Cerebral amyloid angiopathy (CAA) is characterised by the deposition of amyloid beta in the media and adventitia of cortical and leptomeningeal vessels.1 It causes disruption of the vessel architecture, leading to vascular rupture.2 The most frequent form of presentation is lobar parenchymal haemorrhage (single or multiple); transient neurological focal episodes3 and cognitive impairment are other possible manifestations. The appearance of multiple corticosubcortical microbleeds on gradient-echo and susceptibility-weighted imaging (SWI) MRI sequences is a radiological sign of amyloid vasculopathy. Positron emission tomography (PET) studies with amyloid ligands can be useful in the detection of CAA, but are unable to differentiate vascular deposition (typical of CAA) from parenchymal deposition (characteristic of Alzheimer disease).4
The risk of CAA increases with age, and the condition is detected in up to 38% of patients between 80 and 89 years of age and up to 42% of patients over 90 years of age.5 It rarely occurs in patients younger than 50 years of age.
CAA preferentially involves certain parts of the brain, such as the parietotemporal region, with the middle cerebral artery being the most affected. However, unilateral presentation is exceptional and poorly understood.5,6 One hypothesis is that it corresponds to a progressive state of the CAA; another is that it responds to precipitating factors that favour the deposition of amyloid beta in a lateralised pattern.
Although the “experimental seeding” of amyloid beta is well understood,7,8 transmission of amyloid beta pathology in humans has not been reported until very recently. A prion-like mechanism, similar to iatrogenic Creutzfeldt-Jakob disease, is thought to play a role.8–10
We report the case of a patient with history of neurosurgery in his youth, who presented recurrent lobar haematomas suggestive of CAA.
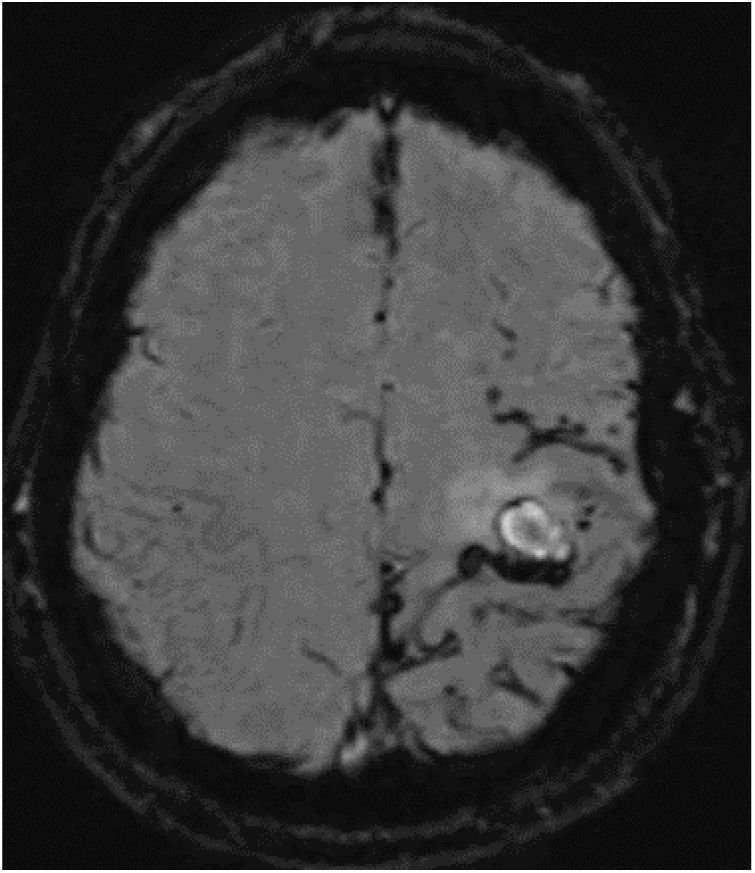
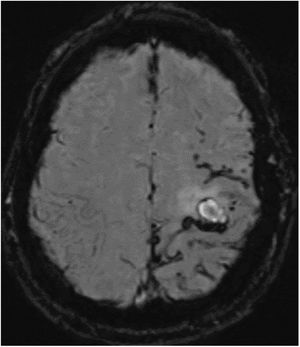
The patient was a 52-year-old white man with no family history of neurodegenerative disease. He was a smoker, and presented sleep apnoea syndrome. At the age of 18 years, he had undergone surgical treatment of an eosinophilic granuloma in the left parietal region, and did not receive subsequent radiotherapy. He was assessed at 44 years of age for cognitive complaints; the neuropsychological study identified no abnormalities. Single photon emission computed tomography revealed cortical hypoperfusion in the posterior temporal lobe bilaterally. At 50 years of age, he had consulted due to self-limited episodes of dysaesthesia in the right arm and hemifacies. Treatment was started with eslicarbazepine acetate due to suspicion of remote symptomatic seizures. A follow-up brain MRI study showed chronic ischaemic lesions, predominantly in the left hemisphere; Doppler ultrasound detected no alterations in blood circulation. At 52 years of age, he was assessed due to sudden onset of weakness in the right hand. A CT study revealed a small subacute parenchymal haematoma in the left frontal convexity. In the brain MRI study (Fig. 1), the SWI sequence showed numerous haemorrhagic foci in the sulci of the left frontoparietal convexity, indicating leptomeningeal siderosis.
Susceptibility-weighted imaging brain MRI sequence, axial plane. Numerous punctiform haemorrhagic foci are observed in the parietal lobe, with fewer foci in the posterior frontal lobe and only isolated foci in the occipital lobe, all in the left hemisphere. A fine hypodense rim is present in the subarachnoid space at the level of the Rolandic fissure, indicating leptomeningeal siderosis.
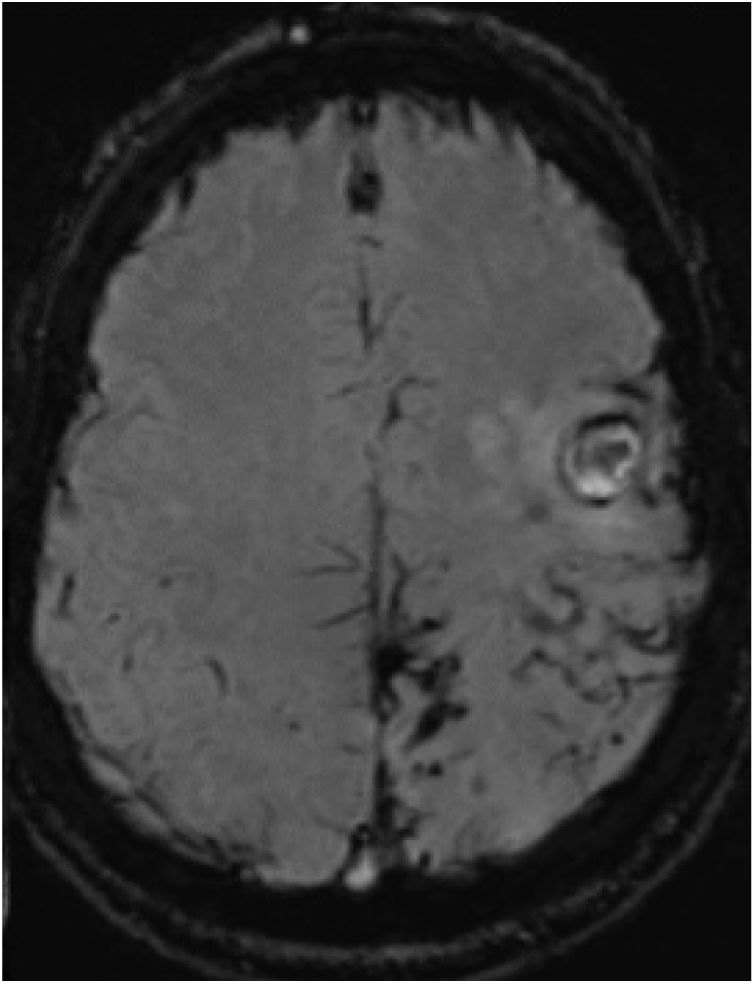
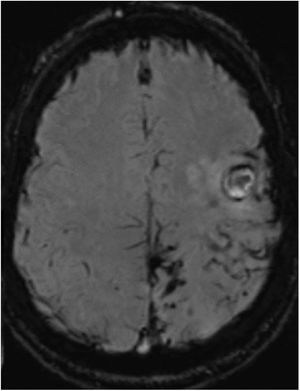
Three months later, the patient presented sudden worsening of the motor sequela; CT revealed 2 acute haemorrhagic foci in the left frontal cortex. In the subsequent MRI study (Fig. 2), SWI revealed persistence of the haemosiderin deposits in the left parietal, posterior frontal, and occipital lobes, similar to those observed in the previous study; no deposits were observed in the right hemisphere. No significant enhancement was observed after gadolinium administration. Vascular sequences did not reveal any significant findings. Coagulation and autoimmunity studies yielded normal findings.
Susceptibility-weighted imaging brain MRI sequence, axial plane, showing persistence of the haemosiderin deposits in the left parietal, posterior frontal, and occipital lobes and the fine subarachnoid rim suggestive of leptomeningeal siderosis. No haemosiderin deposition is visible in the right hemisphere.
An 18F-flutemetamol amyloid-PET study revealed bilateral, diffuse deposition throughout the cerebral cortex. Analysis of cerebrospinal fluid biomarkers and ApoE genotyping were not conducted.
In a clinical exome sequencing study, we analysed 6102 genes. No mutations were found in those genes associated with hereditary AAC, such as APP, PSEN1, PSEN2, ITM2B (or BRI2, encoding integral membrane protein 2B), and CST3 (encoding cystatin 3).
A subsequent neuropsychological study did not detect cognitive impairment. The patient presented moderate difficulties in visuospatial domains, which we considered to be secondary to the surgical lesion in the left parietal lobe.
Our patient would meet the modified Boston criteria for probable CAA,4 with the exception of onset after 55 years of age. The siderosis detected in imaging studies may be explained by the surgical procedure itself, although this would not explain the amyloid-PET findings or the recurrent lobar haematomas.
In young individuals (30-57 years) with early-onset CAA, cases have been reported of history of brain or spinal surgery (with or without a dura mater graft) or another invasive procedure (such as external carotid artery embolisation with dura mater extracts) at least 3 decades earlier.11–14 The reported cases present generalised amyloid deposition and the characteristic clinical and imaging signs of CAA, which predominantly involve the hemisphere ipsilateral to surgery.
While prevalence of the condition is not known, our case supports the hypothesis that amyloid may be transmitted through neurosurgical procedures.9,15
The patient gave verbal (by telephone) rather than written consent to the publication of his case due to restrictions on in-person consultations as a result of the COVD-19 pandemic.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Please cite this article as: Lázaro Romero A, Moreno Loscertales C, Marta Moreno E. Angiopatía amiloide cerebral unilateral tras una neurointervención. Neurología. 2022;37:310–312.