Ischaemic stroke has been reported in patients with COVID-19, particularly in more severe cases. However, it is unclear to what extent this is linked to systemic inflammation and hypercoagulability secondary to the infection.
Materials and methodsWe describe the cases of 4 patients with ischaemic stroke and COVID-19 who were attended at our hospital. Patients are classified according to the likelihood of a causal relationship between the hypercoagulable state and ischaemic stroke. We also conducted a review of studies addressing the possible mechanisms involved in the aetiopathogenesis of ischaemic stroke in these patients.
ResultsThe association between COVID-19 and stroke was probably causal in 2 patients, who presented cortical infarcts and had no relevant arterial or cardioembolic disease, but did show signs of hypercoagulability and systemic inflammation in laboratory analyses. The other 2 patients were of advanced age and presented cardioembolic ischaemic stroke; the association in these patients was probably incidental.
ConclusionsSystemic inflammation and the potential direct action of the virus may cause endothelial dysfunction, resulting in a hypercoagulable state that could be considered a potential cause of ischaemic stroke. However, stroke involves multiple pathophysiological mechanisms; studies with larger samples are therefore needed to confirm our hypothesis. The management protocol for patients with stroke and COVID-19 should include a complete aetiological study, with the appropriate safety precautions always being observed.
Se ha comunicado la asociación de ictus isquémico y COVID-19, con mayor frecuencia en aquellos pacientes más graves. Sin embargo, se desconoce en qué medida podría estar en relación con la inflamación sistémica e hipercoagulabilidad producidas en el contexto de la infección.
MétodosDescripción de cuatro pacientes atendidos en nuestro Centro por ictus isquémico y diagnóstico de COVID-19, clasificándolos según el grado de probabilidad causal entre el estado de hipercoagulabilidad y el ictus isquémico. Revisión de la literatura sobre los posibles mecanismos implicados en la etiopatogenia del ictus isquémico en este contexto.
ResultadosDos pacientes se consideraron con alta probabilidad causal: presentaban infartos corticales, sin patología cardioembólica ni arterial significativa, con parámetros de inflamación sistémica e hipercoagulabilidad; las otras dos pacientes eran de edad avanzada y el ictus isquémico se consideró cardioembólico, con una probable asociación casual de COVID-19.
ConclusionesLa inflamación sistémica, junto con la posible acción directa del virus, provocaría disfunción endotelial, generando un estado de hipercoagulabilidad que podría considerarse una causa potencial de ictus isquémico. Sin embargo, puesto que los mecanismos del ictus pueden ser múltiples, se precisan estudios más amplios que evalúen esta hipótesis. Mientras tanto, el estudio etiológico del ictus en pacientes con COVID-19 debe ser sistemático atendiendo a los protocolos vigentes, con las adaptaciones necesarias en relación con las circunstancias clínicas y epidemiológicas de la actual pandemia.
Numerous neurological manifestations, including ischaemic stroke, have been described in patients with coronavirus disease (COVID-19).1–3 In a series of 214 hospitalised patients with COVID-19 from the Chinese city of Wuhan, ischaemic stroke was reported in 2.8% of patients, rising to 5.7% in the subgroup of patients with severe COVID-19 (n = 88). These patients showed significantly higher d-dimer levels, which suggests that hypercoagulability may have caused stroke in these patients.2 According to data from the COVID-19 registry created by the Spanish Society of Neurology,4 ischaemic stroke is the second most frequent neurological disorder in these patients (22.8%), following confusional syndrome (28.3%). A recent study described the cases of 3 patients with COVID-19 who presented ischaemic stroke and antiphospholipid antibodies, in addition to elevated d-dimer levels and laboratory markers of systemic inflammation.5
Insufficient evidence is available to determine whether hypercoagulability secondary to COVID-19 presents a causal association with ischaemic stroke. To help clarify this matter, we describe 4 patients attended at our hospital due to ischaemic stroke and COVID-19 and present a literature review on the subject.
MethodsWe describe 4 consecutive patients with ischaemic stroke and COVID-19 who were attended between 25 March and 17 April 2020 at a reference centre. The study was approved by the clinical research ethics committee of the Spanish province of Granada. Due to the current SARS-CoV-2 pandemic, patients and/or their legal representatives were informed about the study and gave informed consent by telephone.
We gathered the following data: demographic variables, clinical data at admission, COVID-19-related clinical variables at admission, stroke-related variables, laboratory data at the time of stroke, and data on clinical progression. Stroke aetiology was determined using the TOAST classification criteria.6 Patients were classified according to the likelihood of a causal relationship between hypercoagulability secondary to COVID-19 and ischaemic stroke.
We also conducted a review of studies addressing the possible causal association between SARS-CoV-2 infection and ischaemic stroke.
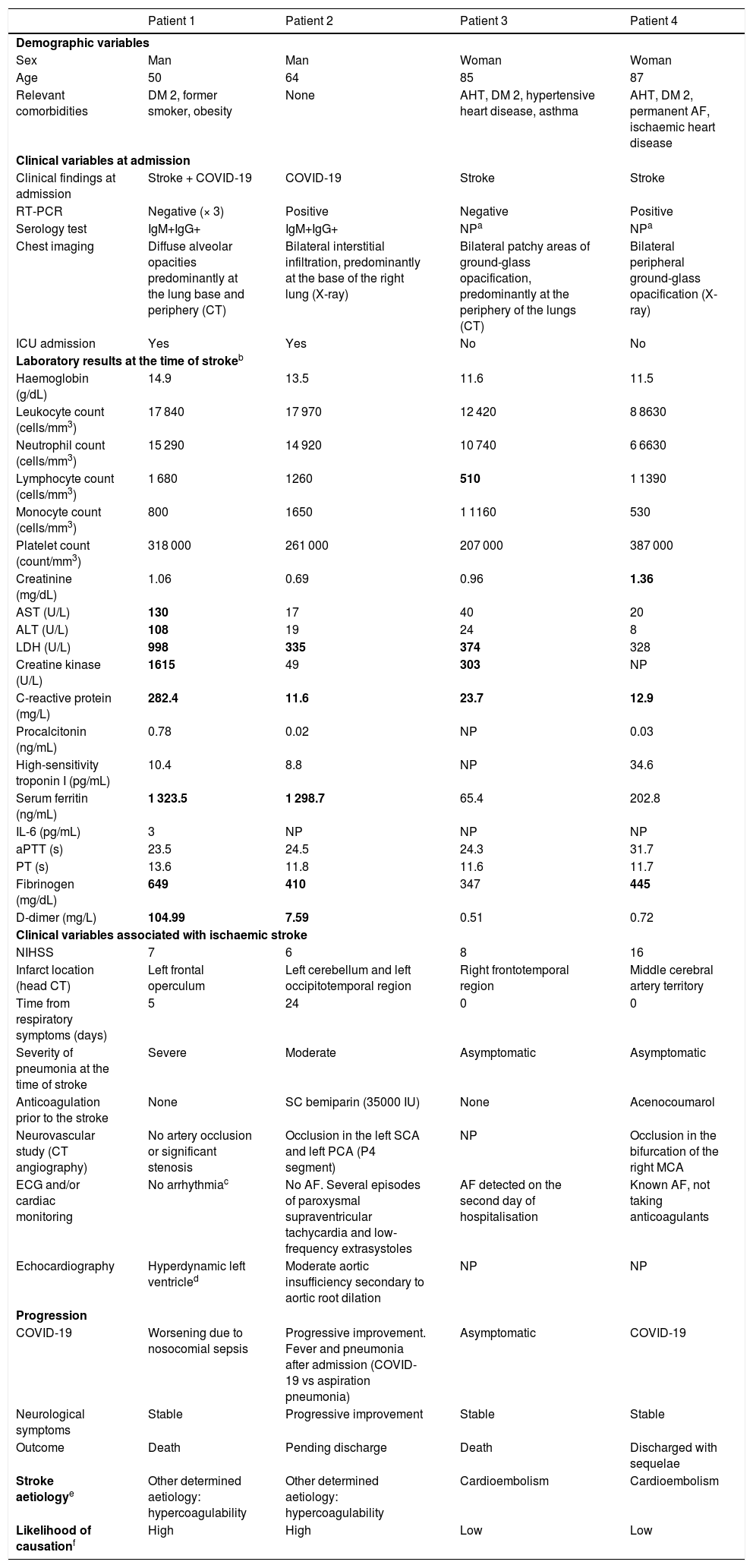
ResultsTable 1 provides clinical and complementary data on the 4 patients included (see also Supplementary material for images and Appendix 1 for a description of the 4 cases), as well as stroke aetiology and likelihood of causation due to hypercoagulability secondary to COVID-19.
Demographic, clinical, and laboratory variables analysed in our patients with ischaemic stroke and COVID-19.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Demographic variables | ||||
| Sex | Man | Man | Woman | Woman |
| Age | 50 | 64 | 85 | 87 |
| Relevant comorbidities | DM 2, former smoker, obesity | None | AHT, DM 2, hypertensive heart disease, asthma | AHT, DM 2, permanent AF, ischaemic heart disease |
| Clinical variables at admission | ||||
| Clinical findings at admission | Stroke + COVID-19 | COVID-19 | Stroke | Stroke |
| RT-PCR | Negative (× 3) | Positive | Negative | Positive |
| Serology test | IgM+IgG+ | IgM+IgG+ | NPa | NPa |
| Chest imaging | Diffuse alveolar opacities predominantly at the lung base and periphery (CT) | Bilateral interstitial infiltration, predominantly at the base of the right lung (X-ray) | Bilateral patchy areas of ground-glass opacification, predominantly at the periphery of the lungs (CT) | Bilateral peripheral ground-glass opacification (X-ray) |
| ICU admission | Yes | Yes | No | No |
| Laboratory results at the time of strokeb | ||||
| Haemoglobin (g/dL) | 14.9 | 13.5 | 11.6 | 11.5 |
| Leukocyte count (cells/mm3) | 17 840 | 17 970 | 12 420 | 8 8630 |
| Neutrophil count (cells/mm3) | 15 290 | 14 920 | 10 740 | 6 6630 |
| Lymphocyte count (cells/mm3) | 1 680 | 1260 | 510 | 1 1390 |
| Monocyte count (cells/mm3) | 800 | 1650 | 1 1160 | 530 |
| Platelet count (count/mm3) | 318 000 | 261 000 | 207 000 | 387 000 |
| Creatinine (mg/dL) | 1.06 | 0.69 | 0.96 | 1.36 |
| AST (U/L) | 130 | 17 | 40 | 20 |
| ALT (U/L) | 108 | 19 | 24 | 8 |
| LDH (U/L) | 998 | 335 | 374 | 328 |
| Creatine kinase (U/L) | 1615 | 49 | 303 | NP |
| C-reactive protein (mg/L) | 282.4 | 11.6 | 23.7 | 12.9 |
| Procalcitonin (ng/mL) | 0.78 | 0.02 | NP | 0.03 |
| High-sensitivity troponin I (pg/mL) | 10.4 | 8.8 | NP | 34.6 |
| Serum ferritin (ng/mL) | 1 323.5 | 1 298.7 | 65.4 | 202.8 |
| IL-6 (pg/mL) | 3 | NP | NP | NP |
| aPTT (s) | 23.5 | 24.5 | 24.3 | 31.7 |
| PT (s) | 13.6 | 11.8 | 11.6 | 11.7 |
| Fibrinogen (mg/dL) | 649 | 410 | 347 | 445 |
| D-dimer (mg/L) | 104.99 | 7.59 | 0.51 | 0.72 |
| Clinical variables associated with ischaemic stroke | ||||
| NIHSS | 7 | 6 | 8 | 16 |
| Infarct location (head CT) | Left frontal operculum | Left cerebellum and left occipitotemporal region | Right frontotemporal region | Middle cerebral artery territory |
| Time from respiratory symptoms (days) | 5 | 24 | 0 | 0 |
| Severity of pneumonia at the time of stroke | Severe | Moderate | Asymptomatic | Asymptomatic |
| Anticoagulation prior to the stroke | None | SC bemiparin (35000 IU) | None | Acenocoumarol |
| Neurovascular study (CT angiography) | No artery occlusion or significant stenosis | Occlusion in the left SCA and left PCA (P4 segment) | NP | Occlusion in the bifurcation of the right MCA |
| ECG and/or cardiac monitoring | No arrhythmiac | No AF. Several episodes of paroxysmal supraventricular tachycardia and low-frequency extrasystoles | AF detected on the second day of hospitalisation | Known AF, not taking anticoagulants |
| Echocardiography | Hyperdynamic left ventricled | Moderate aortic insufficiency secondary to aortic root dilation | NP | NP |
| Progression | ||||
| COVID-19 | Worsening due to nosocomial sepsis | Progressive improvement. Fever and pneumonia after admission (COVID-19 vs aspiration pneumonia) | Asymptomatic | COVID-19 |
| Neurological symptoms | Stable | Progressive improvement | Stable | Stable |
| Outcome | Death | Pending discharge | Death | Discharged with sequelae |
| Stroke aetiologye | Other determined aetiology: hypercoagulability | Other determined aetiology: hypercoagulability | Cardioembolism | Cardioembolism |
| Likelihood of causationf | High | High | Low | Low |
AF: atrial fibrillation; AHT: arterial hypertension; ALT: alanine aminotransferase; aPTT: activated partial thromboplastin time; AST: aspartate aminotransferase; CT: computed tomography; DM 2: diabetes mellitus type 2; ECG: electrocardiography; ICU: intensive care unit; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; NP: not performed; PCA: posterior cerebral artery; PT: prothrombin time; RT-PCR: real-time polymerase chain reaction; SC: subcutaneous; SCA: superior cerebellar artery; TOAST: Trial of ORG 10 172 in Acute Stroke Treatment.
Acute COVID-19 comprises 3 stages: early infection, pulmonary involvement, and severe hyperinflammation.7–9 In some patients, the inflammatory response is highly pronounced, triggering a “cytokine storm” and considerable T cell activation,10 with high levels of such laboratory markers as IL-6, ferritin, and C-reactive protein; these findings are associated with high mortality rates.11 The cytokine storm causes endothelial dysfunction,9,12–14 which may also be favoured by direct viral invasion of endothelial cells through interaction between coronavirus S protein and ACE2 receptors, expressed in the capillary endothelium.15 Endothelial dysfunction increases thrombin synthesis and decreases fibrinolysis, contributing to a hypercoagulable state; this may explain the high rate of thrombotic complications observed in series of patients with COVID-19.8,16 Elevated d-dimer levels in these patients have therefore been proposed as a marker of hypercoagulability and poor prognosis.11,17
Hypercoagulable state has been suggested as an aetiopathogenic mechanism of stroke in patients with severe COVID-19, given that these patients present higher d-dimer levels.2,13 This hypothesis was already considered during the 2002–2004 SARS-CoV epidemic, as ischaemic stroke was observed in middle-aged individuals with few vascular risk factors and severe infection.16 Both SARS-CoV and SARS-CoV-2 present a high binding affinity for ACE2.7 A recent study reported 3 cases of severe COVID-19 and ischaemic stroke; these patients presented antiphospholipid antibodies and laboratory findings compatible with systemic inflammation and coagulopathy.5 Antiphospholipid antibodies constitute the laboratory hallmark of primary antiphospholipid syndrome, but have also been detected in other systemic inflammatory diseases and viral infections, and are associated with higher risk of thromboembolic complications.18 In patients with severe COVID-19, other aetiopathogenic mechanisms of ischaemic stroke should be considered, including cardioembolism secondary to myocardial infarction or haemodynamic mechanisms secondary to cardiogenic or septic shock.16 Microangiopathy associated with endothelial dysfunction has also been proposed as a pathogenic mechanism of ischaemia.19
In patients 1 and 2 of our series (Table 1), the likelihood of a causal relationship between COVID-19 and stroke is high, as these patients presented laboratory markers of systemic inflammation and hypercoagulability and the aetiological study found no evident cause for ischaemic stroke. Patient 1 presented cortical infarction in the absence of artery disease or emboligenic heart disease. Patient 2 presented stroke during hospitalisation, despite treatment with heparin at a prophylactic dose. As reported previously, these findings support the hypothesis of hypercoagulability as a possible cause of ischaemic stroke in patients with COVID-19 and significant coagulopathy.2,5 However, ischaemic stroke may be caused by multiple, complex pathogenic mechanisms, particularly in patients with respiratory involvement. In view of the current pandemic, aetiological studies often cannot be completed or are delayed.20 This underscores the need for consensus documents and adaptations to current stroke management plans, as in the recently published Madrid stroke care plan recommendations.21
In patients 3 and 4, the likelihood that COVID-19 caused stroke was low, for several reasons. Firstly, these patients were of advanced age and had multiple comorbidities; SARS-CoV-2 infection was an incidental finding, and stroke was most likely cardioembolic. Furthermore, in both cases, stroke presented with neurological signs; no symptoms of SARS-CoV-2 infection were observed at stroke onset. These cases show that copresence of COVID-19 and ischaemic stroke does not always imply a causal relationship; therefore, detection of SARS-CoV-2 infection should not interfere with the aetiological study of ischaemic stroke.20,21 Patients with COVID-19 requiring hospitalisation should receive prophylactic anticoagulation with heparin; higher doses should be administered to critically ill patients and those at high risk of thrombosis.13,22 A recent study reported lower mortality rates among patients treated with prophylactic doses of heparin who presented d-dimer levels 6 times greater than the upper limit of normal (>3 mg/L) or a sepsis-induced coagulopathy score ≥ 4.13 This scoring system considers prothrombin time, platelet count, and Sequential Organ Failure Assessment score, and has been proposed as a tool for early detection of sepsis-induced disseminated intravascular coagulation.23 However, in patients with ischaemic stroke and SARS-CoV-2 infection presenting coagulopathy and hyperinflammation, early treatment with intermediate- or therapeutic-dose heparin is controversial due to the associated risk of haemorrhagic transformation.24 In fact, insufficient evidence is available to recommend early anticoagulation therapy20,24: the decision should be made on an individual basis based on the presence of a prothrombotic state and/or systemic inflammation, stroke size, and the risk-benefit balance.
Our study does present several limitations, mainly the small size of the patient series, our limited experience with managing patients with stroke and COVID-19, the difficulties of diagnosing and treating these patients during the pandemic, and the limited scientific evidence on ischaemic stroke in patients with COVID-19.
ConclusionsThe hypercoagulable state associated with the hyperinflammatory response triggered by COVID-19 may be considered a potential cause of ischaemic stroke. However, stroke in these patients may involve numerous pathogenic mechanisms; studies with larger samples are needed to confirm this hypothesis and evaluate the role of endothelial damage secondary to viral invasion. Patients with stroke and COVID-19 should undergo a complete aetiological study, with the appropriate safety precautions always being observed. There is currently no evidence of the benefits of early anticoagulation therapy after ischaemic stroke in patients with COVID-19 and hypercoagulability. Future studies, ideally clinical trials, are needed to provide more robust evidence supporting the use of anticoagulants in these patients. Meanwhile, the risk-benefit balance of this treatment should be assessed on an individual basis.
Conflicts of interestThe authors of this study have no conflicts of interest to declare.
This study has received no specific funding from any public, private, or non-profit organisation.
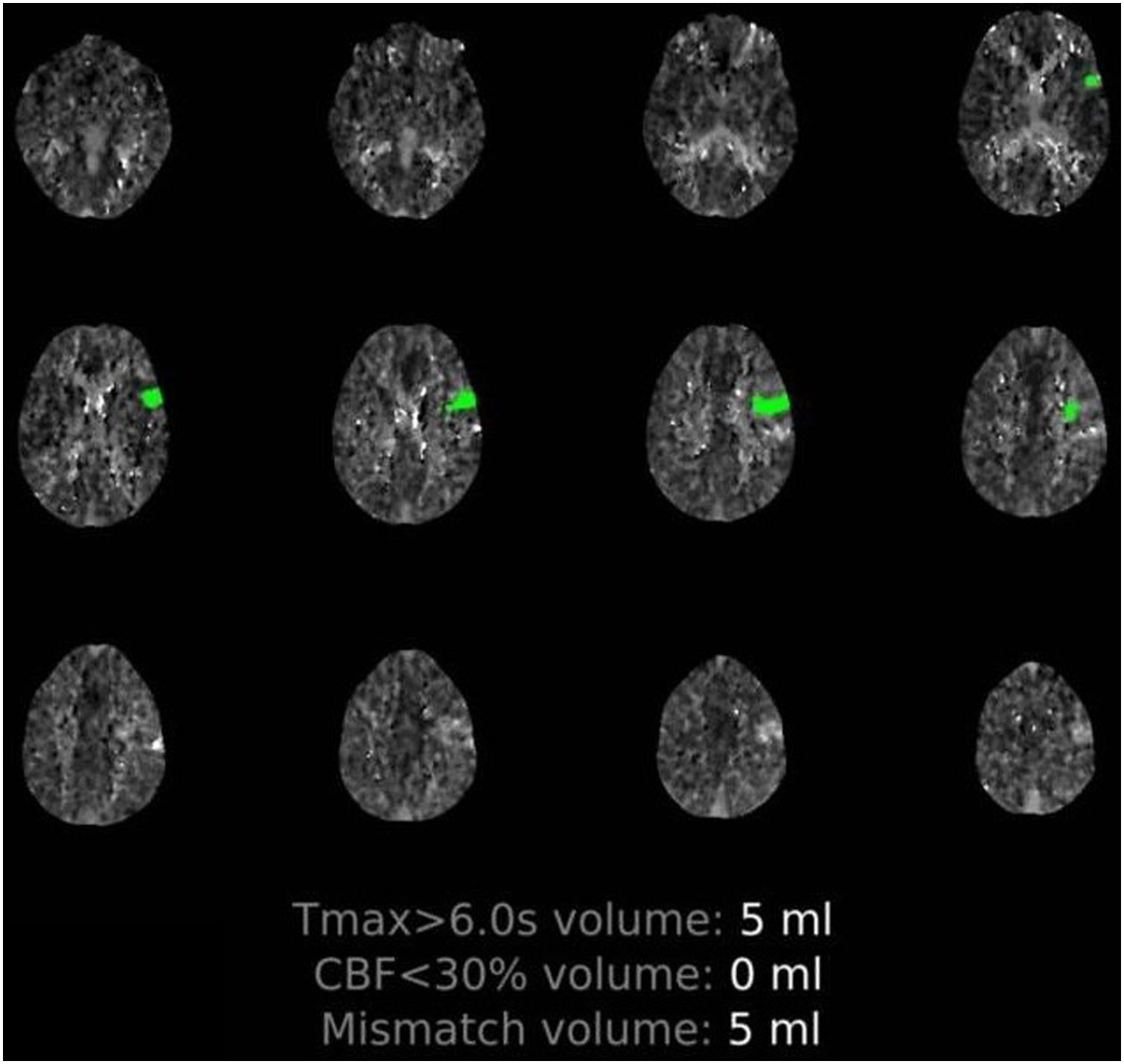
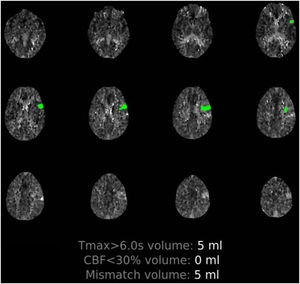
Patient 1. 50-Year-old man with multiple comorbidities (Table 1), presenting sudden-onset language alterations; he presented fever, cough, and dyspnoea 5 days previously. The examination at the emergency department revealed fever and hypoxaemia associated with motor dysphasia and right supranuclear facial palsy. Perfusion imaging detected hypoperfusion in the distal territory of the left MCA (Supplementary material, Fig. 1) and chest CT revealed signs of pneumonia due to COVID-19. The patient received intravenous fibrinolysis and was admitted to the intensive care unit for neurological and pulmonary monitoring. Blood analysis results indicated systemic inflammation and elevated d-dimer levels (Table 1). Although the results of 3 subsequent RT-PCR tests were negative (2 in nasopharyngeal exudate and one in bronchoalveolar lavage), serology tests yielded positive results for IgM and IgG antibodies against SARS-CoV-2. Progression was poor; the patient died 12 days after onset of neurological symptoms due to severe nosocomial sepsis.
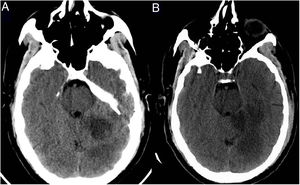
Patient 2. 64-Year-old man with no relevant medical history, admitted due to respiratory insufficiency; RT-PCR results were positive for SARS-CoV-2. He was admitted to the ICU and required high-flow nasal oxygen therapy; respiratory symptoms improved and the patient was transferred to a general ward. Twelve days after admission, he presented language alterations, dizziness, and vomiting; the neurological examination revealed horizontal-torsional nystagmus, dysarthria with intelligible speech, and left-sided dysmetria. An emergency multimodal CT scan revealed occlusion of the distal portion of the left superior cerebellar artery and the P4 segment of the left posterior cerebral artery (Supplementary material, Fig. 2). Intravenous fibrinolysis was ruled out since the patient was outside the therapeutic window. A complete aetiological study yielded positive results for beta-2 glycoprotein 1 IgG antibodies (27 U/mL; normal range, 0–10 U/mL), with negative results for anticardiolipin antibodies. The patient started pulmonary and neurological rehabilitation, progressing favourably.
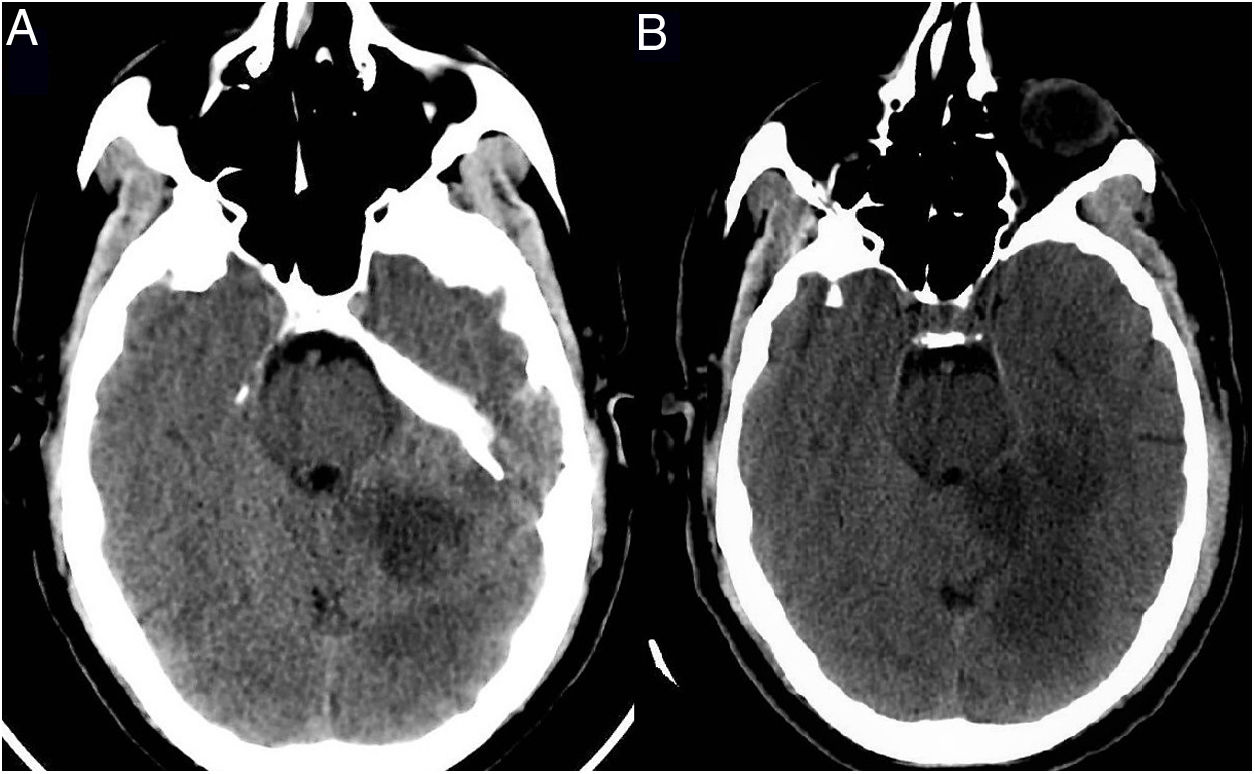
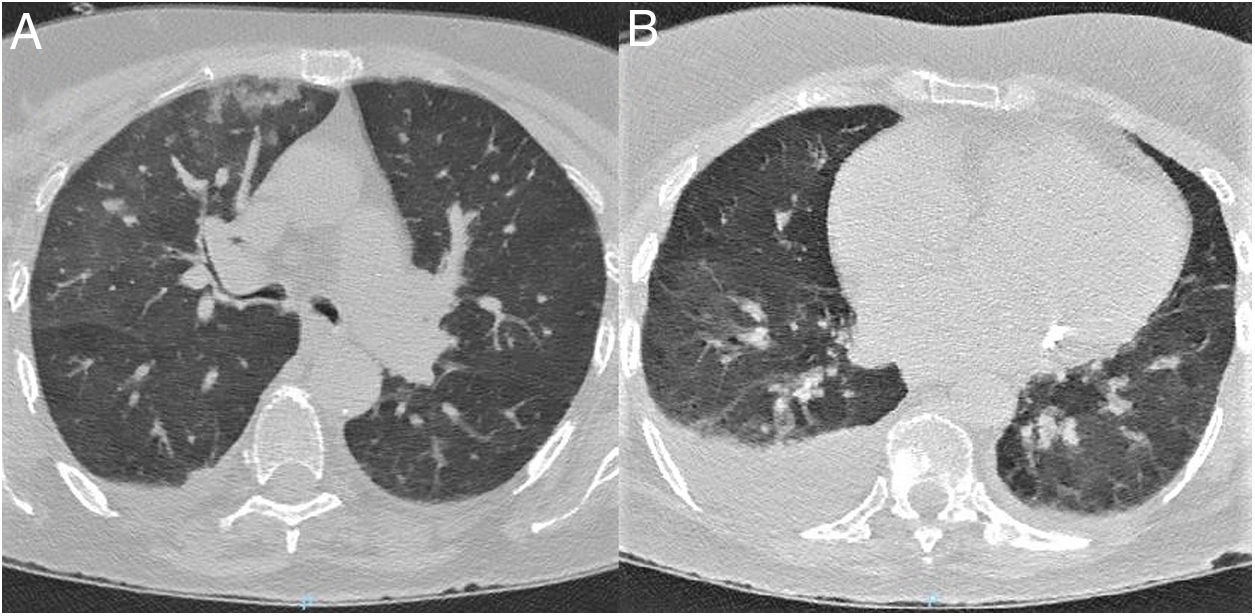
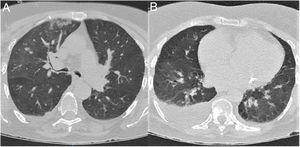
Patient 3. 85-Year-old woman with relevant comorbidities (Table 1) who was found in her home with left limb weakness. Her relatives reported no symptoms of infection or neurological alterations in the preceding days. The examination performed at the emergency department revealed no fever or respiratory symptoms; the patient presented dysarthria, anosognosia, left homonymous hemianopsia, and mild left-sided hemiparesis. A head CT scan revealed subacute ischaemic stroke in the right hemisphere; chest CT findings were suggestive of COVID-19 (Fig. 3). RT-PCR testing of nasopharyngeal exudate yielded negative results. Atrial fibrillation was detected 2 days after admission; the patient worsened, developing fever and symptoms of pneumonia. Clinical, laboratory, and radiological findings were compatible with COVID-19. The patient died due to treatment-resistant respiratory insufficiency.
Chest CT scan at admission. (A) Axial plane at the level of the upper lobes. (B) Axial plane at the level of the lower lobes. Bilateral ground-glass opacities, predominantly on the right side, affecting the central and mainly the peripheral area. The scan also revealed consolidation in the right upper lobe and moderate right pleural effusion.
Patient 4. 87-Year old woman with permanent atrial fibrillation. She had not been taking her prescribed anticoagulation treatment and presented relevant comorbidities (Table 1). She was transferred to our hospital due to symptoms compatible with right hemisphere stroke; multimodal CT revealed occlusion of the right MCA. During her stay at the emergency department, she presented fever; RT-PCR testing of nasopharyngeal exudate yielded positive results for SARS-CoV-2. The patient progressed favourably and was discharged with anticoagulation therapy.
Please cite this article as: Barrios-López JM, Rego-García I, Muñoz Martínez C, Romero-Fábrega JC, Rivero Rodríguez M, Ruiz Giménez JA, et al. Ictus isquémico e infección por SARS-CoV-2, ¿asociación casual o causal? Neurología. 2020;35:295–302.