To determine the frequency of good functional outcomes in patients with NORSE and FIRES treated with immunotherapy.
MethodsWe performed a systematic search of the MedLine and EMBASE databases to gather studies including at least 5 patients with NORSE or FIRES and at least one patient treated with immunotherapy, and reporting functional outcomes. Good functional outcome was defined as a modified Rankin Scale (mRS) score ≤ 2 (or an equivalent measure) at the last available follow-up assessment. Only patients with known functional outcomes were included in the analysis.
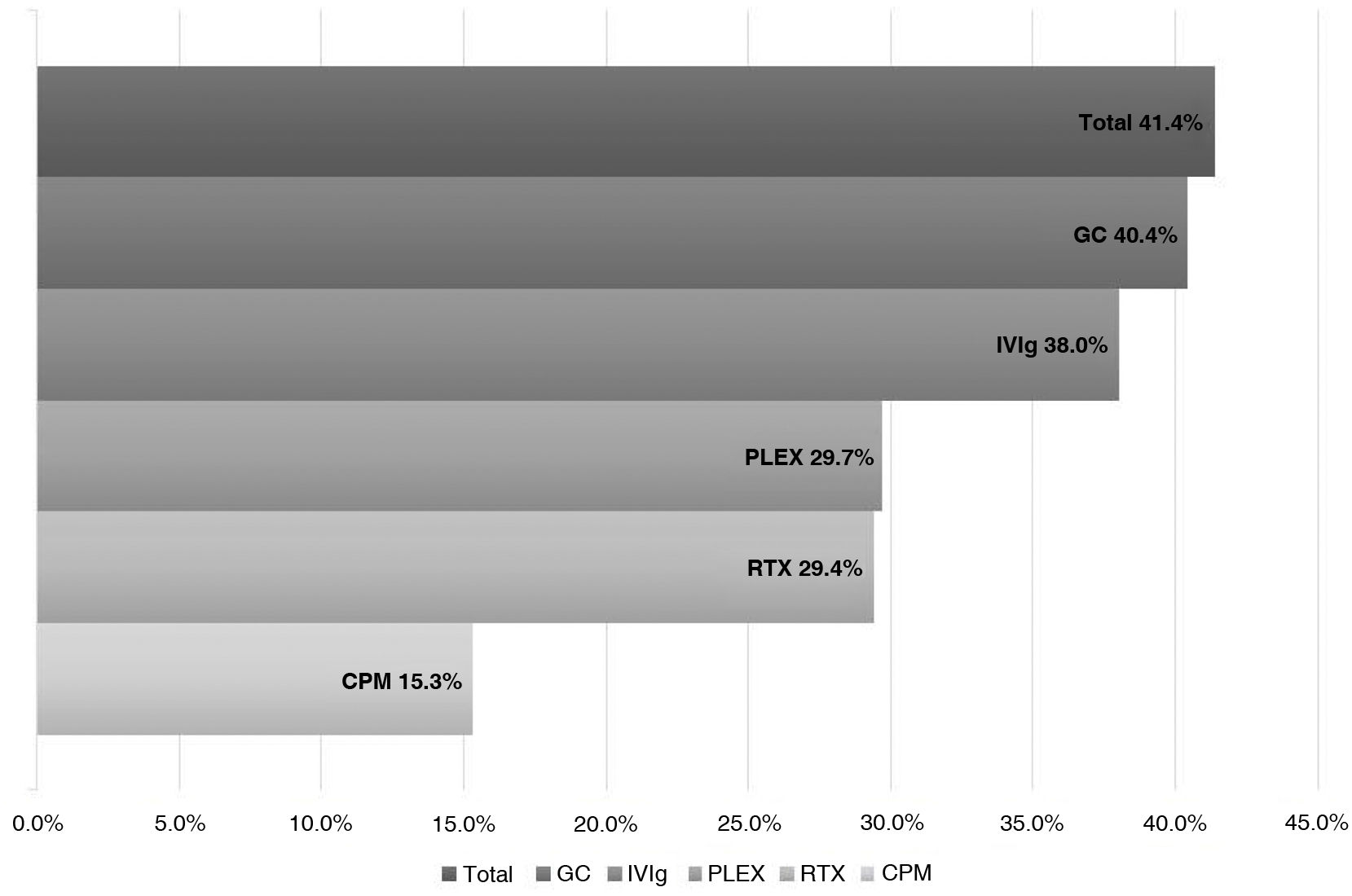
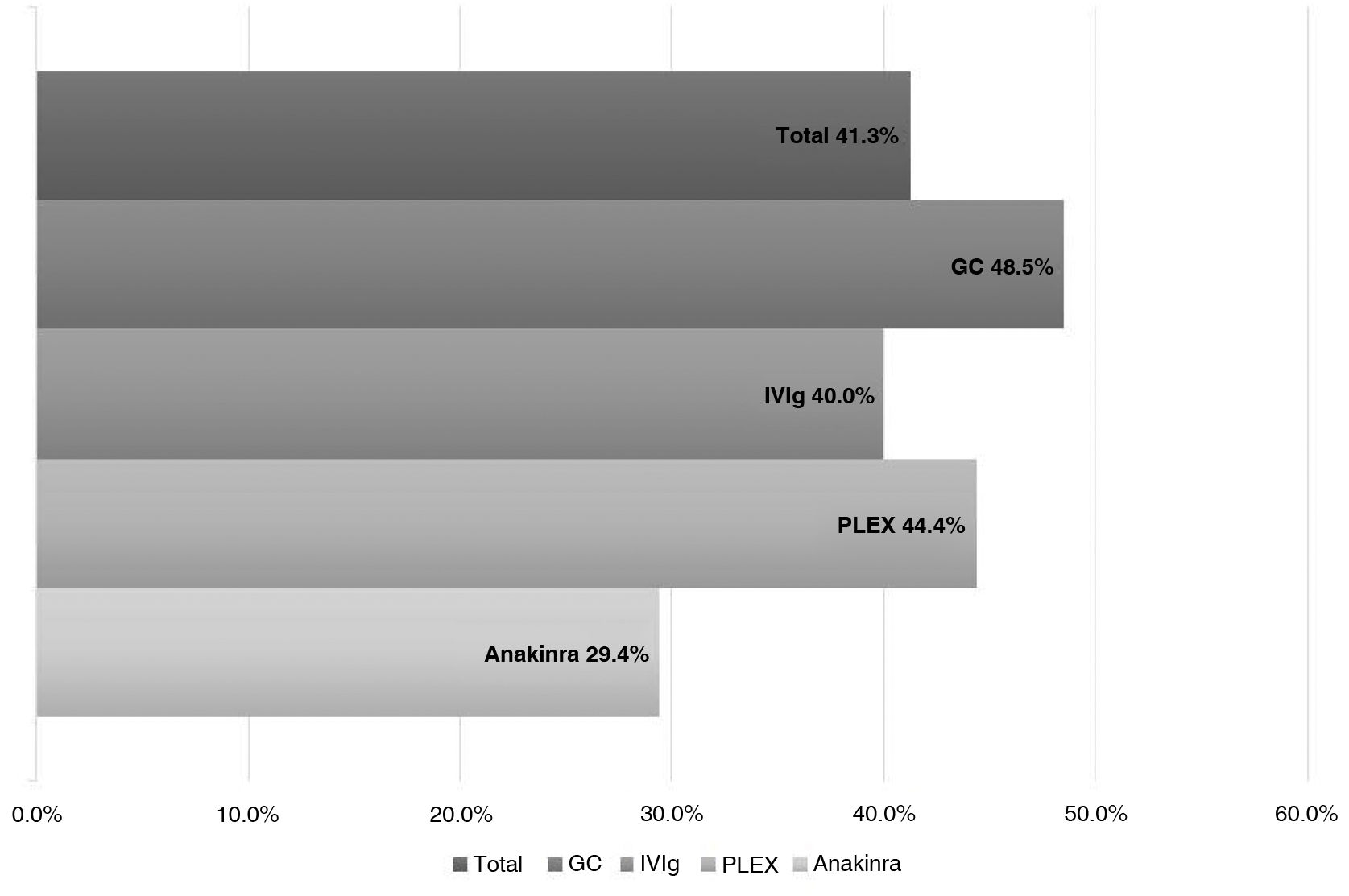
ResultsWe analyzed 16 studies including a total of 161 patients with NORSE. Six studies were carried out only with FIRES patients (n = 64). Of the 161 patients with NORSE, 141 (87.5%) received immunotherapy. Outcome data were available for 135, 56 of whom (41.4%) achieved good functional outcomes. Twenty-four of the 58 patients with FIRES treated with immunotherapy and for whom outcome data were available achieved good functional outcomes (41.3%). Mortality rates in patients with NORSE and FIRES treated with immunotherapy were 20/121 (16.5%) and 6/58 (10.3%), respectively. By type of immunotherapy, good functional outcomes were achieved in 36/89 patients receiving glucocorticoids (40.4%), 27/71 patients receiving IV immunoglobulins (38%), 11/37 patients treated with plasma exchange (29.7%), 5/17 patients receiving rituximab (29.4%), and 2/13 patients receiving cyclophosphamide (15.3%).
ConclusionDespite the lack of randomised clinical trials, immunotherapy is frequently prescribed to patients with NORSE and FIRES. However, rates of functional dependence and mortality remain high in these patients. Second-line therapies achieved lower rates of good outcomes, probably because they were administered to patients with more severe, refractory disease.
Determinar la frecuencia de pacientes con NORSE y FIRES tratados con inmunoterapia (IT) que lograron un buen pronóstico funcional.
MétodosRealizamos una revisión sistemática de la literatura a través de MedLine y EMBASE. Se incluyeron aquellos estudios con ≥ 5 pacientes con NORSE o FIRES, al menos un paciente tratado con IT y donde pudiera extraerse información sobre el buen pronóstico funcional. Un buen pronóstico funcional se definió como una puntuación en la escala Rankin modificada ≤ 2 o equivalente (i. e. independiente) en el último seguimiento disponible. Solo se incluyeron para el análisis los pacientes con pronóstico funcional conocido.
ResultadosSe incluyeron 16 estudios (FIRES 6/16 estudios) con un total de 161 pacientes, de los cuales 141 (87,5%) recibieron IT. De un total de 135 pacientes que recibieron IT y pudo obtenerse datos sobre el pronóstico 56 (41,4%) lograron un buen pronóstico funcional. Los pacientes tratados con IT de los estudios de FIRES lograron un buen pronóstico funcional en 24/58 (41,3%). La mortalidad en los pacientes tratados con IT de los estudios NORSE y FIRES fue de 20/121 (16,5%) y 6/58 (10,3%) respectivamente. Para cada tipo de IT se logró un buen resultado funcional en 36/89 (40,4%) para glucocorticoides, 27/71 (38%) IgIV, 11/37 (29,7%) recambio plasmático, 5/17 (29,4%) rituximab y 2/13 (15,3%) ciclofosfamida.
ConclusiónAunque en el momento actual no existan ensayos clínicos aleatorizados el uso de IT en NORSE y FIRES es frecuente. Sin embargo, a pesar de su uso la mayoría de los pacientes con NORSE y FIRES permanecen en situación de dependencia y la mortalidad es alta. Las terapias de segunda línea obtuvieron una menor frecuencia de buen pronóstico, probablemente porque se utilizaron en pacientes con enfermedad más grave y refractaria.
New-onset refractory status epilepticus (NORSE) is a type of clinical presentation rather than a specific disease, and may be caused by different aetiologies.1 A consensus document2 published in 2018 defined NORSE as refractory status epilepticus manifesting in a patient without active epilepsy or previous neurological disease, in whom no cause can be identified using the “initial battery” of diagnostic tests performed within the first 72 hours. The tests to be included in the “initial battery” remain a subject of debate.3 After performing a more extensive battery of diagnostic tests, aetiological diagnosis may be obtained in up to 50% of cases of NORSE, with the most frequent causes being autoimmune (autoimmune encephalitis) or paraneoplastic diseases.4 NORSE of unknown cause is called cryptogenic NORSE. Cryptogenic NORSE presents certain clinical characteristics that differentiate it from autoimmune NORSE, such as the high prevalence of prodromal fever and poorer long-term outcomes reported in several studies.5
Febrile infection-related epilepsy syndrome (FIRES) is a subtype of NORSE6 with special characteristics that led to its consideration as a distinct entity. Its main characteristic, necessary for diagnosis, is that status epilepticus should be preceded by a febrile infection (for example, influenza) starting between 2 weeks and 24 hours prior to onset of refractory status epilepticus.2 This time period enables clinicians to distinguish it from febrile status epilepticus.7 It typically manifests in paediatric patients (mean age of 8 years) and is more frequent in male patients. Several studies report poor outcomes, with 90% of survivors developing refractory epilepsy; fewer than 20% do not develop sequelae,6 and more than 10% die during the acute phase.8 No aetiology can be identified in the majority of cases of FIRES. The low frequency of antineuronal antibodies and suboptimal response to immunotherapy (IT)9 suggest that FIRES is more likely to be an autoinflammatory process.10
Since a large percentage of NORSE cases are secondary to autoimmune encephalitis, and an autoinflammatory aetiology is assumed in cases of cryptogenic NORSE and FIRES, this entity is usually treated early with IT once the infection has been ruled out.11 However, evidence on its effectiveness is based on retrospective studies and case reports, due to the current lack of clinical trials, with differences between studies in the methods used to assess effectiveness, frequently based on status epilepticus termination, but without assessing the long-term functional outcomes.9 The aim of this systematic review was to summarise the current evidence to determine how many patients with NORSE and FIRES and treated with IT achieve good functional outcomes (functional independence, modified Rankin Scale score [mRS] ≤ 2 or equivalent).
MethodsSearch strategy and study selectionWe performed a systematic review of studies published on the MEDLINE and Embase databases until 1 December 2021, not limiting our search to any language. We used the following MeSH and free text search terms in the search strategy: “NORSE” OR “new onset refractory status epilepticus” OR “FIRES” OR “febrile infection-related epilepsy syndrome” AND “immunotherapy” OR “immunotherapies” OR “therapeutic” OR “therapy” OR “therapies” OR “treatment” OR “treatments” (Appendix, Supplementary Table 1). We also performed a secondary free search of MEDLINE and Embase using the terms NORSE, outcome, and treatment. We also included articles identified during the review of the selected articles. Our review protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Searches were performed by 2 researchers (PCG and NMV), who independently reviewed the titles, abstracts, and selection criteria. Disagreement between reviewers on the inclusion/exclusion of studies was resolved by consensus or with the help of a third reviewer (PSC).
Inclusion and exclusion criteriaWe only included original clinical trials, observational studies (cross-sectional, cohort, case-control studies, and case series), and clinical cases with ≥ 5 patients, which provided prognostic data on functional independence. Depending on the type of participant, we included studies with patients diagnosed with NORSE and/or FIRES according to the 2018 consensus statement of the International League Against Epilepsy2; we excluded those studies not reporting functional outcomes. Regarding the type of intervention, we selected studies including at least one patient receiving some type of IT.
Objective and variablesThe primary outcome measure was response to IT, defined as the number (percentage) of patients with a functional prognosis on the mRS of ≤ 2 or equivalent (functional independence despite sequelae) in the last follow-up visit for which data were available.
As secondary outcomes, we included response to IT in the FIRES subgroup (patients included in the exclusive study of FIRES), mortality rate among patients treated with IT, and response to each individual type of IT. We used the chi-square test to compare the rates of good prognosis and mortality between patients treated with IT and not treated with IT.
Data extraction and risk of biasWe read the full texts of articles whose titles and abstracts met the inclusion criteria. Studies not fulfilling all eligibility criteria were excluded. Any disagreement on the inclusion of a study was resolved by consensus between the 2 reviewers and with the help of a third reviewer. Two reviewers independently extracted data and drafted a report. The level of evidence was assessed using the Scottish Intercollegiate Guidelines Network scoring system.
ResultsSearchOur systematic review identified 639 articles published between 1988 and 2021; we eliminated 163 duplicates, 440 were excluded after reading the title and abstract, and 20 after reading the full text, recording the reason for exclusion (Supplementary Table 2). We finally included 16 articles that met the selection criteria in our systematic review (Fig. 1).
All the included studies were retrospective case series. A total of 6 studies exclusively included patients with FIRES. Of the 161 patients with NORSE described in the different selected studies, a total of 141 (87.5%) patients received IT, including 131 (95.7%) patients for whom functional outcome data were reported. Data on functional prognosis were reported for the 20 (12.4%) patients who did not receive IT. The studies exclusively assessing patients with FIRES included a total of 64 patients, with 63 (98.4%) receiving IT; of these, we included the 58 for whom functional outcome data are reported (Table 1).
Characteristics of the included studies.
| Study | Population | IT | Result | QE |
|---|---|---|---|---|
| Horino et al.12 (2021) | FIRES | 6/6 (100%) | Good outcome with IT: 1/6 (16.6%) | 2– |
| N = 6 | Per IT type: | Good outcome per IT type: | ||
| Male: 5/6 patients (83.3%) | GC: 6/6 (100%) | GC: 1/6 (16.6%) | ||
| Age range: 4-8 years | IVIg: 6/6 (100%) | IVIg: 1/6 (16.6%) | ||
| Intrathecal dexamethasone: 6/6 (100%) | Intrathecal dexamethasone: 1/6 (16.6%) | |||
| Mortality after IT: 0/6 (0%) | ||||
| Nass et al.13 (2021) | FIRES | 6/6 (100%) | Good outcome with IT: 4/6 (66.6%) | 2– |
| N = 6 | Per IT type: | Good outcome per IT type: | ||
| Male: 1/6 patients (16.6%) | GC: 6/6 (100%) | GC: 4/6 (66.6%) | ||
| Age range: 17-51 years | IVIg: 5/6 (83.3%) | IVIg: 3/5 (60.0%) | ||
| PLEX: 3/6 (50.0%) | PLEX: 2/3 (66.6%) | |||
| RTX: 1/6 (16.6%) | RTX: 1/1 (100%) | |||
| CPM: 1/6 (16.6%) | CPM: 1/1 (100%) | |||
| Mortality after IT: 0/6 (0%) | ||||
| Suchdev et al.14 (2021) | NORSE | 3/5 (60%) | Good outcome with IT: 1/3 (33.3%) | 2– |
| N = 5 | Per IT type: | Good outcome per IT type: | ||
| Male: 2 (40%) | GC: 3/5 (60%) | GC: 1/3 (33.3%) | ||
| Age range: 20-56 years | IVIg: 1/3 (33.3%) | IVIg: 0/1 (0%) | ||
| PLEX: 2/3 (66.6%) | PLEX: 0/2 (0%) | |||
| CPM: 2/3 (66.6%) | CPM: 0/2 (0%) | |||
| Mortality after IT: 1/3 (33.3%) | ||||
| Good outcome without IT: 1/2 (50%) | ||||
| Mortality without IT: 0/2 (0%) | ||||
| Aurangzeb et al.15 (2020) | NORSE | 6/7 (85.7%) | Good outcome with IT: 0/5 (0%) | 2– |
| N = 7 | Per IT type: | Good outcome per IT type: | ||
| Male: 4/7 (57.1%) | GC: 6/6 (100%) | GC: 0/5 (0%) | ||
| Mean age (SD): 43 (23.8) years | IVIg: 3/6 (50%) | IVIg: 0/2 (0%) | ||
| PLEX: 3/6 (50%) | PLEX: 0/3 (0%) | |||
| Mortality after IT: 3/5 (50%) | ||||
| Good outcome without IT: 0/1 (0%) | ||||
| Mortality without IT: 0/1 (0%) | ||||
| Gugger et al.16 (2020) | NORSE | 14/20 (70%) | Good outcome with IT: 6/14 (42.8%) | 2+ |
| N = 20 | Per IT type: | Good outcome per IT type: N/A | ||
| Male: 10/20 (50%) | GC: 14/14 (100%) | Mortality after IT: N/A | ||
| Median (Q1-Q3) age in years: 50.5 (29.0-69.5) | IVIg: 3/14 (15%) | Good outcome without IT: 2/6 (33.3%) | ||
| PLEX: 11/14 (55%) | Mortality without IT: N/A | |||
| RTX: 1/14 (5%) | ||||
| CPM: 2/14 (10%) | ||||
| Lai et al.17 (2020) | FIRES | 25/25 (100%) | Good outcome with IT: 6/20 (30%) | 2+ |
| N = 25 | Per IT type: | Good outcome per IT type: | ||
| Male: 17/25 (68%) | GC: 22/25 (88%) | Anakinra: 6/20 (30%) | ||
| Median (Q1-Q3) age in years: 8 (5.0-11.0) | IVIg: 23/25 (92%) | Remaining IT: N/A | ||
| PLEX: 11/25 (44%) | Mortality after IT: 3/20 (15%) | |||
| RTX: 5/25 (2%) | ||||
| CPM: 2/14 (10%) | ||||
| Anakinra: 25/25 (100%) | ||||
| Perea et al.18 (2020) | NORSE | 3/5 (60%) | Good outcome with IT: 1/3 (25%) | 2– |
| N = 5 | Per IT type: | Good outcome per IT type: | ||
| Male: 1/5 (25%) | GC: 2/3 (75%) | GC: 0/2 (0%) | ||
| Age range: 17-79 years | PLEX: 3/3 (100%) | PLEX: 1/3 (25%) | ||
| RTX: 1/3 (25%) | RTX: 0/1 (0%) | |||
| CPM: 1/3 (25%) | CPM: 0/1 (0%) | |||
| Mortality after IT: 1/3 (25%) | ||||
| Good outcome without IT: 0/2 (0%) | ||||
| Mortality without IT: 1/2 (50%) | ||||
| Strohm et al.19 (2019) | NORSE | 12/12 (100%) | Good outcome with IT: 7/12 (58.3%) | 2– |
| N = 12 | Per IT type: | Good outcome per IT type: | ||
| Male: 2/12 (16.6%) | GC: 12/12 (100%) | GC: 7/12 (58.3%) | ||
| Age range: 14-78 years | IVIg: 8/12 (66.6%) | IVIg: 5/8 (62.5%) | ||
| PLEX: 7/12 (58.3%) | PLEX: 3/7 (42.8%) | |||
| RTX: 3/12 (25%) | RTX: 0/3 (0%) | |||
| CPM: 1/12 (8.3%) | CPM: 0/1 (0%) | |||
| Mortality after IT: 1/12 (8.3%) | ||||
| Choi et al.20 (2019) | NORSE | 13/13 (100%) | Good outcome with IT: 6/13 (46.1%) | 2– |
| N = 13 | Per IT type: | Good outcome per IT type: | ||
| Male: 7/13 (53.8%) | GC: 13/13 (100%) | GC: 6/13 (46.1%) | ||
| Median (Q1-Q3) age in years: 45.0 (33.0-50.5) | IVIg: 13/13 (100%) | IVIg: 6/13 (46.1%) | ||
| PLEX: 6/13 (46.2%) | PLEX: 2/6 (33.3%) | |||
| RTX: 2/13 (15.3%) | RTX: 2/2 (100%) | |||
| Mortality after IT: 3/13 (23.1%) | ||||
| Peng et al.21 (2019) | FIRES | 7/7 (100%) | Good outcome with IT: 4/7 (57.1%) | 2– |
| N = 7 | Per IT type: | Good outcome per IT type: | ||
| Male: 4/7 (57.1%) | GC: 5/7 (71.4%) | GC: 3/5 (60%) | ||
| Age range: 1.5-13 years | IVIg: 7/7 (100%) | IVIg: 4/7 (57.1%) | ||
| PLEX: 2/7 (28.5%) | PLEX: 1/2 (50%) | |||
| Mortality after IT: 0/7 (0%) | ||||
| Jun et al.22 (2018) | NORSE | IT: 7/7 (100%) | Good outcome with IT: 1/7 (14.2%) | 2– |
| N = 7 | Per IT type: | Good outcome per IT type: | ||
| Male: 3/7 (42.8%) | GC: 7/7 (100%) | GC: 1/7 (14.2%) | ||
| Age range: 19-61 years | IVIg: 7/7 (100%) | IVIg: 1/7 (14.2%) | ||
| RTX: 6/7 (85.7%) | RTX: 1/6 (16.6%) | |||
| CPM: 2/7 (28.5%) | CPM: 0/2 (0%) | |||
| Tocilizumab: 7/7 (100%) | Tocilizumab: 7/7 (14.2%) | |||
| Mortality after IT: 2/7 (28.5%) | ||||
| Farias-Moeller et al.23 (2018) | FIRES | 5/5 (100%) | Good outcome with IT: 2/5 (40%) | 2– |
| N = 5 | Per IT type: | Good outcome per IT type: | ||
| Male: 3/5 (60%) | GC: 5/5 (100%) | GC: 2/5 (40%) | ||
| Age range: 4-16 years. | IVIg: 5/5 (100%) | IVIg: 2/5 (40%) | ||
| PLEX: 4/5 (80%) | PLEX: 1/4 (25%) | |||
| RTX: 4/5 (80%) | RTX: 1/4 (25%) | |||
| CPM: 1/5 (25%) | CPM: 0/1 (0%) | |||
| Anakinra: 4/5 (80%) | Anakinra: 1/4 (25%) | |||
| Mortality after IT: 0/5 (0%) | ||||
| Iizuka et al.5 (2017) | NORSE | 10/11 (91%) | Good outcome with IT: 2/10 (20%) | 2– |
| N = 11 | Per IT type: | Good outcome per IT type: | ||
| Male: 4/11 (36.3%) | GC: 10/10 (100%) | GC: 2/10 (20%) | ||
| Median (Q1-Q3) age in years: 27.0 (17.0-59.0) | IVIg: 9/10 (90%) | IVIg: 2/9 (22.2%) | ||
| PLEX: 6/10 (60%) | PLEX: 0/6 (0%) | |||
| CPM: 5/10 (50%) | CPM: 1/5 (20%) | |||
| Mortality after IT: 1/10 (10%) | ||||
| Good outcome without IT: 1/1 (100%) | ||||
| Mortality without IT: 0/1 (0%) | ||||
| Patil et al.24 (2016) | FIRES | 14/15 (93.3%) | Good outcome with IT: 7/14 (50%) | 2+ |
| N = 15 | Per IT type: | Good outcome per IT type: | ||
| Male: 12/15 (80%) | GC: 13/14 (92.8%) | GC: 7/13 (53.8%) | ||
| Median (Q1-Q3) age in years: 6.0 (3.0-15.0) | IVIg: 7/14 (50%) | IVIg: 2/7 (28.5%) | ||
| Mortality after IT: 3/14 (21.4%) | ||||
| Good outcome without IT: 1/1 (100%) | ||||
| Mortality without IT: 0/1 (0%) | ||||
| Khawaja et al.25 (2015) | NORSE | 8/11 (72.7%) | Good outcome with IT: 6/8 (75%) | 2– |
| N = 11 | Per IT type: | Good outcome per IT type: N/A | ||
| Male: 2/11 (18.1%) | GC: 7/8 (87.5%) | Mortality after IT: 2/8 (25%) | ||
| Mean age (SD) in years: 48.0 (21.9) | IVIg: 7/8 (87.5%) | Good outcome without IT: 0/3 (100%) | ||
| PLEX: 4/8 (50%) | Mortality without IT: 1/3 (33%) | |||
| RTX: 2/8 (25%) | ||||
| CPM: 1/8 (12.5%) | ||||
| Costello et al.26 (2009) | NORSE | 2/6 (33.3%) | Good outcome with IT: 2/2 (100%) | 2– |
| N = 6 | Per IT type: | Good outcome per IT type: | ||
| Male: 2/6 (33.3%) | GC: 2/2 (100%) | GC: 2/2 (100%) | ||
| Age range: 24-36 years | IVIg: 1/2 (50%) | IVIg: 1 (100%) | ||
| PLEX: 1/2 (50%) | PLEX: 1 (100%) | |||
| Mortality after IT: 0/2 (0%) | ||||
| Good outcome without IT: 1/4 (25%) | ||||
| Mortality without IT: 1/4 (25%) |
CPM: cyclophosphamide; FIRES: febrile infection-related epilepsy syndrome; GC: systemic glucocorticoids; IT: immunotherapy; IVIg: intravenous immunoglobulins; PLEX: plasmapheresis; QE: quality of evidence according to the Scottish Intercollegiate Guidelines Network scoring system; RTX: rituximab.
We observed a larger number of patients presenting good outcomes among those who received IT than among those who did not (56/135 [41.4%] vs 6/20 [30%]; P = .328), as well as a lower mortality rate (20/121 [16.5%] vs 3/14 [21%]; P = .644); however, these differences were not statistically significant.
Broken down by the type of IT received, we observed good outcomes in 36 of the 89 (40.4%) patients receiving systemic glucocorticoids (GC); 27 of the 71 (38%) receiving intravenous immunoglobulins (IVIg); 11 of the 37 (29.7%) receiving plasmapheresis (PLEX); 5 of the 17 (29.4%) receiving rituximab; and 2 of the 13 (15.3%) receiving cyclophosphamide (CPM) (Fig. 2).
Prognosis after immunotherapy in febrile infection-related epilepsy syndromeOf the 58 patients receiving IT, 24 (41.3%) patients presented good outcomes, 34 (58.6%) poor outcomes, and 6 (10.3%) died.
According to the type of IT received, we observed good outcomes in 17 of the 35 (48.5%) patients receiving systemic GC; 12 of the 30 (40%) receiving IVIg; 4 of the 9 (44.4%) receiving PLEX; and 7 of the 24 (29.1%) receiving anakinra (Fig. 3).
DiscussionThe aim of our systematic review was to determine the functional outcomes of patients with NORSE and FIRES and treated with IT. According to our analysis, the majority of patients with NORSE presented poor functional outcomes despite the use of IT (58.5%). The percentage of patients with good outcomes was higher among those who received IT than those who did not (41.1% vs 30%), although this difference was not statistically significant, probably partly due to the low number of patients who did not receive IT.
The majority of patients (87.5%) included in our study received some type of IT. This percentage, which is somewhat higher than those reported in other studies with a large number of patients,4 may be partly due to the fact that our inclusion criteria established that studies must include at least one patient receiving IT. However, it is true that IT is frequently used in NORSE despite the lack of high-quality evidence, revealing the need for further well-designed clinical trials of the effectiveness and safety of IT in NORSE and FIRES, particularly in the light of the fact that several retrospective studies observed no benefits for prognosis. For example, the study by Gaspard et al.,4 in which 62% of patients received some kind of IT, reported no prognostic differences associated with its use, although there was a tendency not to treat patients with cryptogenic NORSE, which may be of inflammatory aetiology and benefit from IT. Another series of 92 patients with paediatric NORSE also found no differences.27 In a study on NORSE in 46 paediatric patients, including 40 with cryptogenic NORSE, 16 of which corresponded to FIRES,7 the use of IT was associated with poorer outcomes, which was attributed to the fact that the majority of patients who received IT were in the FIRES group, which was considered to have poor outcomes. In contrast, other studies have reported better outcomes with the use of IT25; together with the fact that many cases are of confirmed or suspected immune-mediated origin, this has contributed to the extended use of IT in NORSE.
Furthermore, we observed that the mortality rate among patients treated with IT is approximately 50% higher in patients with NORSE (16.5%) than in those with FIRES (10.3%). FIRES is considered a homogeneous subcategory of autoinflammatory cause, whereas NORSE can be associated with a range of aetiologies. This may lead us to think that the lower mortality rate in FIRES may partly be explained by the effectiveness of a targeted intervention. Similar differences in mortality rates between paediatric and adult patients with NORSE have been described in the literature, with reported rates of 12% and 16%-27%, respectively; this may be explained by the fact that FIRES was the most frequent form of presentation of NORSE in paediatric patients.11
Regarding the different types of IT, all second-line therapies showed a lower probability of good outcomes than first-line options (systemic corticosteroids, IVIg, and PLEX). This is probably because second-line therapies are used in patients with more severe and refractory conditions, and therefore poorer baseline prognosis. For example, the study by Lai et al.17 analyses the effectiveness of anakinra in 25 patients with FIRES refractory to several first-line therapies, obtaining good outcomes in only 30% of patients. We should also consider that a possible increase in infectious complications due to the use of second-line therapies may contribute to a lower probability of good outcomes, although we do not address this in our study.
Our study has several limitations. On the one hand, according to our inclusion criteria, we did not select studies including large numbers of patients, as the included studies provided sufficient data to obtain our main outcome variable on functional outcomes.4,7,27 This demonstrates the need for a future consensus to identify the most relevant variables in determining the effectiveness of therapies in NORSE and specifically, the scales and scores to be used to evaluate prognostic outcomes. On the other hand, we could not obtain the sequence or individual types of IT used in each patient from most of the included studies, which prevented us from performing a statistical analysis to determine the presence of significant differences between the different types of IT, or to extract individual data on patients with FIRES in NORSE studies. Lastly, follow-up periods vary between studies, resulting in differences in the time at which data on functional outcomes was recorded. However, we considered the most recently available data for each patient, as these are the most reliable in reflecting the patient’s progression.
In conclusion, the use of IT in NORSE and FIRES is very frequent, despite the lack of well-designed clinical trials supporting its effectiveness and safety. Furthermore, IT may improve the likelihood of functional independence compared to patients who do not receive IT; however, further studies with a higher level of evidence are needed. Second-line therapies showed a lower probability of good outcomes, probably because they are used in patients with refractory and more severe disease. Despite the use of IT, the majority of patients with NORSE and FIRES are dependent and present a high mortality rate.
Conflicts of interestThe authors have no conflicts of interest to declare.