The conclave of acute onset movement disorders in diabetes mellitus (DM) is ever-evolving.1 Even, on many occasions, movement disorder itself has been the presenting manifestation of previously undiagnosed DM, especially reported from developing countries like India, where health-related awareness is considered still inadequate.1,2 Amid several kinds of movement disorders in DM, hemichorea/hemiballism is a well-recognized entity, unlike others such as hemifacial spasm (HFS) and upper limb monochorea, which have been rarely reported.2–6 Further, a combination of both HFS and predominantly upper limb monochorea is unheard of in diabetic striatopathy.
Authors hereby report a case of a previously healthy elderly man from rural India presenting with recent onset left-sided HFS. This case was evaluated clinico-radiologically and treated accordingly without any improvement for two weeks, following which he came to our clinic having a predominantly left upper limb monochorea. He was finally diagnosed to be a case of diabetic striatopathy and managed successfully. This single case, again, emphasizes upon the fact that blood glucose status should be checked at point-of-care especially in cases of both common and rare types of recent onset movement disorders.
Case reportA 61-year-old male visited the clinic of the department of General Medicine at Burdwan Medical College & Hospital, Burdwan, West Bengal, India, as he had been experiencing increasing involuntary intermittent twitching of the muscles of the left side of the face, including the left upper eyelid, for last one month. Alongside that, he was having sudden onset involuntary semi-purposeful dancing movements (flowing from distal to proximal limb) predominantly affecting his left upper-limb for the last 36h.
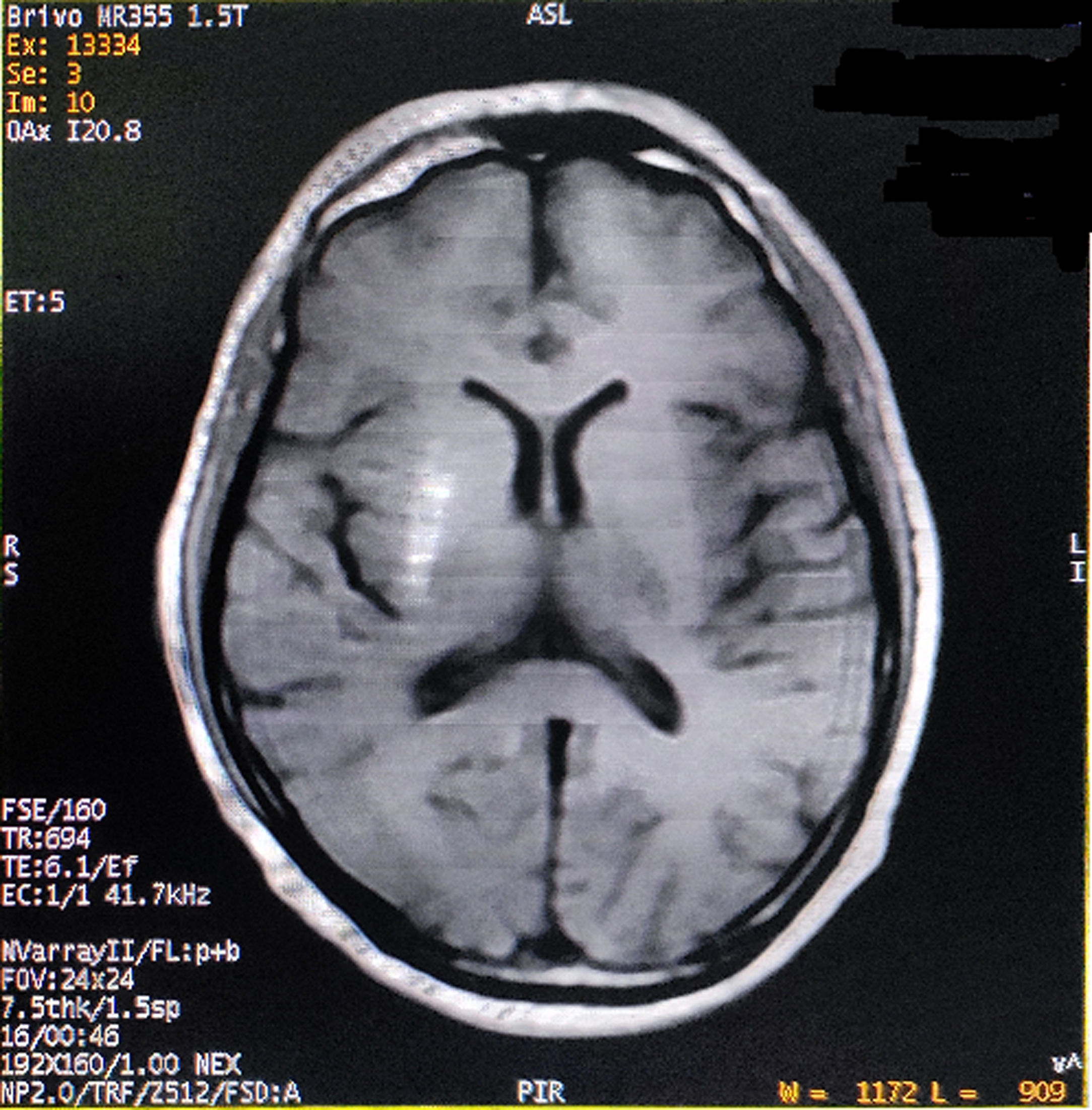
He had consulted several physicians and one neurologist for the last one month before visiting us. The initial phenomenon was diagnosed as left-sided HFS, but etiologic diagnosis remained elusive. On probing, he complained of malaise, increased thirst, and excessive frequency of urination and generalized weakness for last few days. Past medical, surgical and family history was unremarkable, except both his parents were having type-2 DM. His vital signs were all stable. Neurological examination displayed left-sided HFS along with predominantly left upper limb monochorea and subtle truncal chorea (video, see supplementary data associated with this article). His cognitive functions were intact. The patient's oropharyngeal swab test for SARS-CoV-2, by qualitative real-time reverse-transcriptase–polymerase-chain-reaction assay, was negative. Measured capillary blood glucose level was 640mg/dL. Complete blood cell count, liver, kidney and thyroid function tests were normal. Arterial blood gas analysis, serum electrolytes, C-reactive protein, urine and plasma ketones, electrocardiography, chest-X-ray, abdominal ultrasound, and blood and urine cultures were negative too. Cerebrospinal fluid (CSF) study excluded infectious etiologies. HbA1c was 8.1%. Type-1 DM was excluded as the tests for anti-GAD 65 antibodies, anti-islet cell antibodies, anti-insulin antibodies, and anti-IA2 (protein tyrosine phosphatase) antibodies were negative. On the other hand, serum fasting C-peptide level was 3.80 ng/ml (normal 0.81–3.85). Computed tomography (CT) scan of the brain and temporal bones with intravenous contrast was normal. Brain magnetic resonance imaging (MRI) revealed right striatal hyperintensity on T1-weighted imaging (Fig. 1). Magnetic resonance angiogram of the brain was otherwise normal. Acute symptomatic hyperglycemia associated with predominantly left upper limb monochorea and left-sided HFS with striatal abnormalities on neuroimaging pointed towards a diagnosis of diabetic striatopathy due to non-ketotic hyperglycemia. Continuous intravenous insulin infusion and rapid rehydration with intravenous fluids and were started.
After two days, blood glucose level stabilized. The abnormal movements reduced in frequency and intensity, but persisted with sustained normalization of blood glucose levels. He was discharged after seven days with a basal-bolus regime of insulin and diabetic medical nutrition therapy. The patient was followed-up at regular intervals with achievement of good glycemic control and no evidence of exacerbation of abnormal movements (though subtle infrequent unilateral HFS and mild choreiform movements in the left upper limb were persisting). On follow-up visit after six months, a new brain MRI was performed showing partial resolution of the striatal lesion.
DiscussionAcute metabolic insults, like hyperglycemic, can result in glutamatergic and dopaminergic excitotoxicity and thereby can cause direct neuronal damage, particularly at striatum, a metabolically vulnerable structure.7,8 Depletion of gamma-amino-butyric acid (GABA) may result from anerobic cerebral metabolism in uncontrolled hyperglycemia.7,8 Altered GABA metabolism in striatal neurons, cytotoxicity, hyperviscocity, obliterative angiopathy, and hyperglycemia itself might lead to gemistocyte accumulation in striatal neurons.7,8 This in turn gives rise to globus pallidus internus-mediated disinhibition of thalamus, leading to genesis of hyperkinetic movements.7,8
The commonest etiology of HFS is vascular ectasia at its root exit zone compressing the nerve.9 In our case, brain MRI and magnetic resonance angiogram failed to reveal any such pathologies. Uncontrolled DM is a potential risk factor for fulminant infection and hence, particularly relevant in this case. However, absence of tenderness over ipsilateral hemiface, absence of any features of otorhinolaryngeal infection, normal CT scan of the brain and temporal bones, and normal CSF study ruled out these etiologies. Since there are literature supporting the existence of acute onset HFS in cases of uncontrolled DM,2–5 we ascribed the unilateral facial spasm in this case to diabetic striatopathy itself.
Monochorea is an extremely rare entity in clinical practice and more so in cases of acute hyperglycemia-induced movement disorders.6,10,11 The majority of previous reported cases of monochorea have resulted from cerebrovascular insults.10,11 The subthalamic nucleus, putamen, thalamus, caudate and rarely pallidum, and pons have been somatotopically localized for origin of monochorea, but the evidence is neither yet accurate nor conclusive.6,10,11 Even in this case, the left upper limb monochorea may have originated from the striatal lesion. Other differential diagnoses that were considered in this case were (1) acute euglycemic metabolic chorea,12,13 (2) toxin-induced chorea (Mn, carbon monoxide),14 (3) vascular-insult induced chorea,10,11 (4) senile chorea, and (5) de novo COVID-19 associated hemichorea-hemiballismus.8 However, all of these were excluded due to lack of substantial evidence in favor.
In conclusion, the combination of HFS and upper limb monochorea is extremely rare. The usual causes of HFS and upper limb chorea are to be excluded from relevant investigations and therapeutics to be planned accordingly. However, potentially reversible metabolic etiologies are often missed. Hence, clinicians should be aware of existence of such potentially reversible metabolic perturbations leading to such complex movement disorders amenable to conservative treatment.
Authors’ contributionsAll authors contributed to the study conception. Patient information, diagnosis and management and data collection was carried out by RG. The first draft of the manuscript was written by RG and edited by DR, DR, SD, and JBL. All the authors read and approved the final manuscript.
Ethical compliance statementInformed consent was obtained from the patient for inclusion into this study. We also confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no relevant financial or non-financial interests to declare.
The following are the supplementary data to this article:
Video showing involuntary intermittent twitching of the left half of face and left upper eyelid suggestive of hemifacial spasm. In addition, involuntary semi-purposeful dancing movements (flowing from distal to proximal limb) affecting predominantly his left upper-limb (left upper limb monochorea) is observed. Subtle truncal chorea can also be noticed.