Tolosa-Hunt syndrome (THS) is an idiopathic condition included in the differential diagnosis of painful ophthalmoplegia. The condition is caused by granulomatous infiltration of the lateral wall of the cavernous sinus and characterised by one or more episodes of unilateral orbital pain associated with paralysis of one or more oculomotor nerves. Magnetic resonance imaging (MRI) reveals granulomatous infiltration of the cavernous sinus. The condition is treated with corticosteroids, leading to the complete resolution of symptoms within 72hours. Up to half of patients experience recurrences even several months or years after the first episode; recurrences are usually ipsilateral. However, spontaneous remission may also occur. We present a case of spontaneous remission and subsequent recurrence of THS.
Our patient was a 31-year-old Peruvian woman who had been living in Spain for the past 5 years. She had a history of idiopathic right-sided peripheral facial paralysis in 2009, which resolved completely, and a 2-year history of episodic migraine without aura. The patient was receiving oral contraceptives. She visited the emergency department in October 2011 due to a one-week history of binocular diplopia and headache. Diplopia became worse with right horizontal gaze and did not change with distance. Headache was right hemicranial and pulsatile; it was associated with photophobia and had similar features to those of her usual episodes of migraine, increasing progressively until reaching a score of 8/10 on the visual analogue scale. The patient also reported paraesthesia in the area around the mouth and the right side of the jaw. No proptosis or any other eye abnormalities were reported.
In August of the same year, the patient had experienced a similar episode, which lasted a week and resolved spontaneously, leaving no sequelae. The results from the physical examination and the patient's vital signs were normal. A neuro-ophthalmological examination revealed anisocoria (right pupil: 4mm; left pupil: 3mm); changes in illumination did not affect pupil size. Both direct and consensual light reflexes were normal. We also observed ptosis in the right eye and dysconjugate gaze in the primary gaze position; esotropia in the right eye and hypertropia in the left (positive cover-uncover test, negative alternate cover test). Examination of ocular movements revealed limited abduction and supradextroversion of the right eye; diplopia was most marked in these gaze positions. In the following days, the right eye also displayed limited adduction, infradextroversion, and infralaevoversion. The rest of the neurological examination (including examination of the eye fundus, visual acuity, and the remaining cranial nerves) found no abnormalities. In summary, the results of the neuro-ophthalmological evaluation were compatible with paralysis of the right third and sixth cranial nerves.
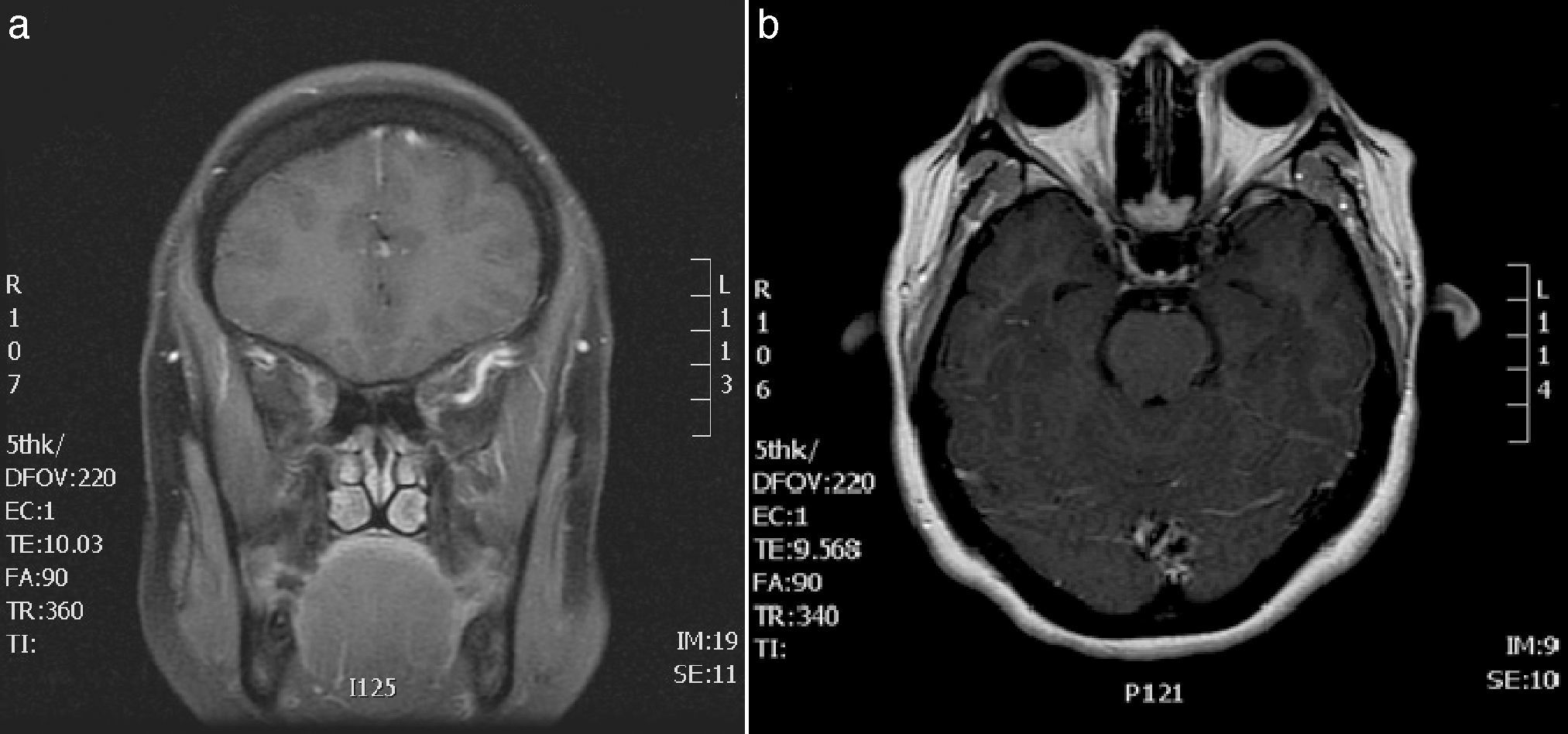
In view of her symptoms, the patient was diagnosed with recurrent painful ophthalmoplegia. A complete blood count and biochemical study (including determination of the erythrocyte sedimentation rate [ESR] and an angiotensin-converting enzyme [ACE] test) only revealed hypertriglyceridaemia. The results from serology tests for Borrelia and syphilis and an immunological study (antinuclear, anti-neutrophil cytoplasmic, and anti-thyroid autoantibodies) were negative. A CSF analysis (biochemical analysis, culture, and Venereal Disease Research Laboratory test) disclosed no abnormalities. Post-contrast MRI sequences displayed asymmetrical uptake in the orbital apex and the lateral wall of the right cavernous sinus (Fig. 1). No asymmetries were found in the calibre or signal intensity of the optic nerve.
(A) Brain MRI scan: gadolinium-enhanced T1-weighted coronal sequence showing signal alterations in the right cavernous sinus. (B) Brain and orbital MRI scan: gadolinium-enhanced T1-weighted axial sequence revealing asymmetrical contrast uptake in the orbital apex and the lateral wall of the right cavernous sinus.
The patient was diagnosed with THS based on her symptoms and neuroimaging results, and after other aetiologies were ruled out. We started treatment with intravenous methylprednisolone dosed at 1g every 24hours for 3 days, followed by decreasing doses of oral prednisone. Within 24hours from treatment onset, headache and diplopia improved progressively, eventually resolving completely.
THS is a rare, idiopathic, inflammatory process of the cavernous sinus. The syndrome affects both sexes equally and is more frequent in middle-aged individuals. Some authors, however, have reported cases of THS in children.1 THS is characterised by unilateral orbital pain that may radiate to the retro-orbital, frontal, temporal, and even occipital regions. Pain is associated with diplopia due to involvement of one or more oculomotor nerves, and may also affect the ipsilateral first or second branches of the trigeminal nerve.2,3 Cranial nerve involvement may occur simultaneously or up to 2 weeks after headache. Involvement of other cranial nerves, such as cranial nerve II, the third branch of cranial nerve V, cranial nerve VII, or the ipsilateral sympathetic pupillary pathway, has also been described.4 Bilateral cranial nerve involvement is very infrequent. In 2004, the International Headache Society reviewed the 1998 diagnostic criteria for headache disorders.5
Brain and orbital MRI is the imaging technique of choice in patients with painful ophthalmoplegia. Patients with THS display an enlarged cavernous sinus which appears as isointense signal on T1/T2-weighted sequences and gadolinium uptake in that area. A lack of MRI findings, however, does not rule out a diagnosis of THS: the literature reports some cases of THS in patients with typical symptoms and normal neuroimaging results.6 An MRI study of the cavernous sinus and orbital apex is highly sensitive in detecting the characteristic inflammatory lesions observed in THS and is very useful for following up these patients.7
Corticosteroids are the treatment of choice according to the diagnostic criteria for THS.5 There are no conclusive data on the most appropriate dose, frequency of administration, treatment duration, administration route, or type of corticosteroid. In clinical practice, these patients are usually treated either with an intravenous bolus of methylprednisolone dosed at 500-1000mg/day for 3-5 days or with oral prednisone dosed at 1mg/kg per day.8 Treatment duration ranges from a few weeks to several months and should be adapted to each patient's clinical response. Recurrence is less likely in patients receiving high corticosteroid doses (500-1000mg/day for several days, followed by daily doses of 1mg/kg for several weeks).9
THS must be diagnosed by exclusion, which requires adequate differential diagnosis.10 Patients should undergo thorough testing, including a complete blood count (ESR and ACE), chest radiography, serology and immunology tests, a lumbar puncture, and a neuroimaging study. Differential diagnosis should include other entities associated with painful ophthalmoplegia and/or affecting the cavernous sinus, superior orbital fissure, or orbit,10,11 as well as those conditions manifesting with headache and ophthalmoplegia or fluctuating/episodic diplopia (Table 1).12
Disorders manifesting with episodic or fluctuating diplopia
| Myasthenia gravis |
| Neuromyotonia |
| Superior oblique myokymia |
| Thyroid-associated orbitopathy |
| Decompensated heterophoria |
| Convergence spasm |
| Cyclic spasms |
| Intermittent ophthalmoplegia |
| Tolosa-Hunt syndrome |
In the present case, a history of episodic migraine combined with a previous self-limiting episode of similar characteristics initially pointed to ophthalmoplegic migraine. However, MRI findings and the resolution of pain and ophthalmoplegia within 12hours from treatment onset led us to conclude that the patient had recurrent THS.
Although spontaneous remission does occur at times, recurrence is more frequent (40%-50% of patients). Recurrences are usually ipsilateral, and to a lesser extent contralateral; bilateral recurrence is infrequent. Follow-up of these patients is recommended due to the frequency of THS recurrence. Recurrences normally occur within the first months following the initial episode, although the literature reports cases of recurrences over a decade after the first episode.14 Clinical and radiological follow-up of these patients is essential. Such conditions as sarcoidosis, vasculitis, and lymphoma may initially respond to treatment with corticosteroids; in these cases, subsequent progression of the disease will lead us to the correct diagnosis.15
Spontaneous resolution of THS, as in our case, is infrequent and has rarely been reported in the recent literature. The cases of spontaneous THS resolution published in the literature were reported before corticosteroids were established as the treatment of choice for THS. New diagnostic criteria, combined with recent advances in neuroimaging, will very likely enable earlier diagnosis of THS and administration of corticosteroid treatment, resulting in fewer cases of spontaneous resolution. However, spontaneous resolution of THS is not unlikely. The condition should therefore be suspected in patients experiencing an episode of self-limiting painful ophthalmoplegia and subsequent recurrence of symptoms.
Please cite this article as: Carreón E, Muñiz S, Di Capua D, Porta-Etessam J. Síndrome de Tolosa-Hunt con remisión espontánea y recurrencia. Neurología. 2018;33:68–70.