in migraine patients: Depression (diagnosed according to HADS), patients older than 46.5 years old, menopause and female gender. The optimal model obtained in the testing set through a kNN algorithm with these selected features was a k=5 model (5NN). This 5NN was able to significantly (p=0.005) distinguish between migraine patients with sexual dysfunction (accuracy (95% CI): 0.75 (0.62–0.85); sensitivity: 0.83, specificity: 0.64, kappa: 0.48; CCR: 0.79) in the testing set (60 initial samples).
DiscussionSexuality is a relevant component of our perception of quality of life and function. Migraine is a chronic neurological disorder that affects all spheres of personal, social, work and could even affect sexual life. The MIGREX study demonstrates that sexual dysfunction is a common health problem in migraine patients especially in older females and those with more severe migraine phenotypes and psychiatric comorbidities, but we did not find a clear association between migraine characteristics and sexual dysfunction.
The prevalence of sexual dysfunction among migraineurs attended at Spanish Headache Clinics is 41.8% (women: 44.7%, men: 25.0%). Our results are in consonance with epidemiological data available in the general population as well as migraine populations. About 40–45% of adult women and 20–30% of adult men have sexual dysfunction, and the prevalence of all sexual dysfunction domains increases as men and women become older.20,29 Regarding migraine population, previous studies have rated sexual dysfunction prevalence in migraine patients between 30–40% for females30,31 and 20–60% for male patients.32,33 However, over 90% of headache female patients (including migraine and tension type headache) attended in a tertiary headache centers reported sexual dysfunction.34,35 Is noteworthy that studies including tension type headache patients have found even higher sexual dysfunction rates compared with migraine.30,35 Whether there are differences in prevalence of sexual dysfunction in migraine patients compared to non-headache patients is controversial, as some studies have demonstrated an increased prevalence of sexual dysfunction in headache and migraine patients than in the general population.30,35,36 However, other studies have failed to find differences between headache patients and controls regarding sexual activity, desire or satisfaction, although sexual pain disorder might be more frequent.31,37 Because of the lack of control group in our study we cannot determine if sexual dysfunction prevalence in migraine patients is or not higher than in the general population but our results do not seem to differ from previously reported sexual dysfunction prevalence in migraine patients neither prevalence in middle-aged female population.38,39
Interpretation of migraine clinical data is a challenging issue. This is because the medical data is nonlinear, non-normal, correlation structured with other comorbidities, and complex in nature.40,41 Thus, machine learning can help health professionals make a preliminary judgment about migraine patients according to other comorbidities and their daily physical examination data, and it can serve as a reference for researchers.
In the general population, risk factors for sexual dysfunction are general health status, presence of diabetes mellitus and cardiovascular disease, concurrence of other genitourinary disease, psychiatric or psychological disorders, other chronic diseases and socio-demographic conditions.29 Contrary to our hypothesis, risk factors for sexual dysfunction in migraine patients do not differ from general risk factors for sexual dysfunction as only older age, female gender, menopause and the presence depression were associated with sexual dysfunction in the classification model. Nonetheless, we have to keep in mind that migraine and particularly chronic migraine, is a chronic disease that frequently is comorbid with psychiatric disorders as well as cardiovascular risk factors and other pain disorders,42,43 what makes migraine patients a very sensitive population to suffer from sexual dysfunction.
Older age and female gender has been associated to sexual dysfunction in our sample. It is already known that low sexual desire, erection/lubrication and orgasmic dysfunction increases with age, especially in women.29 This positive relationship between age and sexual dysfunction has also been confirmed for women with migraine.19,44,35
In the MIGREX cohort, presence of depression was statistically associated with sexual dysfunction. Depression has been strongly associated with male and female sexual dysfunction, particularly symptoms such as loss of interest, low self-esteem, decrease in energy, and inability to experience pleasure, all of which can compromise sexual performance.45 Concomitant depression increases the risk of sexual dysfunction, and even in the absence of a clinical depression, negative mood has been found to impair sexual function.8,46 In a previous study, depression was the most important factor that predicted sexual dysfunction in migraine patients,47 while this relationship has not been proven in other studies.35 Presence of anxiety has also been linked to sexual dysfunction in our sample although its association was not as stronger as the features selected in the classification model. Some studies performed in migraine male and female patients have not found this association so far.34
Our primary hypothesis was that sexual dysfunction was more prevalent among patients with more severe migraine phenotypes. It is true that, in our cohort, sexual dysfunction prevalence increases together with migraine frequency as well as other headache features, preventive treatment and comorbidities. However, this association was not significant in the classification model, what means that they are factors correlated to other main basal characteristics, such as depression, in the pathophysiology of sexual functioning. Other studies that also fail to find association between sexual dysfunction and headache frequency.30,35,48 Linked to headache frequency, migraine preventative medications have also been proved to affect sexual functioning, although it was not spontaneously referred and well tolerated in the majority of patients.49 Other comorbidities as cardiovascular risk factors as well as other pain syndromes as fibromyalgia that also have been linked to sexual dysfunction in previous studies and were more common in the group of patients with sexual dysfunction.6,50,51
This study has several strengths. The use of ASEX scale facilitates sexual dysfunction screening for all domains in a quick and non-intrusive way and the anonymous and self-applicable format gives veracity to our results but previous studies have used diverse definitions and even different tools to screen for sexual dysfunction which makes it difficult to compare. In our study, patients have been evaluated by headache neurologists and we have included validated screening scales for anxiety and depression in order to ensure proper data collection. We have included a large sample of migraine patients attended in Spanish headache centers, which may be not comparable to all migraine patients but the most disabled group. The use of machine learning algorithms has shown to be worthy of use in the study of complex disorders when the primary diagnosis is just clinical. The lack of control group is the principal limitation of our study, also, the high proportion of women corresponding with typical demographics of migraine patients attended in headache units, limits dawing conclusions in male population. Nevertheless, the good quality of data and the scarce evidence about migraine and SD relationship makes our study valuable.
ConclusionsThe MIGREX study demonstrates that sexual dysfunction is a frequent disorder in migraine patients visited in a headache clinic. Our results seem to indicate that sexual dysfunction is more common in female and older migraine patients and those with more severe migraine phenotypes and comorbidities. However, the only clinical factors that predict sexual dysfunction in migraine patients are higher age, female gender, menopause and current depression, suggesting that sexual dysfunction in migraine patients is more linked to patient demographic factors and comorbidities than migraine clinical features per se.
Considering the impact which this condition can have on the patient's quality of life, correct screening and diagnosis of sexual dysfunction in migraine patients may change the therapeutic approach of these patients and improve patient's quality of life.
Ethics approvalThe study protocol had been approved by the Vall d’Hebron Ethics Committee (PR (AG)65/2018), before any subject recruitment, and all participants provided informed written consent before data collection.
Consent to participateAll participants provided informed written consent before data collection.
Consent for publicationAll authors fully comply with and approve the version to be published.
Availability of data and materialAnonymized data will be shared by request from any qualified investigator.
Code availabilityPipeline code analysis will be shared by request from any qualified investigator.
Author contributionsAll authors made substantial contributions to conception and study design and data collection. VJG contributed to analysis and interpretation of data. MTF and EB wrote first draft. MTF, VJG and PPR critically revised and finally approved the version to be published.
FundingNo funding was received.
Conflicts of interestThe authors have no conflicts of interest in this paper.