Haemorrhagic transformation is a major complication of acute ischaemic stroke (AIS). We sought to determine the predictors and clinical impact of intracranial haemorrhage (ICH) after revascularisation therapy.
MethodsWe conducted a retrospective, single-centre study including 235 patients with AIS who underwent intravenous recombinant tissue plasminogen activator (IV-rtPA) therapy and/or endovascular treatment. A binary logistic regression model was used to determine the variables associated with ICH, parenchymal haematomas (PH), modified Rankin Scale (mRS) scores, and mortality.
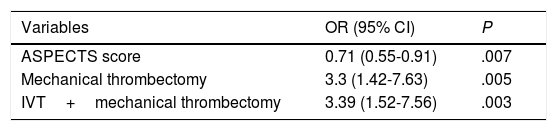
ResultsICH was detected in 57 (30 with PH) of 183 patients included. Mechanical thrombectomy, either alone (OR 3.3 [1.42-7.63], P=.005) or in combination with IV-rtPA (OR 3.39 [1.52-7.56], P=.003), was associated with higher risk of ICH, while higher Alberta Stroke Program Early CT scores (OR 0.71 [0.55-0.91], P=.007) were associated with lower risk. Patients with older age (OR 1.07 [1.02-1.13], P=.006) and occlusion of the terminal branch of the internal carotid artery (OR 4.03 [1.35-11.99], P=.012) had a higher risk of PH, while the use of IV-rtPA alone (OR 0.24 [0.08-0.68], P=.008) was associated with lower risk of PH. Only PH was associated with disability as measured by the mRS (OR 3.2 [1.17-8.76], P=.02) and higher mortality (OR 5.06 [1.65-15.5], P=.005).
ConclusionsGreater understanding about the predictors of ICH, mRS scores, and mortality could enable better selection of patients and treatments.
La transformación hemorrágica es una complicación importante del ictus isquémico agudo (IIA). El propósito del trabajo es analizar el impacto clínico y los factores predictores de las hemorragias intracraneales (HIC) tras terapia revascularizadora.
MétodosAnálisis retrospectivo monocéntrico de 235 pacientes con IIA tratados mediante trombólisis intravenosa (TIV) o tratamiento endovascular (TE). Se ha realizado un modelo de regresión logística binaria para determinar los factores asociados con las HIC, las hemorragias parenquimatosas (HP), la escala mRS y la mortalidad.
ResultadosDe los 183 pacientes incluidos, 57 tuvieron HIC (30 HP). El TE mecánico (OR 3,3 [1,42-7,63], p=0,005) y la TIV junto con TE mecánico (OR 3,39 [1,52-7,56], p=0,003) se han asociado a mayor riesgo de HIC, mientras que valores altos de ASPECTS (OR 0,71 [0,55-0,91], p=0,007) se han asociado a menor riesgo. Mayor edad (OR 1,07 [1,02-1,13], p=0,006) y la oclusión de la carótida interna terminal (OR 4,03 [1,35-11,99], p=0,012) han sido factores predictores de HP, mientras que haber recibido TIV exclusivamente (OR 0,24 [0,08-0,68], p=0,008) se ha asociado con menor riesgo. Solo las HP se han asociado a valores invalidantes de mRS (OR=3,2 [1,17-8,76], p=0,02) y mayor mortalidad (OR 5,06 [1,65-15,5], p=0,005).
ConclusionesUna mejor comprensión de los factores predictores de HIC, mRS y mortalidad puede permitir una mejor selección de pacientes y tratamientos.
Cerebrovascular accidents constitute the second leading cause of death worldwide, with 6.24 million deaths in 2015.1 The European Stroke Association and the American Heart Association/American Stroke Association recommend administering intravenous thrombolysis (IVT) within 3hours of ischaemic stroke onset, with the highest level of evidence,2,3 and within 4.5hours in selected cases.4 Mechanical thrombectomy should be performed within 6hours of symptom onset in patients with anterior circulation stroke secondary to large-vessel occlusion, and can be combined with IVT within 4.5hours of stroke onset in eligible patients.5 Mechanical thrombectomy achieves high rates of recanalisation and functional independence in patients with posterior circulation stroke. Intracranial haemorrhages (ICH) constitute one of the most severe hyperacute complications of revascularisation6; fear of this complication is one of the main reasons that patients refuse this treatment, leading to poorer outcomes.7 The incidence of ICH varies considerably between studies (2%-30%) due to variability in diagnostic criteria.8,9
In view of the great impact of haemorrhagic transformation on prognosis, and the lack of consensus on its definition, this study aimed to analyse ICH incidence and prognosis in patients with acute ischaemic stroke treated with IVT or with mechanical thrombectomy or pharmacological thrombolysis in our centre, and to determine whether the variables available at the time of revascularisation can predict haemorrhagic transformation.
Patients and methodsWe performed a retrospective observational study with a sample of 235 consecutive patients attended between 1 January 2014 and 30 September 2016.
The patients included were aged 18 years or older and had history of acute ischaemic stroke treated with revascularisation therapy according to the acute stroke management protocol of Hospital Universitario Reina Sofía in Córdoba, Spain.10 We excluded 34 patients with posterior circulation stroke, 17 patients with artery dissections, and one who presented stroke due to septic emboli, in order to obtain a more homogeneous sample comparable to those used in other published series. We opted to exclude patients with posterior circulation stroke due to the differences with carotid artery or middle cerebral artery (MCA) strokes in terms of management. All patients underwent head CT studies within 22-36hours of treatment, or earlier in case of neurological deterioration.4 The final sample included 183 patients.
We gathered data on demographic variables (age and sex) and cerebrovascular risk factors (diabetes mellitus, arterial hypertension, atrial fibrillation, hyperlipidaemia, history of coronary artery disease, history of stroke, peripheral vascular disease, use of anticoagulants or antiplatelets, and alcohol/tobacco use). The National Institutes of Health Stroke Scale (NIHSS) was used to determine each patient's neurological status upon arrival at the emergency department; the scale was administered by a neurologist or an emergency department specialist.4 A radiologist with over 15 years’ experience in neuroradiology determined the Alberta Stroke Program Early CT score (ASPECTS) using the non-contrast head CT images11 obtained at baseline and identified the location of the thrombus using CT angiography of the supra-aortic trunks and intracranial arteries. CT angiography results were classified as follows: no occlusion, thrombus in the extracranial segment of the internal carotid artery (ICA), in the terminal branch of the ICA, in the M1 segment of the MCA, in the M2 segment of the MCA, in the M3 segment of the MCA, or tandem occlusion. We also gathered data on the time elapsed from symptom onset to treatment. The treatment or procedure was classified as follows: IVT (rtPA dosed at 0.9mg/kg, to a maximum of 90mg), mechanical thrombectomy, pharmacological thrombolysis (intra-arterial urokinase, initial bolus of 250000IU in 10minutes, followed by 1000IU/min, to a maximum of 1000000IU), mechanical thrombectomy plus pharmacological thrombolysis, IVT plus mechanical thrombectomy, IVT plus pharmacological thrombolysis, IVT plus mechanical thrombectomy and pharmacological thrombolysis (for the latter 2 treatment combinations, maximum urokinase dose was 500000IU), IVT plus angiography without endovascular treatment, or diagnostic angiography without endovascular treatment. We also evaluated disability at discharge using the modified Rankin Scale (mRS), which was administered by a neurologist; scores ≥ 3 points were considered to indicate dependence.4,12
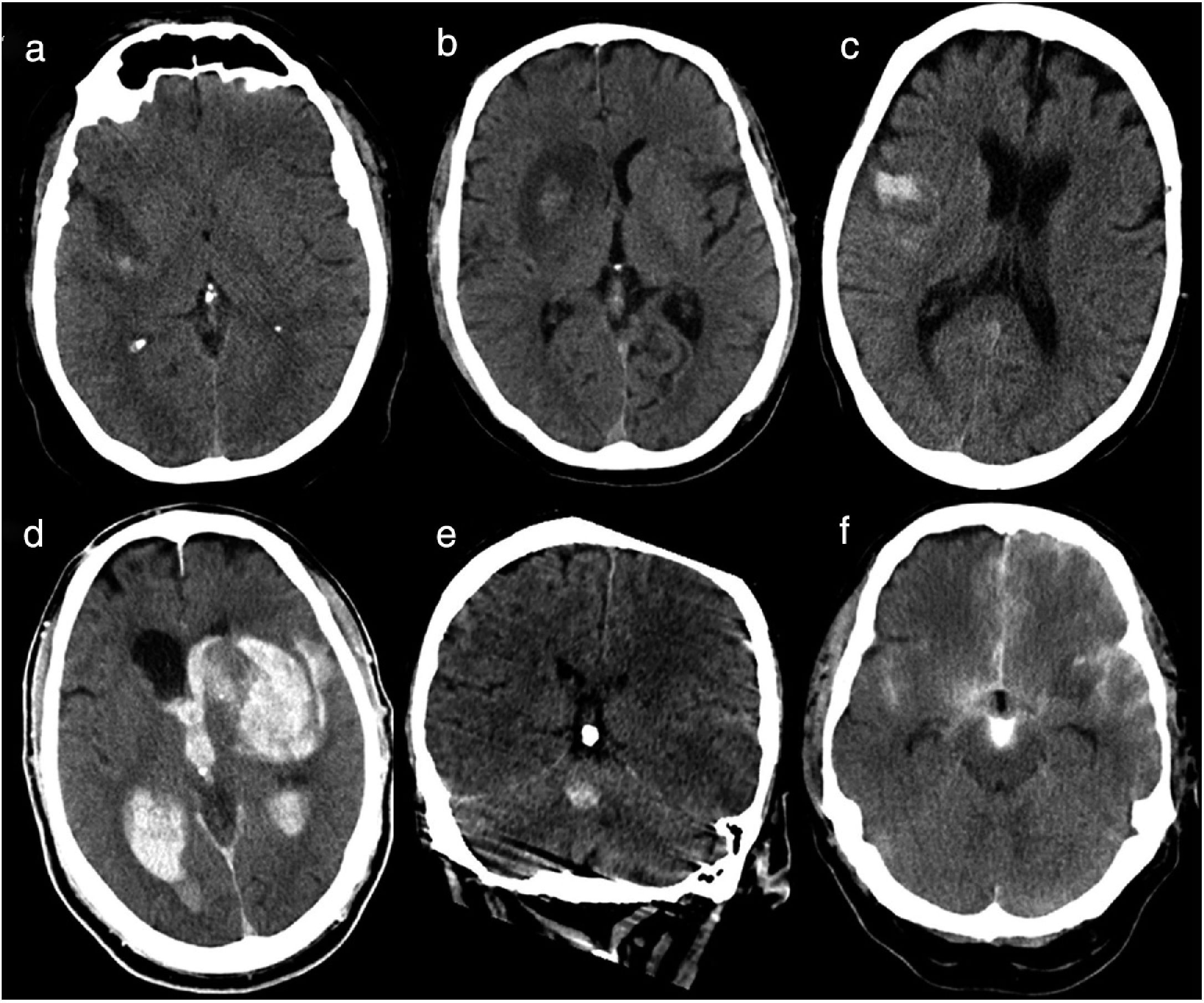
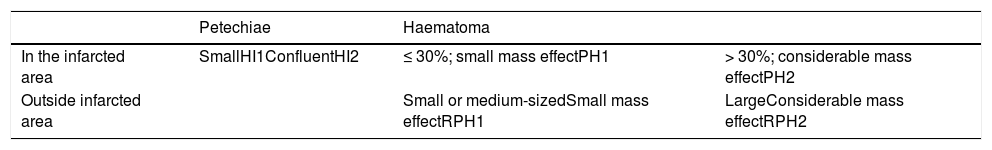
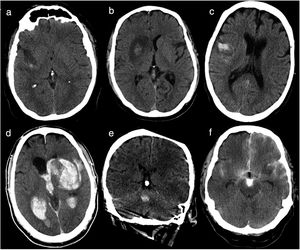
Haemorrhagic transformation was classified according to the classification of ICH proposed by Fiorelli et al.13 (Table 1, Fig. 1): haemorrhagic infarction type 1 (HI1), haemorrhagic infarction type 2 (HI2), parenchymal haematoma type 1 (PH1), parenchymal haematoma type 2 (PH2), remote parenchymal haematoma type 1 (RPH1), and remote parenchymal haematoma type 2 (RPH2). We also considered patients with subarachnoid haemorrhage (SAH) and those displaying contrast extravasation, defined as a hyperdense area with > 90 Hounsfield units on the follow-up CT scan14; contrast extravasation is the main differential diagnosis for ICH after mechanical thrombectomy.15 Some patients displayed more than one type of haemorrhage; a single patient could therefore be classified under several categories. Follow-up CT images were independently analysed by 2 radiologists with 5 and 3 years’ experience, respectively; disagreements were discussed with a third radiologist with over 15 years’ experience in neuroradiology.
Types of haemorrhages as classified by Fiorelli et al.13 Haemorrhages are classified according to whether they present in the form of petechiae or haematoma, and whether they are located in or away from the infarcted area.
| Petechiae | Haematoma | ||
|---|---|---|---|
| In the infarcted area | SmallHI1ConfluentHI2 | ≤ 30%; small mass effectPH1 | > 30%; considerable mass effectPH2 |
| Outside infarcted area | Small or medium-sizedSmall mass effectRPH1 | LargeConsiderable mass effectRPH2 |
HI1 (haemorrhagic infarction type 1): small petechiae around the infarcted area. HI2 (haemorrhagic infarction type 2): confluent petechiae in the infarcted area, with no mass effect. PH1 (parenchymal haematoma type 1): haematoma covering ≤ 30% of the infarcted area; may present a mild mass effect. PH2 (parenchymal haematoma type 2): haematoma covering > 30% of the infarcted area, with evident mass effect. RPH1 (remote parenchymal haematoma type 1): small or medium-sized parenchymal haematoma not associated with the infarct; may have a mild mass effect. RPH2 (remote parenchymal haematoma type 2): extensive, confluent parenchymal haematoma not associated with the infarct, with evident mass effect.
Baseline head CT scan performed within 36hours of revascularisation. (a) Haemorrhagic infarction type 1: small petechiae around the infarcted area. (b) Haemorrhagic infarction type 2: confluent petechiae in the infarcted area with no mass effect. (c) Parenchymal haematoma type 1: haematoma in ≤ 30% of the infarcted area, with no mass effect. (d) Parenchymal haematoma type 2: parenchymal haematoma open to the ventricular system, observed in > 30% of the infarcted area, with a significant mass effect. (e) Remote parenchymal haematoma type 1: small haemorrhage with no association with the infarcted area (left middle cerebral artery territory). (f) Haemorrhagic infarction type 1+subarachnoid haemorrhage+contrast uptake: small petechiae around the infarcted area, haematoma in the subarachnoid space, and contrast uptake.
To identify the predictors of ICH and PH, we performed a univariate analysis, using the chi-square test and Fisher exact test for qualitative variables and the t test for quantitative variables. The 2 dependent variables (ICH and PH) were modelled using binary logistic regression. To create the regression line, we analysed the independent variables that displayed an association with the dependent variables; these were identified using contingency tables and the chi-square test, with the critical value established at 0.1. Having obtained these variables, we conducted a binary logistic regression analysis, following the enter method, to obtain the regression line. To validate the regression line we calculated the Cox and Snell R2 coefficient, the Nagelkerke R2 coefficient, and the percentage of correct predictions of the model. The latter is the most important value for validation; regression lines with a percentage below 50% should not be used.
We also designed other binary logistic regression models to determine the prognosis and mortality rates associated with ICH and PH.
Statistical analysis was performed using SPSS version 23 for Windows.
ResultsA total of 183 patients met our inclusion criteria. Mean age (standard deviation) was 67 (12) years; 63% of patients were men. Mean NIHSS score upon arrival at the emergency department was 14 (5) points; mean ASPECTS score was 8.4 (1.4) points.
CT angiography revealed no alterations in 22% of patients; thrombi were located in the extracranial segment of the ICA in 12%, in the terminal branch of the ICA in 12%, in MCA segment M1 in 31%, in MCA segment M2 in 8%, and in MCA segment M3 in 2%; and tandem occlusions were found in 14% of patients.
Regarding treatment, 35% of patients received IVT, 23% underwent mechanical thrombectomy, 1% received pharmacological thrombolysis, 3% mechanical thrombectomy plus pharmacological thrombolysis, 27% IVT plus mechanical thrombectomy, 3% IVT plus pharmacological thrombolysis, 4% IVT plus mechanical thrombectomy and pharmacological thrombolysis, 4% received IVT and underwent angiography but no endovascular procedure, and 1% underwent angiography and no endovascular procedure. Mean time from symptom onset to treatment onset was 174.2 (81.8) minutes.
Haemorrhagic transformation was observed in 31.1% of patients; 16.4% were PH (PH2: 14.2%). HI2 was the most common type of haemorrhagic infarction, accounting for 8.7% of cases. Symptomatic ICH, (i.e., ICH accompanied by neurological deterioration [NIHSS≥ 4 points]) was observed in 10.9% of patients, 80% of whom had PH2. Fourteen percent of the patients who received IVT developed ICH (6% with symptomatic ICH), as did 41% of those who underwent endovascular treatment with or without IVT (13% with symptomatic ICH). SAH and contrast extravasation were observed in 3.2% and 1.6% of patients, respectively; both complications were associated with haemorrhagic infarction in all cases.
At discharge, 45.9% of patients were dependent according to the mRS. Sixteen (25%) of the patients who received IVT were discharged with mRS scores indicating dependence, 4 of whom (6%) presented ICH. Sixty-eight (58%) of the patients who underwent endovascular treatment with/without IVT were discharged with mRS scores ≥ 3 points, of whom 33 (28%) presented ICH. The overall mortality rate was 16.9%. Four (6%) of the patients who received IVT died, of whom 2 (3%) presented ICH. Twenty-seven (23%) of the patients who received endovascular treatment with/without IVT died, 16 of whom (14%) presented ICH.
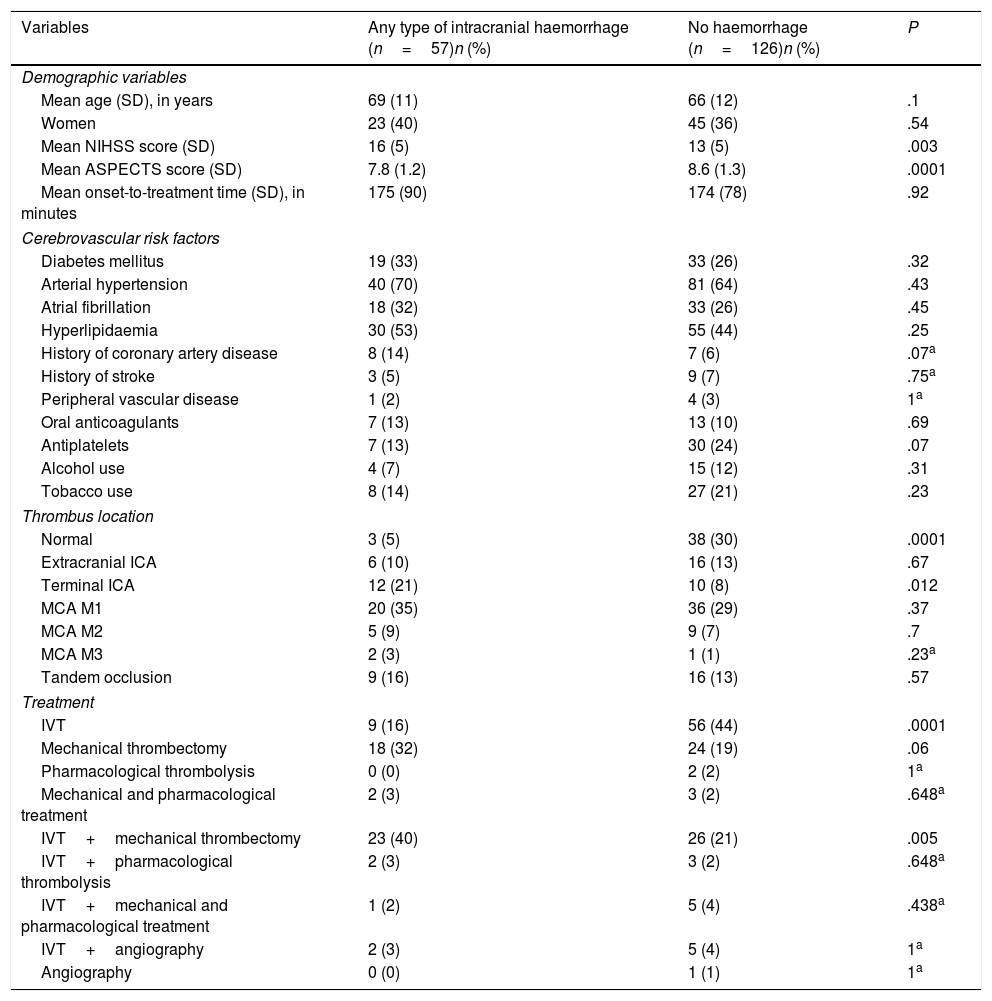
Table 2 shows the univariate analysis of the predictors of any type of ICH. Mechanical thrombectomy (OR=3.3; 95% CI, 1.42-7.63; P=.005) and IVT plus mechanical thrombectomy (OR=3.39; 95% CI, 1.52-7.56; P=.003) were found to be independent predictors of ICH in the binary logistic regression model (Table 3), whereas high ASPECTS scores (OR=0.71; 95% CI, 0.55-0.91; P=.007) were independently associated with lower risk of ICH. The model created to predict ICH has a percentage of correct predictions of 72.4%.
Univariate analysis of predictors of any type of intracranial haemorrhage after revascularisation.
| Variables | Any type of intracranial haemorrhage (n=57)n (%) | No haemorrhage (n=126)n (%) | P |
|---|---|---|---|
| Demographic variables | |||
| Mean age (SD), in years | 69 (11) | 66 (12) | .1 |
| Women | 23 (40) | 45 (36) | .54 |
| Mean NIHSS score (SD) | 16 (5) | 13 (5) | .003 |
| Mean ASPECTS score (SD) | 7.8 (1.2) | 8.6 (1.3) | .0001 |
| Mean onset-to-treatment time (SD), in minutes | 175 (90) | 174 (78) | .92 |
| Cerebrovascular risk factors | |||
| Diabetes mellitus | 19 (33) | 33 (26) | .32 |
| Arterial hypertension | 40 (70) | 81 (64) | .43 |
| Atrial fibrillation | 18 (32) | 33 (26) | .45 |
| Hyperlipidaemia | 30 (53) | 55 (44) | .25 |
| History of coronary artery disease | 8 (14) | 7 (6) | .07a |
| History of stroke | 3 (5) | 9 (7) | .75a |
| Peripheral vascular disease | 1 (2) | 4 (3) | 1a |
| Oral anticoagulants | 7 (13) | 13 (10) | .69 |
| Antiplatelets | 7 (13) | 30 (24) | .07 |
| Alcohol use | 4 (7) | 15 (12) | .31 |
| Tobacco use | 8 (14) | 27 (21) | .23 |
| Thrombus location | |||
| Normal | 3 (5) | 38 (30) | .0001 |
| Extracranial ICA | 6 (10) | 16 (13) | .67 |
| Terminal ICA | 12 (21) | 10 (8) | .012 |
| MCA M1 | 20 (35) | 36 (29) | .37 |
| MCA M2 | 5 (9) | 9 (7) | .7 |
| MCA M3 | 2 (3) | 1 (1) | .23a |
| Tandem occlusion | 9 (16) | 16 (13) | .57 |
| Treatment | |||
| IVT | 9 (16) | 56 (44) | .0001 |
| Mechanical thrombectomy | 18 (32) | 24 (19) | .06 |
| Pharmacological thrombolysis | 0 (0) | 2 (2) | 1a |
| Mechanical and pharmacological treatment | 2 (3) | 3 (2) | .648a |
| IVT+mechanical thrombectomy | 23 (40) | 26 (21) | .005 |
| IVT+pharmacological thrombolysis | 2 (3) | 3 (2) | .648a |
| IVT+mechanical and pharmacological treatment | 1 (2) | 5 (4) | .438a |
| IVT+angiography | 2 (3) | 5 (4) | 1a |
| Angiography | 0 (0) | 1 (1) | 1a |
ASPECTS: Alberta Stroke Program Early CT score; ICA: internal carotid artery; IVT: intravenous thrombolysis; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; SD: standard deviation.
Independent predictors of any type of intracranial haemorrhage after revascularisation.
| Variables | OR (95% CI) | P |
|---|---|---|
| ASPECTS score | 0.71 (0.55-0.91) | .007 |
| Mechanical thrombectomy | 3.3 (1.42-7.63) | .005 |
| IVT+mechanical thrombectomy | 3.39 (1.52-7.56) | .003 |
ASPECTS: Alberta Stroke Program Early CT score; CI: confidence interval; IVT: intravenous thrombolysis; OR: odds ratio.
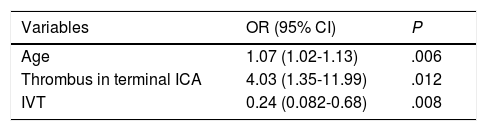
Table 4 shows the results of the univariate analysis of predictors of PH. Older age (OR=1.07; 95% CI, 1.02-1.13; P=.006) and thrombus location in the terminal branch of the ICA (OR=4.03; 95% CI, 1.35-11.99; P=.012) were found to be independent predictors of PH in the binary logistic regression model, whereas treatment with IVT (OR=0.24; 95% CI, 0.08-0.68; P=.008) was independently associated with lower risk of PH (Table 5). The model created to predict PH showed a percentage of correct predictions of 80.1%.
Univariate analysis of predictors of parenchymal haematoma after revascularisation.
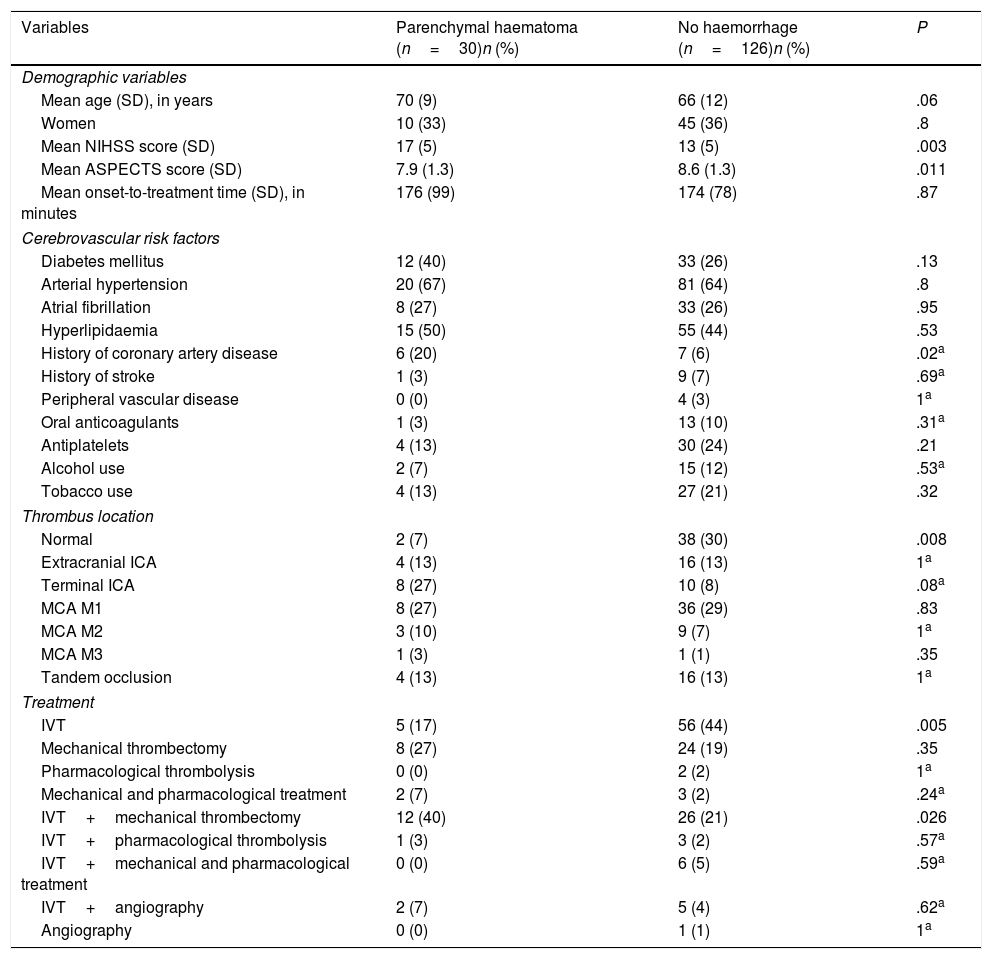
| Variables | Parenchymal haematoma (n=30)n (%) | No haemorrhage (n=126)n (%) | P |
|---|---|---|---|
| Demographic variables | |||
| Mean age (SD), in years | 70 (9) | 66 (12) | .06 |
| Women | 10 (33) | 45 (36) | .8 |
| Mean NIHSS score (SD) | 17 (5) | 13 (5) | .003 |
| Mean ASPECTS score (SD) | 7.9 (1.3) | 8.6 (1.3) | .011 |
| Mean onset-to-treatment time (SD), in minutes | 176 (99) | 174 (78) | .87 |
| Cerebrovascular risk factors | |||
| Diabetes mellitus | 12 (40) | 33 (26) | .13 |
| Arterial hypertension | 20 (67) | 81 (64) | .8 |
| Atrial fibrillation | 8 (27) | 33 (26) | .95 |
| Hyperlipidaemia | 15 (50) | 55 (44) | .53 |
| History of coronary artery disease | 6 (20) | 7 (6) | .02a |
| History of stroke | 1 (3) | 9 (7) | .69a |
| Peripheral vascular disease | 0 (0) | 4 (3) | 1a |
| Oral anticoagulants | 1 (3) | 13 (10) | .31a |
| Antiplatelets | 4 (13) | 30 (24) | .21 |
| Alcohol use | 2 (7) | 15 (12) | .53a |
| Tobacco use | 4 (13) | 27 (21) | .32 |
| Thrombus location | |||
| Normal | 2 (7) | 38 (30) | .008 |
| Extracranial ICA | 4 (13) | 16 (13) | 1a |
| Terminal ICA | 8 (27) | 10 (8) | .08a |
| MCA M1 | 8 (27) | 36 (29) | .83 |
| MCA M2 | 3 (10) | 9 (7) | 1a |
| MCA M3 | 1 (3) | 1 (1) | .35 |
| Tandem occlusion | 4 (13) | 16 (13) | 1a |
| Treatment | |||
| IVT | 5 (17) | 56 (44) | .005 |
| Mechanical thrombectomy | 8 (27) | 24 (19) | .35 |
| Pharmacological thrombolysis | 0 (0) | 2 (2) | 1a |
| Mechanical and pharmacological treatment | 2 (7) | 3 (2) | .24a |
| IVT+mechanical thrombectomy | 12 (40) | 26 (21) | .026 |
| IVT+pharmacological thrombolysis | 1 (3) | 3 (2) | .57a |
| IVT+mechanical and pharmacological treatment | 0 (0) | 6 (5) | .59a |
| IVT+angiography | 2 (7) | 5 (4) | .62a |
| Angiography | 0 (0) | 1 (1) | 1a |
ASPECTS: Alberta Stroke Program Early CT score; ICA: internal carotid artery; IVT: intravenous thrombolysis; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; SD: standard deviation.
Independent predictors of parenchymal haematoma after revascularisation.
| Variables | OR (95% CI) | P |
|---|---|---|
| Age | 1.07 (1.02-1.13) | .006 |
| Thrombus in terminal ICA | 4.03 (1.35-11.99) | .012 |
| IVT | 0.24 (0.082-0.68) | .008 |
CI: confidence interval; ICA: internal carotid artery; IVT: intravenous thrombolysis; OR: odds ratio.
After controlling for all the variables measured, ICH was not associated with mRS scores ≥ 3 at discharge or with higher mortality rates. After adjusting for all the variables measured, PH was found to be associated with mRS scores ≥ 3 (OR=3.2; 95% CI, 1.17-8.76; P=.02) and higher mortality rates (OR=5.06; 95% CI, 1.65-15.5; P=.005).
DiscussionThe studies published in the literature propose different definitions of haemorrhagic transformation and different methodologies for patient selection, which makes it difficult to analyse and extrapolate findings.
Rate of haemorrhagic transformationNo standard definition has been established for ICH after reperfusion therapy, and none of the existing definitions reliably predict mortality or outcomes.16 Furthermore, many patients with severe stroke, and particularly those undergoing endovascular treatment, may require sedation, which makes it difficult to perform a proper neurological examination.12 These 2 factors have motivated the use of a purely radiological concept of haemorrhagic transformation, as in the ECASS I study13 rather than a clinico-radiological definition; according to this interpretation, the condition results in higher rates of ICH but not necessarily poorer prognosis. The rate of haemorrhagic transformation in our sample was 31.1%, or 10.9% if we consider patients with neurological deterioration; these rates are in line with those reported by Seet and Rabinstein,8 who conclude that variations in the reported rates of haemorrhagic transformation may be explained by differences in the definition criteria used. By type of treatment, 14% of patients who received IVT and 41% of those who underwent endovascular treatment with or without IVT developed ICH. These rates are similar to those reported in a recent meta-analysis comparing 1320 patients receiving medical treatment (ICH rate of 19%) against 1499 patients receiving endovascular treatment (ICH rate of 35%).17
Factors associated with haemorrhagic transformationIdentifying patient profiles associated with greater risk of ICH may enable the implementation of measures for early prevention12; some strategies have already been proposed, including close monitoring of arterial blood pressure.18 The main purpose of our study was to identify predictors of ICH and PH.
In our sample, mechanical thrombectomy and IVT prior to mechanical thrombectomy were independent predictors of ICH, whereas high ASPECTS scores were independently associated with lower risk of ICH. In a study of 1122 patients receiving endovascular treatment, diabetes mellitus, treatment with mechanical thrombectomy, and higher baseline NIHSS scores were found to be independent predictors of ICH.12 Our results differ from those of other studies into the predictors of symptomatic ICH after IVT19,20 mainly due to the statistical power of our study and the fact that our sample included patients receiving pharmacological and endovascular treatment. High ASPECTS scores have been associated with lower risk of haemorrhagic transformation; this association was unsurprising, considering that higher ASPECTS scores result in a lower likelihood of complications.21 There is much controversy on whether administering IVT before mechanical thrombectomy increases the risk of ICH22; this factor was found to be the most important predictor of ICH in our model.
Factors associated with parenchymal haematomaSome authors only consider parenchymal haematomas documented by CT to be haemorrhagic transformation9; this explains the importance of analysing patients with PH on an individual basis. In our study, older age and thrombus location in the terminal branch of the ICA were found to be independent predictors of PH, whereas being treated with IVT exclusively was independently associated with lower risk of PH. There is extensive evidence on the variables that increase the risk of PH after IVT (older age, high NIHSS score, high systolic blood pressure, male sex, long onset-to-treatment time, and treatment with aspirin).20,23 However, evidence on the predictors of PH after endovascular treatment is insufficient.12,22 According to Nogueira et al.,12 the independent predictors of PH after endovascular treatment include pharmacological thrombolysis, atrial fibrillation, and high NIHSS scores, whereas Kaesmacher et al.22 report long onset-to-treatment time, lower ASPECTS scores, and wake-up stroke as predictors. Given the wide range of predictors proposed in the literature, new prospective studies should be conducted to analyse the associated factors and predictors of ICH after endovascular treatment.
Prognosis and mortalityAfter controlling for all the variables measured, ICH was not associated with disability (mRS≥3) at discharge or with higher mortality rates; the opposite was true of PH. Our results underscore the clinical importance of PH and support the use of a more restrictive definition of ICH. However, some studies question the “benign” nature of haemorrhagic infarction after both IVT24 and endovascular treatment.12,22 Future research into this area should aim to standardise the definition of ICH, given that it is unclear whether we should opt for a more conservative definition, encompassing haemorrhagic infarction and asymptomatic ICH, or a more restrictive one.
LimitationsOur retrospective observational study has a number of limitations. Firstly, we included patients receiving medical treatment, endovascular treatment, and a combination of both, whereas other studies focus on a single type of treatment. Secondly, including patients receiving medical treatment prevented us from evaluating revascularisation with the modified Thrombolysis In Cerebral Infarction (mTICI) scale, for example.25 Furthermore, there is a risk of selection bias, as some patients treated with endovascular treatment or combination therapy were outside the time window for IVT or showed no improvements after IVT, which may have reflected more severe stroke or poorer prognosis. Despite these limitations, we feel that our sample is large enough and sufficiently representative to satisfy the purposes of our study.
ConclusionsDetermining the predictors and consequences of ICH after revascularisation of acute ischaemic stroke is essential to the early identification of patients at risk of ICH and the implementation of prevention strategies. Our data suggest that ICH is not associated with poorer prognosis or higher mortality rates, unlike in the case of PH. Furthermore, mechanical thrombectomy and IVT prior to mechanical thrombectomy were independent predictors of ICH, whereas high ASPECTS scores were independently associated with lower risk of ICH. Older age and thrombus location in the terminal segment of the ICA were found to be independent predictors of PH, whereas being treated with IVT alone was independently associated with lower risk of PH.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Jurado PB, Roldán Romero E, Pérez Montilla ME, Valverde Moyano R, Bravo Rey IM, Delgado Acosta F, et al. Incidencia, pronóstico y predicción de la transformación hemorrágica tras el tratamiento revascularizador del ictus. Neurología. 2021;36:589–596.