Coronavirus disease 2019 (COVID-19) has been linked to a wide range of neurological alterations. In many patients, SARS-CoV-2 induces an abnormal immune response, causing multisystem hyperinflammation. Some cases of autoimmune diseases triggered by COVID-19 have been described,1–4 including anti–n-methyl-d-aspartate (NMDA.0) receptor encephalitis.5–9 We present a case of anti–NMDA-receptor encephalitis of possible post-infectious aetiology in a patient with COVID-19.
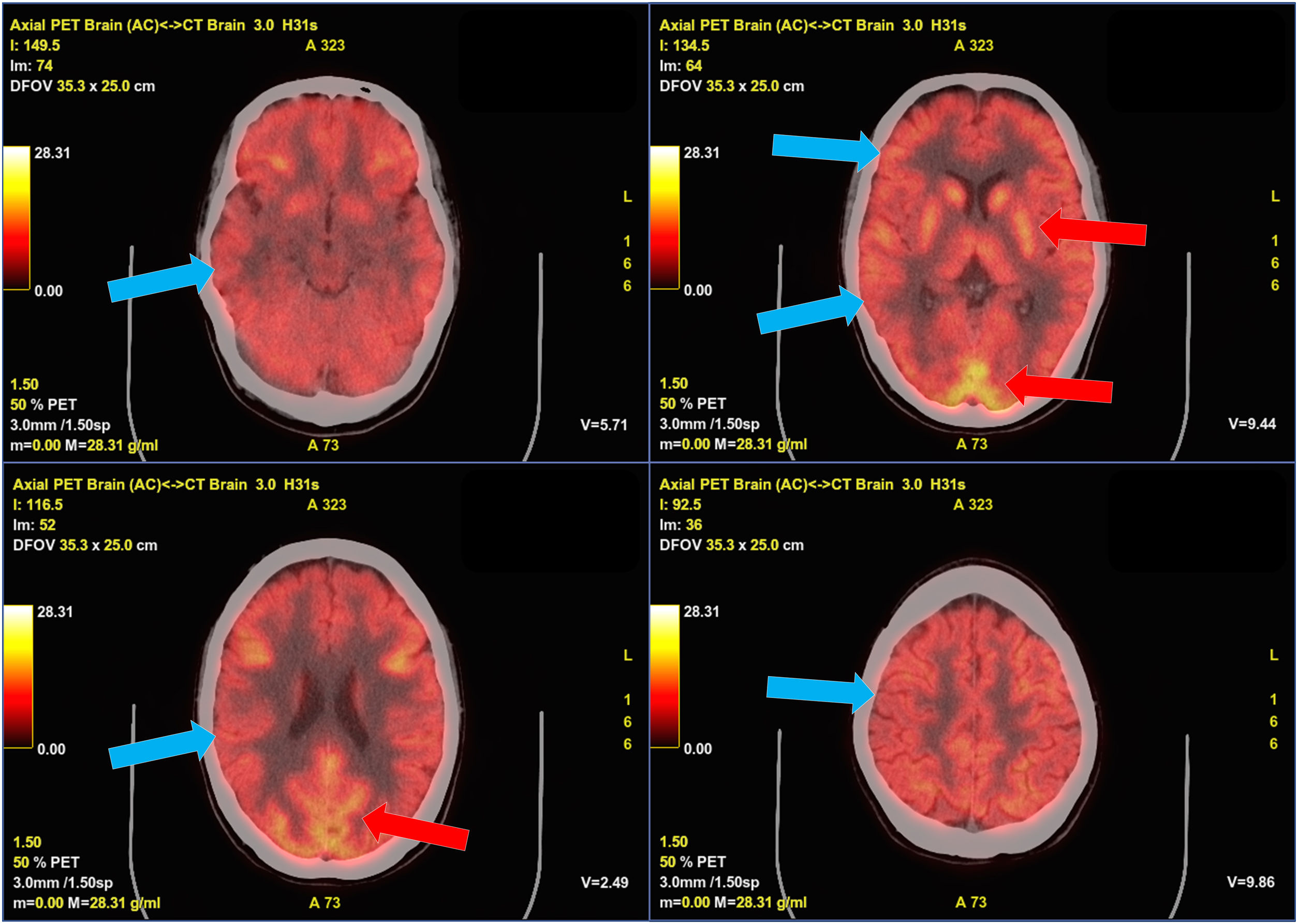
Our patient was a 22-year-old woman errase diagnosed with non-lesional focal right frontal lobe epilepsy at the age of 20 years being seizure-free for the last year treated in monotherapy. In January 2022 she presented COVID-19, with mild flu-like symptoms. Five days after symptom onset, she presented 4 focal to bilateral tonic-clonic seizures and a second antiseizure drug was started. Following this episode, the patient presented severe anxiety, dysphoric mood, and insomnia, and subsequently developed language block, which gradually progressed to motor aphasia, persisting despite adjustment of antiseizure treatment. Two weeks later, she presented 3 focal to bilateral tonic-clonic seizures within several hours, followed by a neurological worsening, developing bradyphrenia and delusions with visual hallucinations and psychomotor agitation; the patient was admitted to hospital. She did not present fever, systemic symptoms, consciousness alterations, or movement disorders. EEG revealed frontal intermittent rhythmic delta activity (FIRDA), with no other relevant findings (Fig. 1). Brain MRI and blood analysis results were normal. We initially suspected seizure control decompensation due to COVID-19, followed by postictal psychosis. The patient was started on benzodiazepines and antipsychotics. Psychiatric symptoms improved and the patient presented no further seizures, but dysphasia and bradyphrenia persisted. Four weeks a lumbar puncture was performed 4 weeks after symptom onset. CSF analysis revealed 7 cells per mm3, with normal protein and glucose levels; CSF cytology and microbiology studies (serology, cultures, PCR) yielded negative results. Anti–NMDA-receptor antibodies tested positive in the CSF and serum. Treatment with methylprednisolone dosed at 1 g/day for 5 days was started, achieving a partial improvement. Subsequently, intravenous immunoglobulins (0.4 g/kg/day for 5 days) were prescribed, gradually improving to her baseline status. A gynaecological examination and whole-body CT and FDG-PET studies were normal, ruling out an occult tumor; however, the FDG-PET study detected diffuse hypometabolism in the cerebral cortex (Fig. 2). The patient was started on rituximab (2 infusions of 1 g, 15 days apart).
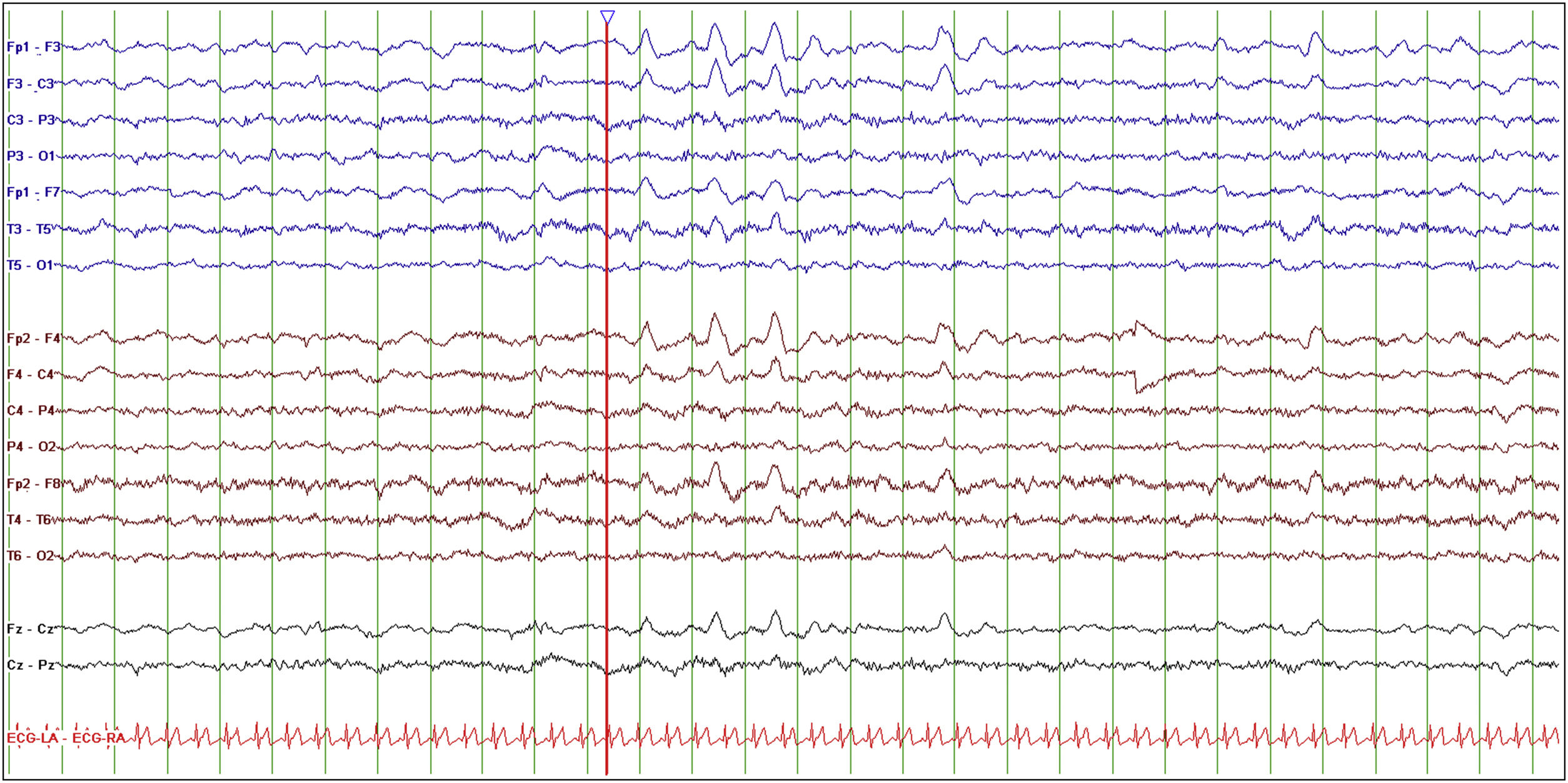
Baseline EEG study.
The study was performed with the patient awake, calm, and with her eyes closed. Setup: longitudinal bipolar montage, LFF 0.5 Hz, HFF 30 Hz, sensitivity 10 μV/mm, timebase 15 mm/s. EEG reveals normal background activity with frontal intermittent rhythmic delta activity, both in isolation and in trains lasting several seconds (red line).
Autoimmune encephalitis may be caused by immune cross-reactivity. Exposure of the immune system to an epitope mimicking an antigen present in the nervous system could lead to the formation of antineuronal antibodies. In addition to some recent cases of SARS-CoV-2,5–9 several other viruses have been associated with the development of anti–NMDA-receptor antibodies, although most cases occurred after herpes simplex encephalitis.10 However, its association with bacterial or parasitic infections has also been described.11,12 In the case of COVID-19, the structural similarities between the NMDA receptor GluN1 subunit with the the SARS-CoV-2 nonstructural protein 8 may explain the association between this infection and anti–NMDA-receptor encephalitis.13 However, in several recently published cases of anti–NMDA-receptor encephalitis in the context of COVID-19, the chronology of this association is questionable, since symptoms of encephalitis started several weeks before the symptoms or diagnostic confirmation of COVID-19,6–9 which casts doubt on whether SARS-CoV-2 was the trigger of the dismune process. However, it should be noted that antibody production times during a post-infectious dysimmune process are not well established. Furthermore, one of the cases was associated with a diagnosis of ovarian teratoma5 and several other cases provide no information on whether studies were performed to detect occult malignancy; therefore, the association with COVID-19 may be merely coincidental.
Anti–NMDA-receptor encephalitis is the most common type of autoimmune encephalitis.14 It frequently affects young women (female-to-male ratio of 4:1; median age, 21 years).14,15 No trigger factor is identified in over half of cases, although in young women it is frequently associated with ovarian teratoma (58%).14 It has been suggested that the presence of certain human leukocyte antigen haplotypes, such as HLA-DRB1*16:02 and HLA-B*07:02, may predispose to anti–NMDA-receptor encephalitis.16 The condition typically manifests with psychiatric symptoms such as delusions, hallucinations, agitation, insomnia, and catatonia. Patients subsequently develop bradyphrenia, language disorders, seizures, dysautonomia, hypoventilation, and movement disorders. In our patient, the initial clinical symptoms were mild but typical. The symptoms of non-paraneoplastic anti–NMDA-receptor encephalitis are frequently milder than those of paraneoplastic cases.15 Brain MRI findings are normal in 70% of cases; however, EEG, brain PET, and CSF analysis yield abnormal results in most patients.14,15 Treatment includes immunotherapy with corticosteroids, immunoglobulins, and/or plasmapheresis, in addition to tumour resection, when applicable. Rituximab and cyclophosphamide are recommended in refractory cases.14 Recurrence is reported in approximately 12% of cases, with the risk being higher in non-paraneoplastic cases and in patients not receiving immunotherapy.15
In conclusion, post–COVID-19 anti–NMDA-receptor encephalitis must be suspected in patients with compatible symptoms and history of SARS-CoV-2 recent infection. The description of several cases of anti–NMDA-receptor encephalitis and other immune-mediated neurological syndromes after SARS-CoV-2 infection suggests a potential association between COVID-19 and some immune-mediated disorders.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThe study has not received any public or private funding.