Electroencephalography (EEG) is a useful assessment tool in critical care units, especially in the case of apparently unexplained changes in the patient's level of consciousness. Herpes simplex encephalitis (HSE) is a serious disease with multiple prognostic factors; the most important of these is probably time elapsed to starting antiviral treatment.1 Another important factor is the presence of seizures, which affect a significant number of patients2 and are responsible for a cascade of pathophysiological events that seem to promote brain damage.3,4 A study by Carrera et al.5 showed that nearly one third of the patients with central nervous system infections and monitored with continuous EEG (cEEG) experienced seizures; in more than half of the cases, seizures had no clinical manifestation. Furthermore, presence of seizure activity with no clinical changes and a pattern of periodic lateralised epileptiform discharges (PLED) were independent variables of poor prognosis.
We describe the case of a patient with HSE with an aggressive course and complications consisting of epilepsy-related alterations in the level of consciousness. We would like to underscore the importance of frequent and periodic EEG studies in these patients for follow-up, management, and probably also in predicting prognosis.
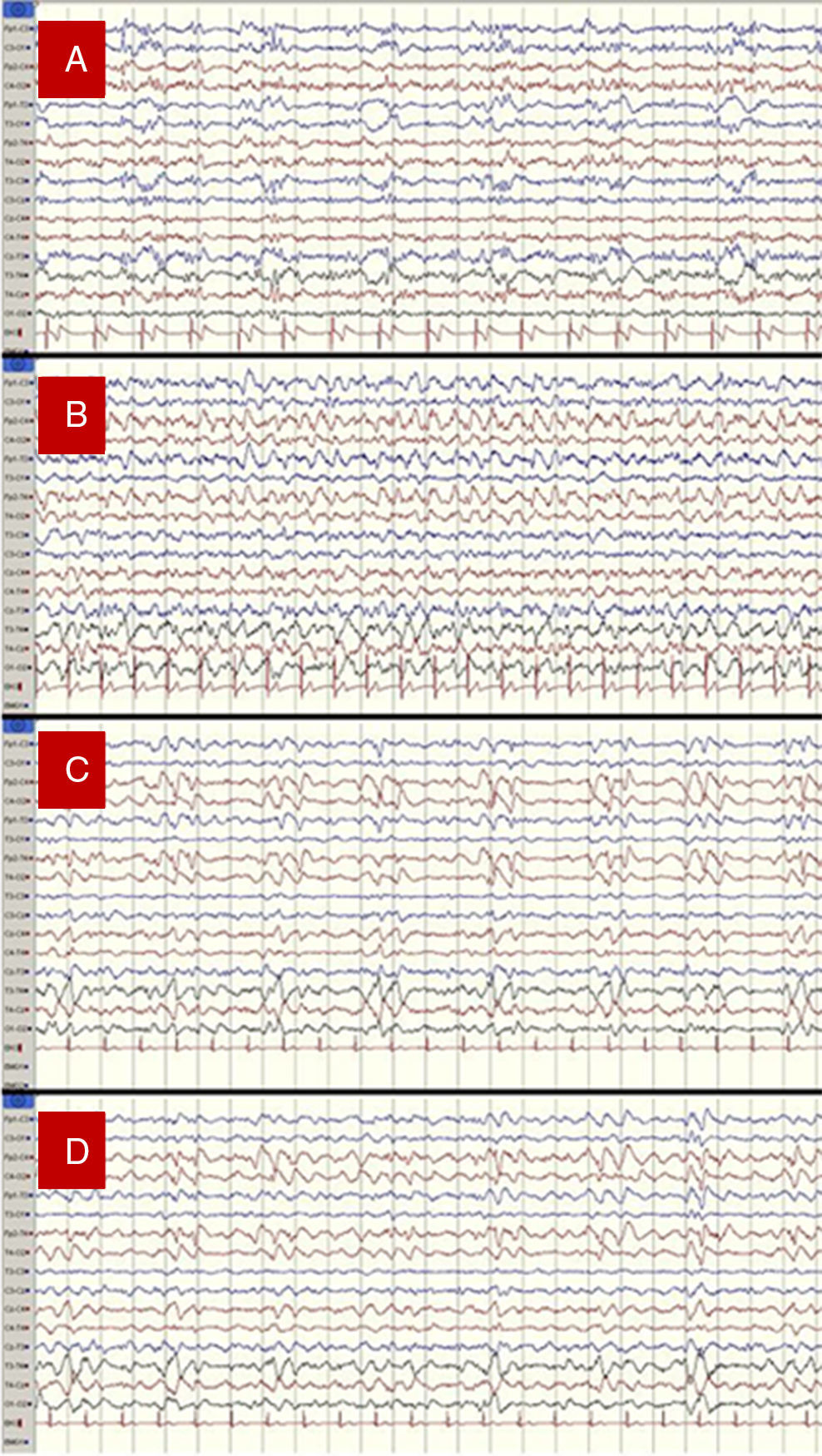
Our patient was a 56-year-old woman with no relevant medical history who presented fever, progressive disorientation, and decreased verbal fluency upon waking. A cranial CT scan and a CSF analysis conducted at her local hospital yielded normal results. She was admitted and treated with aztreonam, with no significant improvements. On the third day she was transferred to our hospital, a reference centre; she then presented stupor, sensorimotor deficits on the right side of her body, and abolished plantar reflex in the ipsilateral foot. An additional cranial CT scan also displayed no alterations. However, a subsequent lumbar puncture yielded abnormal CSF (96cells/mm3 [75% lymphocytes], protein levels 76mg/dL, and glucose levels 52mg/dL [glycaemia 137mg/dL]). We initiated treatment with acyclovir and applied our hospital's EEG monitoring protocol for these cases: at least 2 EEG recordings (3 on some occasions) every 24hours. The first EEG recording revealed a PLED pattern suggesting HSE. Subsequent EEG recordings evidenced rapid and severe progression of the process and some additional complications (Fig. 1).
- 1.
1st day of hospital stay: PLED in the left hemisphere, predominantly in temporal areas. We started treatment with levetiracetam.
- 2.
3rd day of hospital stay: non-convulsive seizures (NCS), which persisted and evolved to generalised non-convulsive status epilepticus (NCSE) that was more pronounced in the right hemisphere. Intravenous administration of propofol resulted in a pattern of seizure remission and relapse. We then supplemented the antiepileptic treatment with phenytoin.
- 3.
4th day of hospital stay: PLED in the right hemisphere. No critical episodes were recorded.
- 4.
6th day of hospital stay: PLED in the right hemisphere plus persistent, fluctuating epileptic activity with critical characteristics and some long-lasting episodes, all of which were more marked in the right hemisphere. We added lacosamide to antiepileptic treatment.
(A) 1st day of hospital stay: PLED in the left hemisphere, predominantly in temporal areas. (B) 3rd day: generalised status epilepticus, predominantly in the right hemisphere. (C) 4th day: PLED in the right hemisphere. (D) 6th day: PLED plus critical epileptic activity in the right hemisphere.
EEG recordings evidenced slow progression; this was subsequently confirmed by an additional CT scan revealing patchy hypodensities in the basal frontal and temporal area bilaterally but predominantly on the left side (Fig. 2A). Antioedema therapy and treatment with corticosteroids delivered some degree of improvement. The patient was transferred to the neurology department a week later. After 21 days, she presented poor attention, apathy, left gaze preference, right-sided neglect, signs of frontal release, global dysphasia, right homonymous hemianopsia, and mild right hemiparesis. Epileptic activity decreased substantially according to subsequent follow-up EEG recordings. A PCR study conducted a few days later confirmed the diagnosis of HSE due to HSV-1, and an MRI scan displayed the extension of the structural lesion (Fig. 2B and C).
Nearly one third of the patients admitted to the neurointensive care unit and undergoing cEEG monitoring have NCS (in the form of NCSE in 75%).6 Furthermore, 8% of all comatose patients, regardless of their medical histories, experience NCS.7 Since such a high percentage of patients has no epileptic symptoms, doctors must maintain a high level of suspicion and perform EEG studies to detect these patients. Numerous studies have underscored the harmful effects of ictal activity, which is associated with poor prognosis especially in cases of NCSE.8,9 The above suggests that EEG (and especially cEEG monitoring) is useful for identifying NCS and NCSE,10,11 which might otherwise go undetected. Likewise, EEG enables early treatment of this complication which naturally leads to better outcomes by helping minimise cognitive-behavioural sequelae, which are frequent in patients with NCSE.3
The question of which patients should be monitored, and for how long, is controversial. Since 2012, some guidelines have recommended at least 48 hours of cEEG monitoring for all comatose patients12; according to one earlier study, some 80% of seizures occur in the first 24 hours and up to 87% in the first 48 hours of coma onset.4 To date, there are no guidelines clearly establishing a methodology for cEEG monitoring.13
Frequent use of short conventional EEG studies cannot replace cEEG monitoring.14 However, it constitutes an acceptable alternative when long-term EEG monitoring cannot be performed15; this is the case in our hospital, where we have designed a protocol for monitoring critical patients with acute neurological processes, especially status epilepticus. The protocol includes non-video EEG monitoring by an on-site EEG specialist who establishes the association between electric and clinical findings and immediately issues a report. Monitoring takes place 2 to 3 times per day: on weekdays, at the beginning (8.00-9.00) and end (14.00-15.00) of the morning shift, and at the end of the afternoon shift (19.00-20.00); during the weekend, patients are monitored every 24 hours (or more frequently if necessary) by the on-call neurologist. EEG recordings take about 30 minutes to complete,15 although they may require more time depending on the patient's condition. As we anticipate the possibility of implanting cEEG monitoring for critical neurological patients in our centre, we feel that this methodology achieves more satisfactory results than those from isolated EEG recordings performed every 24 or 48 hours. A thorough prospective study aiming to determine the accuracy of this methodology is currently underway.
EEG is helpful for early diagnosis and detection of seizures, especially in cases of NCSE. We feel that use of this technique had an impact on the management and outcome of our patient.
FundingThe authors received no funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study has not been presented at the SEN's Annual Meeting or at any other conferences or congresses.
Please cite this article as: Grande-Martin A, Pardal-Fernández JM, García-López FA. Utilidad de observaciones EEG frecuentes en el manejo de una paciente con encefalitis herpética. Neurología. 2017;32:193–195.





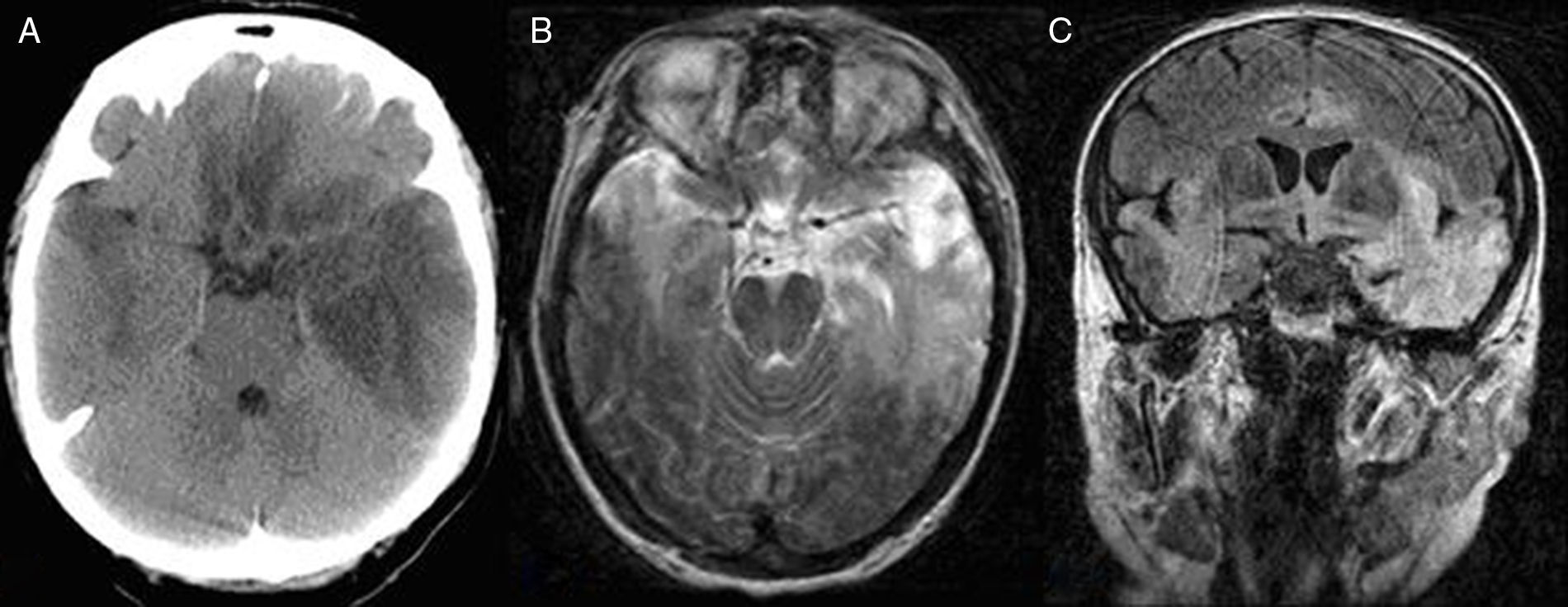
![Bilateral asymmetric temporal, basal frontal, insular, and cingulate involvement, predominantly in the left hemisphere, in CT (A) and MR images (T2-weighted axial sequence [B] and coronal FLAIR sequence [C]). Bilateral asymmetric temporal, basal frontal, insular, and cingulate involvement, predominantly in the left hemisphere, in CT (A) and MR images (T2-weighted axial sequence [B] and coronal FLAIR sequence [C]).](https://static.elsevier.es/multimedia/21735808/0000003200000003/v1_201703310206/S2173580817300391/v1_201703310206/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
