Altered states of consciousness have traditionally been associated with poor prognosis. At present, clinical differences between these entities are beginning to be established.
MethodOur study included 37 patients diagnosed with vegetative state/unresponsive wakefulness syndrome (UWS) and 43 in a minimally conscious state (MCS) according to the Coma Recovery Scale–Revised (CRS-R). All patients were followed up each month for at least 6 months using the CRS-R. We recorded the time points when vegetative state progressed from ‘persistent’ to ‘permanent’ based on the cut-off points established by the Multi-Society-Task-Force: 12 months in patients with traumatic injury and 3 months in those with non-traumatic injury. A logistic regression model was used to determine the factors potentially predicting which patients will emerge from MCS.
ResultsIn the UWS group, 23 patients emerged from UWS but only 9 emerged from MCS. Of the 43 patients in the MCS group, 26 patients emerged from that state during follow-up. Eight of the 23 patients (34.7%) who emerged from UWS and 17 of the 35 (48.6%) who emerged from MCS recovered after the time points proposed by the Multi-Society-Task-Force. According to the multivariate regression analysis, aetiology (P<.01), chronicity (P=.01), and CRS-R scores at admission (P<.001) correctly predicted emergence from MCS in 77.5% of the cases.
ConclusionsUWS and MCS are different clinical entities in terms of diagnosis and outcomes. Some of the factors traditionally associated with poor prognosis, such as time from injury and likelihood of recovery, should be revaluated.
Los estados alterados de conciencia han sido considerados tradicionalmente como cuadros clínicos de pronóstico infausto. En la actualidad, sabemos que dichos estados engloban distintas entidades clínicas cuyo perfil diferencial empieza a reconocerse.
MétodoSe incluyeron 37 pacientes con el diagnóstico de estado vegetativo o síndrome de vigilia sin respuesta (SVSR) y 43 en estado de mínima conciencia (EMC) de acuerdo con la Coma Recovery Scale-Revised (CRS-R). Todos los pacientes fueron evaluados mensualmente con la CRS-R durante al menos 6meses. Se evaluó el momento de superar cada estado considerando los puntos de corte de «irreversibilidad» (12 meses para los casos de origen traumático y 3para los no traumáticos), tradicionalmente establecidos por la Multi-Society-Task-Force. Se empleó un modelo de regresión logística para determinar las variables predictoras de superar el EMC.
ResultadosUn total de 23 pacientes en SVSR superaron este estado, pero solo 9superaron el EMC. De los 43 pacientes en EMC al ingreso, 26 lograron superarlo. Ocho de los 23 (34,7%) pacientes que superaron el SVSR y 17 de los 35 (48,6%) que superaron el EMC lo hicieron más allá del punto de «irreversibilidad». La etiología (p<0,01), la cronicidad (p=0,01) y la puntuación en la CRS-R (p<0,001) predijeron la salida de EMC en el modelo multivariante con un 77,5% de acierto.
ConclusionesTanto el SVRS como el EMC son entidades clínicamente diferenciadas en términos diagnósticos y pronósticos. Algunos criterios clásicos relacionados con el mal pronóstico de estos estados en términos de tiempo y posibilidades de recuperación deben ser reevaluados.
“Permanent vegetative state” may be one of the diagnoses with the worst prognostic connotations in the sphere of neurology. Although the term “vegetative life” is attributed to Arnaud,1 it was in 1971 that Jennett and Plum generalised the term “vegetative state” (VS) with the clinical definition used today.2 The term “vegetative,” referring to an “organic body capable of growth and development but devoid of sensation and thought,” was selected by the authors from the Oxford English Dictionary definition. The initial clinical description of these cases included the absence of any adaptive response to the external environment, and the absence of any evidence of a functioning mind that is either receiving information or integrating and performing directed behaviours in patients with long periods of alertness.
In 1944, the New England Journal of Medicine published a consensus document on VS. This document, still considered a reference, presented the conclusions of 5 important American medical associations included in the Multi-Society Task Force (MSTF).3,4 The MSTF document listed the criteria established in Jennett and Plum's description, and also proposed adding the prognostic adjective “permanent” for cases of hypoxic-ischaemic, metabolic, or congenital aetiology with clinical symptoms lasting more than 3 months, or of post-traumatic aetiology with symptoms lasting more than 12 months. Despite criticism,5 most medical societies from both sides of the Atlantic eventually adopted the MSTF criteria, ratifying the periods of 3 months (non-traumatic cases) and 12 months (traumatic cases) as critical points for recovering consciousness.6–8
A new critical trend has emerged in the last decade, with an attempt to replace the clinical term VS with others with less negative connotations, such as “unresponsive wakefulness syndrome” (UWS).9 In parallel, the scientific evidence supporting the criteria for irreversibility in these cases has been systematically questioned.9–12 Reasons for this include the following: 1) the description of new clinical entities such as the minimally conscious states (MCS),13 subdivided into MCS− and MCS+ according to the level of verbal expression and comprehension14; 2) the publication of several cases of medium- and long-term recovery12,15–22; and 3) the critical review of methodological aspects including sample size, follow-up periods, and the diagnostic and follow-up assessment criteria used in many of the studies on which the MSTF based their conclusions.9,23
The aim of this study is to assess the clinical progression of a sample of patients with altered levels of consciousness, including the typical range of diagnoses observed in these cases, and specifically focusing on the MSTF's temporal criteria for irreversibility. Our hypothesis is that these patients present a heterogeneous, long-lasting recovery pattern, whose prognosis may be improved by correct clinical characterisation.
Material and methodsOur study included all patients older than 16 attended between January 2013 and January 2016 within a hospital network of 4 centres specialised in the rehabilitation of patients with brain injury and diagnosed with UWS, MCS−, or MCS+ according to their score on the Spanish-language version of the Coma Recovery Scale–Revised (CRS-R)24 at admission. All patients were included in a multidisciplinary rehabilitation programme and were assessed weekly using the CRS-R until they emerged from MCS or completed a minimum of 6 months of follow-up.
Statistical analysisThe clinical and demographic characteristics of the groups of patients in UWS, MCS−, and MCS+ were compared using one-way ANOVA for quantitative variables and chi-square (and Fisher F-test when necessary) for qualitative variables. We determined the percentage of patients who emerged from UWS and MCS after more than 3 and 12 months since diagnosis, according to aetiology. We conducted a longitudinal study and compared the clinical and demographic variables of patients who emerged from MCS during the rehabilitation process (t test and chi-square).
Finally, we used a univariate regression model to assess the prognostic value of clinical variables including neurological status at admission (UWS vs MCS− vs MCS+), CRS-R score at admission, and aetiology (traumatic vs non-traumatic), as well as other demographic variables (age, sex, chronicity, years of schooling), as independent variables, and emergence from UWS as the dependent variable. Variables were included in the multivariate analysis using the forward stepwise method; all variables that obtained P<.1 in the univariate analysis were considered candidate variables.
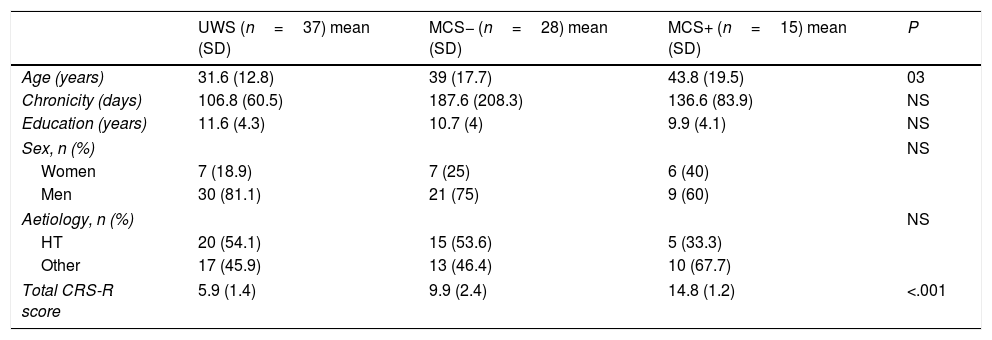
ResultsDescriptive studyA total of 94 patients met our inclusion criteria. Of these, 5 died during the follow-up period and 9 did not fulfil the minimum follow-up of 6 months after admission. The final sample included 80 patients: 20 women (25%), and 60 men (75%) with a mean age (standard deviation) of 37.5 (16.5) years (range, 16-83). Chronicity, defined as the time elapsed between the appearance of the lesion and the onset of treatment at our service, was 136.4 (128.5) days. Regarding aetiology, 40 patients (50%) presented brain injury secondary to head trauma, 23 (28.7%) a brain haemorrhage, 15 (18.9%) anoxia, and 2 patients (2.5%) brain ischaemia. According to the CRS-R score at admission, 37 patients presented UWS, 28 MCS−, and 15 MCS+. There were no significant differences in the demographic variables, with the exception that patients with UWS were younger than patients with MCS+ (P<.05). Logically, given that the CRS-R was used to establish the diagnosis between groups, scores on the scale showed significant differences between groups (Table 1).
Comparison between the 3 groups of patients included in the study.
| UWS (n=37) mean (SD) | MCS− (n=28) mean (SD) | MCS+ (n=15) mean (SD) | P | |
|---|---|---|---|---|
| Age (years) | 31.6 (12.8) | 39 (17.7) | 43.8 (19.5) | 03 |
| Chronicity (days) | 106.8 (60.5) | 187.6 (208.3) | 136.6 (83.9) | NS |
| Education (years) | 11.6 (4.3) | 10.7 (4) | 9.9 (4.1) | NS |
| Sex, n (%) | NS | |||
| Women | 7 (18.9) | 7 (25) | 6 (40) | |
| Men | 30 (81.1) | 21 (75) | 9 (60) | |
| Aetiology, n (%) | NS | |||
| HT | 20 (54.1) | 15 (53.6) | 5 (33.3) | |
| Other | 17 (45.9) | 13 (46.4) | 10 (67.7) | |
| Total CRS-R score | 5.9 (1.4) | 9.9 (2.4) | 14.8 (1.2) | <.001 |
CRS-R: Coma Recovery Scale–Revised; HT: head trauma; MCS: minimally conscious states; SD: standard deviation; UWS: unresponsive wakefulness syndrome.
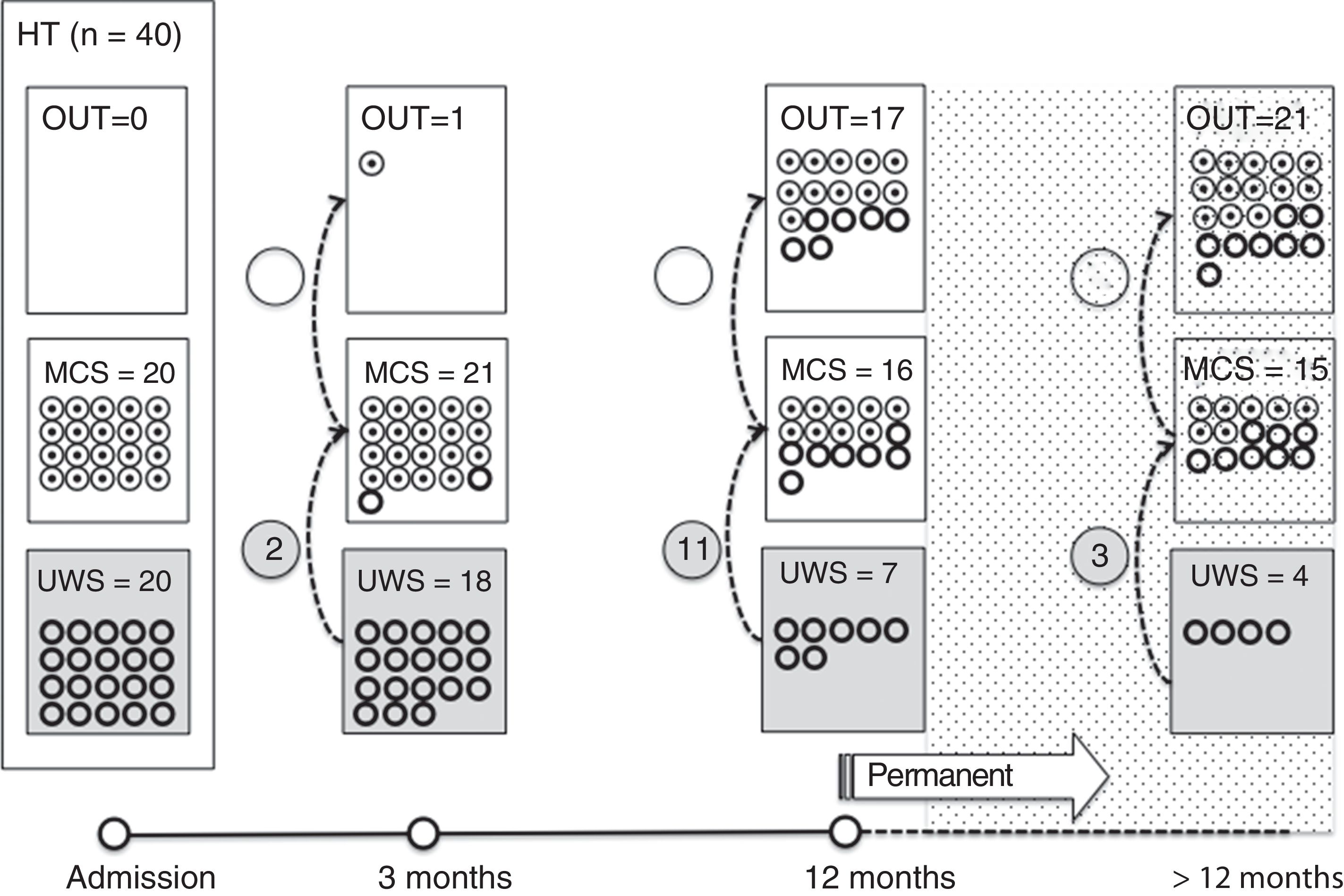
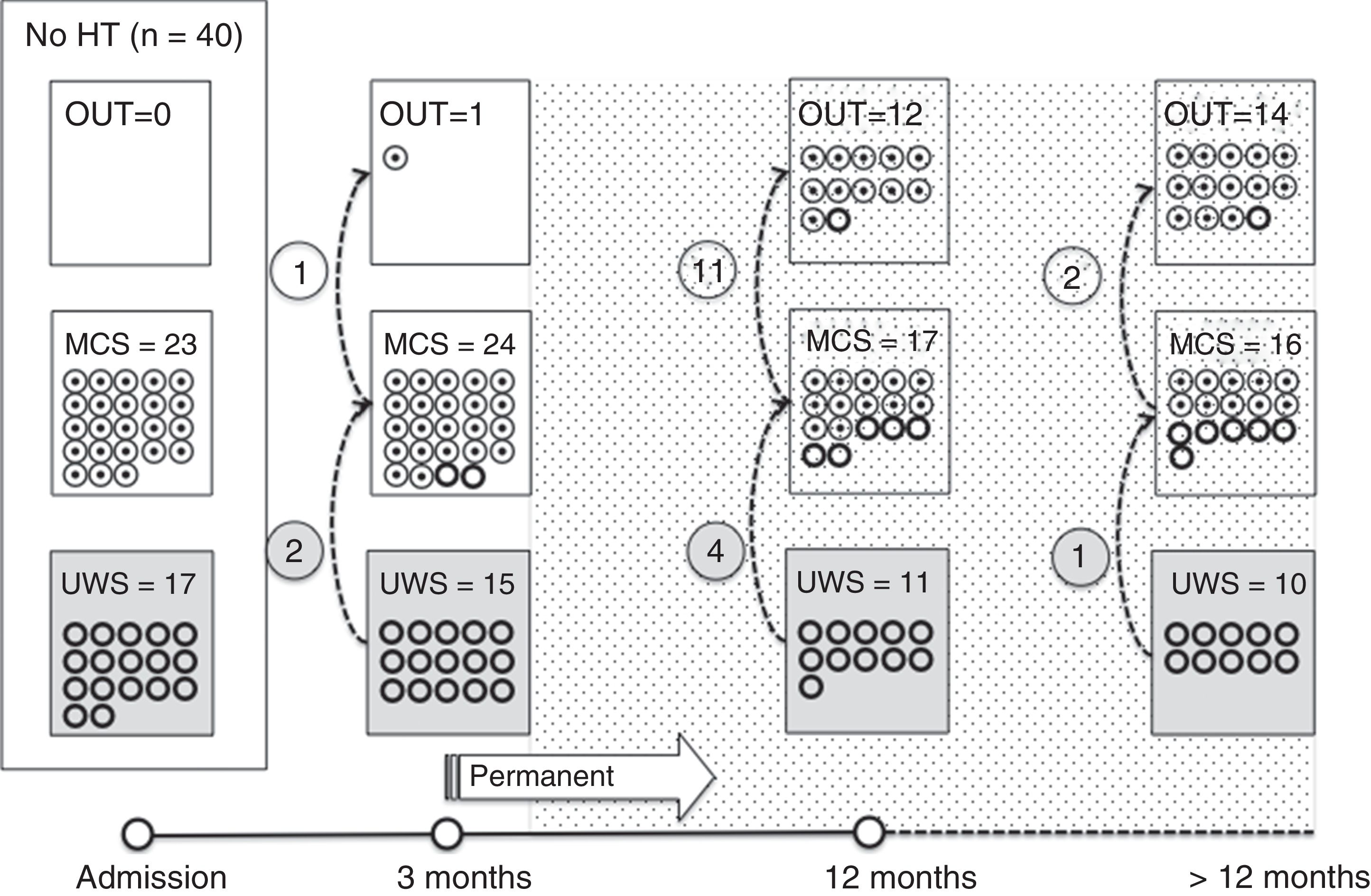
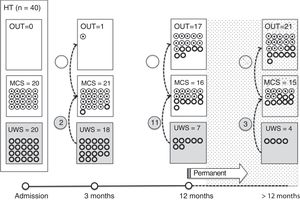
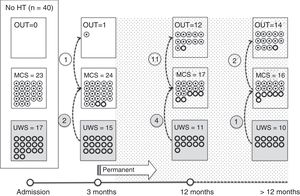
Of the 37 patients with UWS at admission, 23 (62.2%) emerged from this state in a mean of 190.6 (123.2) days after the accident and 70.4 (75) days after admission to our service. Sixteen of these 23 patients (69.9%) had suffered head trauma. Of these 16, 13 emerged from UWS in the 12 months following trauma, with 3 emerging after the critical period was over (Fig. 1). Of the 23 patients who emerged from UWS, 7 had non-traumatic damage; only 2 of this group emerged in the critical 3-month period after the lesion (Fig. 2). A total of 8 patients (34.7%) emerged from UWS beyond the cut-off point considered permanent in the MSTF criteria (Figs. 1 and 2).
Of the patients with MCS, 35 from the initial sample recovered from this state; specifically, 9 of the 37 (24.3%) patients with UWS at onset, 14 of the 28 (50%) with MCS− at admission, and 12 of the 15 patients (80%) with MCS+ at admission emerged from MCS a mean of 247.8 (162.3) days after the accident and 142.5 (139.4) days after admission. Of these 35 patients, 21 (60%) had suffered head trauma (Fig. 1). Of this group, 17 emerged during the 12 months following the trauma and 4 emerged after the critical period was over. Of the 35 patients who emerged from MCS, 14 (40%) had non-traumatic injuries; only one emerged more than 3 months after the injury, considered a critical period in prognostic terms (Fig. 2). In total, 17 of the 35 patients (48.6%) who emerged from MCS did so beyond the cut-off point considered “permanent” in the MSTF criteria (Figs. 1 and 2).
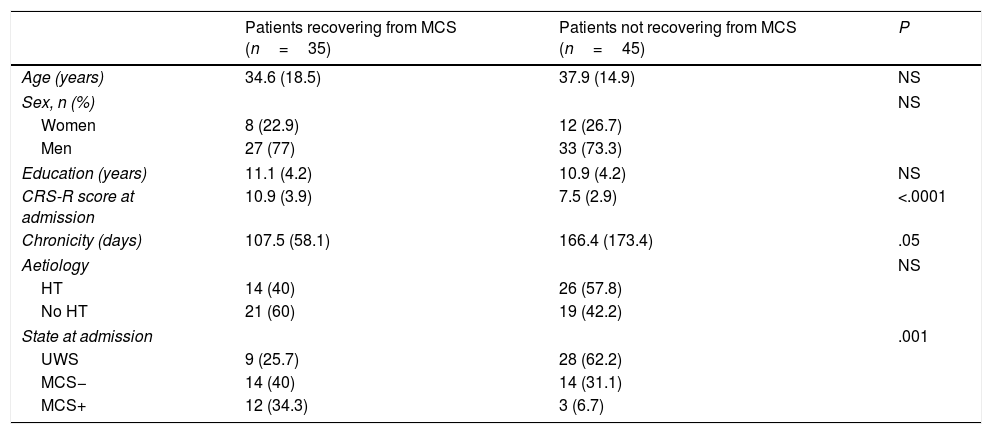
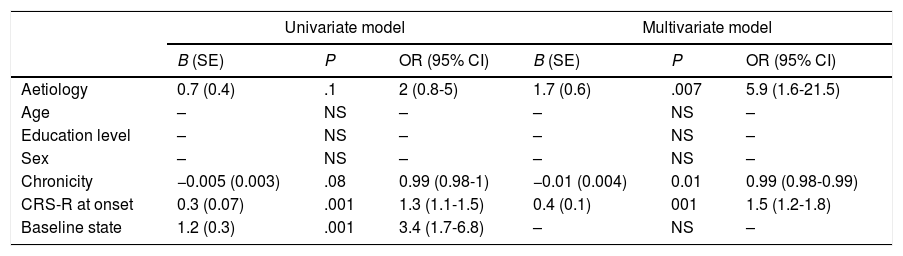
Predictive studyPatients who emerged from MCS presented slightly lower chronicity (P=.058) and higher CRS-R scores at admission (P<.001) than those who did not (Table 2). Similarly, the percentage of patients emerging from MCS was higher among those who presented MCS at admission than those who presented UWS (P<.001). The univariate logistic regression model included chronicity (P=.08), aetiology (traumatic vs non-traumatic) (P=.1), CRS-R score at admission (P<.001), and status at admission (UWS vs MCS− vs MCS+) (P<.001) as significant variables. The multivariate model included the first 3 of these variables, with an overall percentage of correct predictions of 77.5% (Table 3).
Comparisons between the group of patients who emerged from minimally conscious states and those who did not recover consciousness.
| Patients recovering from MCS (n=35) | Patients not recovering from MCS (n=45) | P | |
|---|---|---|---|
| Age (years) | 34.6 (18.5) | 37.9 (14.9) | NS |
| Sex, n (%) | NS | ||
| Women | 8 (22.9) | 12 (26.7) | |
| Men | 27 (77) | 33 (73.3) | |
| Education (years) | 11.1 (4.2) | 10.9 (4.2) | NS |
| CRS-R score at admission | 10.9 (3.9) | 7.5 (2.9) | <.0001 |
| Chronicity (days) | 107.5 (58.1) | 166.4 (173.4) | .05 |
| Aetiology | NS | ||
| HT | 14 (40) | 26 (57.8) | |
| No HT | 21 (60) | 19 (42.2) | |
| State at admission | .001 | ||
| UWS | 9 (25.7) | 28 (62.2) | |
| MCS− | 14 (40) | 14 (31.1) | |
| MCS+ | 12 (34.3) | 3 (6.7) | |
CRS-R: Coma Recovery Scale–Revised; HT: head trauma; MCS: minimally conscious states; NS: not significant;UWS: unresponsive wakefulness syndrome.
Predictive model for emergence from minimally conscious states. Univariate and multivariate logistic regression.
| Univariate model | Multivariate model | |||||
|---|---|---|---|---|---|---|
| B (SE) | P | OR (95% CI) | B (SE) | P | OR (95% CI) | |
| Aetiology | 0.7 (0.4) | .1 | 2 (0.8-5) | 1.7 (0.6) | .007 | 5.9 (1.6-21.5) |
| Age | – | NS | – | – | NS | – |
| Education level | – | NS | – | – | NS | – |
| Sex | – | NS | – | – | NS | – |
| Chronicity | −0.005 (0.003) | .08 | 0.99 (0.98-1) | −0.01 (0.004) | 0.01 | 0.99 (0.98-0.99) |
| CRS-R at onset | 0.3 (0.07) | .001 | 1.3 (1.1-1.5) | 0.4 (0.1) | 001 | 1.5 (1.2-1.8) |
| Baseline state | 1.2 (0.3) | .001 | 3.4 (1.7-6.8) | – | NS | – |
CI: confidence interval; CRS-R: Coma Recovery Scale–Revised; NS: not significant; OR: odds ratio; SE: standard error.
Our results contradict the typical negative prognosis attributed to patients in altered states of consciousness in terms of time and likelihood of recovery.3,4 Considering the total sample, almost 2 out of 3 patients admitted with UWS recovered consciousness. Furthermore, almost half of the patients included in our study emerged from MCS during the follow-up period. Our data also show that the classical temporal criteria of 3 and 12 months, considered critical periods to regard these states as “permanent,” should be interpreted with extreme caution. In fact, one-third of patients with UWS at admission in our sample emerged from this state after these periods. With regard to this, data on patients with UWS of non-traumatic origin were particularly interesting. Analysis of our results revealed that up to 77% of these patients emerged from this state beyond the traditional 3-month period established by the MSTF as the cut-off point for irreversibility.
The late recovery of this type of patients, as observed in this study, is not exceptional. In fact, one of the main criticisms of the MSTF conclusions in terms of time is that the recommendations are based solely on the analysis of 5 verified cases of long-term recovery.20 For example, a recent review of clinical cases published up to 2012, with much stricter selection criteria than those established by the MSTF, detected more than 15 cases of late recovery, even years after the event causing the injury.20 In addition to isolated reports of patients with late recovery,12,15,16,20,22 published data are available from long-term longitudinal studies of samples of patients with UWS. Most of these studies report late recovery in up to 74% of patients, although these percentages are variable.17,21,25 Our results support this concept of “late recovery” and other authors’ criticism of the temporal cut-off points proposed by the MSTF and universally accepted as one of the most important prognostic indicators in this population. This fact is especially relevant if we consider that establishing the prognosis of these patients based exclusively on temporal criteria may lead to treatment being restricted, which may further limit the already difficult recovery of these patients.
According to our results, and in line with previous studies, the group of patients with MCS in our sample presented a higher probability of recovery from this state than those with UWS at admission.21,24–26 It is unanimously accepted that in comparison with patients with UWS, patients with MCS present a longer and more favourable recovery window. Almost half of the patients who emerged from MCS did so beyond the cut-off point considered “permanent” in the MSTF criteria, in accordance with aetiology. To date, very few longitudinal studies include patients with MCS, probably because this diagnosis is such a new concept, as well as the well-known difficulties with recruitment and follow-up of these patients. In follow-up studies of over 12 months, the percentage of patients emerging from this state ranges from 33% to 89%12,21,24–26; these figures are higher than those described in patients with UWS. In light of the findings described above, it seems clear that accurate diagnosis is crucial to establishing a correct prognosis. Today, standardised tools such as the CRS-R enable the correct clinical characterisation of these states, minimising the percentage of diagnostic error.24 Our data supports this: both initial diagnosis and CRS-R score were predictors with a strong and significant prognostic value, displacing even traditional predictive variables such as age. In fact, age, aetiology, and chronicity are the 3 variables that have traditionally been conferred a higher prognostic value in these states, with younger patients with lesions of traumatic origin and less chronicity being considered to present the best prognosis in terms of recovery of consciousness. The limited number of studies conducted in patients with MCS seem to confirm the value of these 3 variables.21,26 The relative homogeneity of our sample in terms of age (50% of the sample was between 25 and 50 years old), and the fact that the percentage of patients older than 50 among patients who had suffered brain damage of traumatic vs non-traumatic origin was 0% vs 40%, may explain aetiology displacing age in the final predictive model.
Our results should be interpreted in the clinical context of our study. All patients in our sample were part of a special programme that includes hospital care and prevention of complications, associated with a multidisciplinary work programme mainly based around physiotherapy and multisensory stimulation for a minimum of 2hours per day. Recently, the question of how to address chronicity care has given rise to an ethical, social, and financial debate on the healthcare limits and resources that should be dedicated to these patients.27 This debate is even more necessary in a population in which diagnostic errors are frequent, medical complications are common, clinical changes are slow, and therapeutic resources are frequently costly and limited.28
In summary, it seems clear that among the clinical entities included in the diagnostic spectrum of altered states of consciousness, UWS and MCS are clinically distinct entities in diagnostic and prognostic terms. Beyond temporal criteria, the aetiology associated with a correct clinical characterisation of these states is now one of the strongest predictors of progression. Therefore, some of the criteria classically associated with poor prognosis of these states, particularly those related to chronicity, should be reevaluated. Our study group is currently focusing on the description of new biomarkers that, together with the already known clinical variables, may help us improve current diagnostic models and increase the accuracy of the predictive models, and may in the long term enable us to stratify and personalise therapeutic strategies. Until then, we recommend replacing the term VS with UWS due to the former's negative connotations, as well as mentioning the aetiology and the date of the event leading to the injury, and avoiding as far as possible making prognostic clinical judgements in terms of “permanence” or “irreversibility.”
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Noé E, Olaya J, Colomer C, Moliner B, Ugart P, Rodriguez C, et al. Validez actual del diagnóstico de «estado vegetativo permanente»: estudio longitudinal en una muestra clínica de pacientes en estados alterados de conciencia. Neurología. 2019;34:589–595.
Part of this study was presented as an oral communication at the 33rd Annual Meeting of the Valencian Society of Neurology (February 2016) and the 14th Congress of the Spanish Society of Neurorehabilitation (November 2016).