Designs for determining nociceptive response in rodents are of great use in neurology and experimental neuroscience. Immersing mice's tails in warm water is one of the most widely used procedures to evaluate this response; however, a wide range of temperatures are used in different studies. Knowing the temperature that produces a powerful nociceptive response in the tail of BALB/c mice is extremely useful.
MethodsEight 2-month-old male BALB/c mice were used. A 14-cm high beaker was filled with water up to 13cm. The animals’ tails were immersed in the container with a starting temperature of 36°C. The water temperature was raised in 1°C increments until we identified the temperatures that produced nociceptive responses. That response was determined by counting the time taken before the mouse shook its tail to remove it from the water.
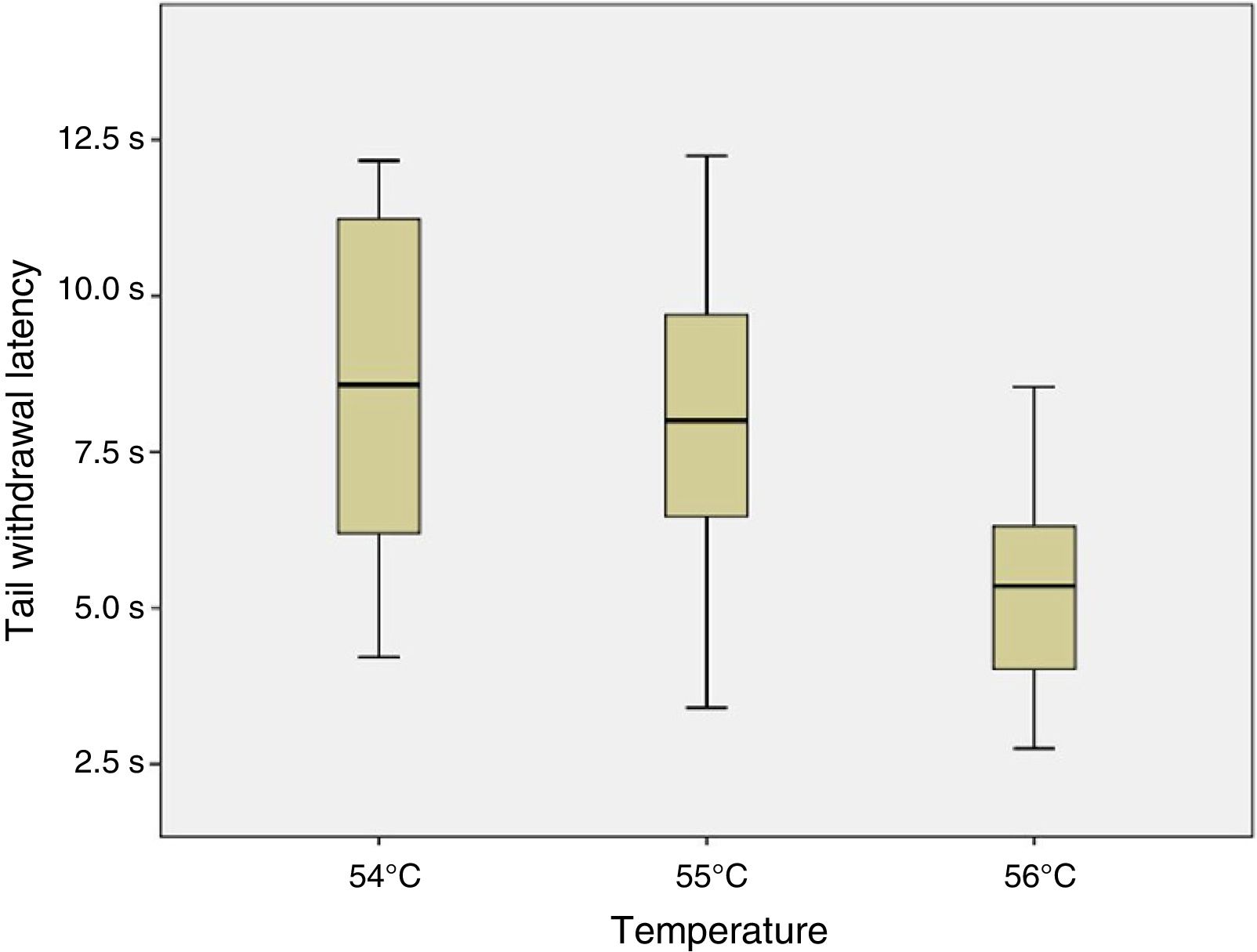
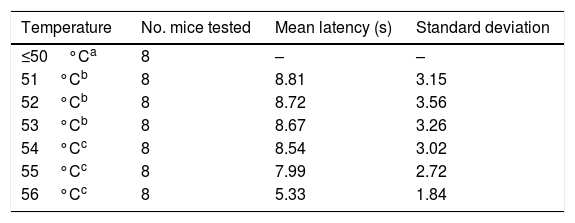
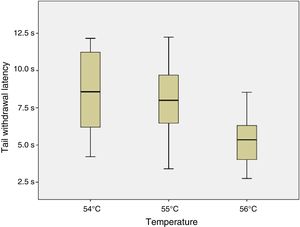
ResultsSix of the 8 mice began shaking their tails at the temperature of 51°C. All animals removed their tails from the water at the temperatures of 54°C, 55°C, and 56°C, taking a mean time of 8.54, 7.99, and 5.33seconds, respectively. ANOVA applied to the response times for each of the 3 temperatures indicated revealed a value of F=2.8 (P=.123).
ConclusionsThe response time was statistically similar for the temperatures of 54°C, 55°C, and 56°C; however, the data were less dispersed for the latter temperature.
Los diseños para determinar la respuesta nociceptiva en roedores son de gran utilidad en neurología y en neurociencias experimentales. El paradigma de inmersión de la cola de ratón en agua temperada es uno de los más empleados para evaluar dicha respuesta; sin embargo, existe amplia variación en la temperatura utilizada en las diversas investigaciones. Resulta sumamente útil determinar la temperatura que produce una mejor respuesta nociceptiva sobre la cola de ratones de la cepa Balb/c.
MétodosSe emplearon 8 ratones machos Balb/c de 2 meses de edad. Un beaker de 14cm de alto se llenó de agua hasta 13cm. Partiendo desde los 36°C se empezó a sumergir la cola del animal dentro del recipiente. Se comenzó a elevar en 1°C el agua hasta encontrar las temperaturas que produzcan las respuestas nociceptivas. Dicha respuesta se determinó contabilizando el tiempo que el ratón tardó en sacudir su cola retirándola del agua.
ResultadosLos ratones empezaron a sacudir su cola a los 51°C (6 de los 8 roedores). El total de la muestra retiró su cola del agua a los 54, 55 y 56°C en el tiempo promedio de 8,54, 7,99 y 5,33s, respectivamente. Al aplicar ANOVA a los tiempos de las 3 temperaturas señaladas se obtuvo el valor F=2,8 y p=0,123.
ConclusionesEl tiempo de respuesta fue similar estadísticamente ante las temperaturas de 54, 55 y 56°C; sin embargo se encontró menor dispersión de los datos ante esta última.
Pain is a highly subjective, multifaceted experience influenced by psychological and emotional factors.1 Rodent experimental models of nociception are pivotal in various areas of the health sciences.2–4 Pain research is particularly relevant in biomedicine5–8 since pain is a frequent manifestation of a wide range of diseases.9–11
The latency of tail withdrawal from a thermal stimulus in mice is one of the most widely used paradigms in experimental research. Several sources of thermal stimulation have been used, including a light beam or hot water.5,12,13 Given the wide range of temperatures used in the tail immersion test,14–19 our study aimed to determine the optimal temperature for triggering a nociceptive response in the tails of male albino BALB/c mice.
Material and methodsAnimalsWe used 8 male albino BALB/c mice (body weight, 30-35g); animals were obtained from the laboratory animal facilities of the Peruvian National Institute of Health (Chorrillos, Lima province, Peru). The animals were housed in the Universidad Nacional Mayor de San Marcos medical school's vivarium, where they were allowed to adapt for one week. Food and water were available ad libitum, the light/dark cycle was 12:12, and humidity and temperature (22±2°C) were kept within the parameters stipulated in the Guide for the Care and Use of Laboratory Animals.20
ProcedureThe protocol was approved by the Research Ethics Committee of the Faculty of Medicine of Universidad Nacional Mayor de San Marcos. All evaluations were conducted in a single day, between 08:00 and 12:00, using an Isolab® beaker (14cm high×11cm diameter) and a Mastrad® digital thermometer (Fig. 1). The beaker was filled with water at a temperature of 36°C up to a depth of 13cm; the water was heated with a Thomas® electric boiler. Starting at 36°C, water was heated in 1°C increments until mice responded to the thermal stimulus; the experiment was repeated once with each animal. During each trial, we immersed two-thirds of the tail into the water until the animal withdrew its tail with a sudden flick (Figs. 2 and 3). The maximum immersion time was 15 seconds; animals not withdrawing their tails within that time period were considered not to have responded to the thermal stimulus. All trials were filmed with a Sony® CX330 video camera and stored on a microSD® card. Video footage was subsequently played on a Lenovo® Yoga 2 laptop and tail withdrawal latencies for each trial were determined using a digital stopwatch.
Data analysisWe performed a statistical analysis with measures of central tendency (mean and standard deviation). For inferential data analysis, we used repeated-measures analysis of variance (ANOVA) with the SPSS® statistical software, version 23 for Windows®.
ResultsTrials started with water at 36°C. Some animals responded to the nociceptive stimulus at 51°C; however, it was not until 54°C that all animals withdrew their tails from the water (Table 1).
Given that data displayed a normal distribution (Shapiro–Wilk test: 54°C, P=.296; 55°C, P=.947; and 56°C, P=.892) and homoscedasticity, we applied a one-factor repeated-measures ANOVA analysis to response times at 54°C, 55°C, and 56°C. As shown in Fig. 4, no significant differences were observed between tail withdrawal latencies for the 3 temperatures (F=2.8; P=.123).
DiscussionOur study used 8 male albino BALB/c mice from the same litter. All animals responded to the nociceptive stimulus at temperatures 54°C, 55°C, and 56°C.
Several experimental studies in mice report nociceptive responses at temperatures ranging from 48°C to 55°C.15,16,19,21 In our study, response to nociceptive stimulation was observed at temperatures 51°C and higher; this is higher than the temperature used by de Prá et al.12 and Bhalla et al.15 These researchers evaluated nociceptive response to water at 48°C, although they used genetically modified mice. In contrast, Desroches et al.16 and Brown et al.17 tested nociceptive responses to water at 52°C in genetically modified animals. The temperatures tested vary greatly between studies.
Using non–genetically modified animals, Melo et al.19 tested tail withdrawal latency at 51°C in female mice; in our study, the same temperature caused a response in some of our animals (6 of the 8 mice withdrew their tails from the water). Yemitan and Adeyemi14 tested nociceptive responses to water at 55°C in male and female adult mice; Griseb et al.13 and García et al.21 used the same temperature for the tail immersion test, although they used male mice only.
As no statistically significant differences were observed between the 3 temperatures analysed, we conclude that they all may yield similar results. However, data were less dispersed for 56°C (standard deviation of 1.84, vs 2.72 for 55°C and 3.02 for 54°C), which may be relevant when selecting the temperature to be used for an experiment.
FundingThis study was funded by the research vice-chancellorship at Universidad Nacional Mayor de San Marcos.
Conflicts of interestThe authors have no conflicts of interest to declare with regard to this study.
Please cite this article as: Aguirre Siancas EE, Lam Figueroa NM, Delgado Rios JC, Ruiz Ramirez E, Portilla Flores OS, Crispín Huamaní LJ, et al. Determinación de la temperatura de respuesta nociceptiva sobre la cola de ratones albinos de la cepa Balb/c. Neurología. 2021;36:584–588.