Many diseases associated with hyperkinetic movement disorders manifest in women of childbearing age. It is important to understand the risks of these diseases during pregnancy, and the potential risks of treatment for the fetus.
ObjectivesThis study aims to define the clinical characteristics and the factors affecting the lives of women of childbearing age with dystonia, chorea, Tourette syndrome, tremor, and restless legs syndrome, and to establish guidelines for management of pregnancy and breastfeeding in these patients.
ResultsThis consensus document was developed through an exhaustive literature search and a discussion of the content by a group of movement disorder experts from the Spanish Society of Neurology.
ConclusionsWe must evaluate the risks and benefits of treatment in all women with hyperkinetic movement disorders, whether pre-existing or with onset during pregnancy, and aim to reduce effective doses as much as possible or to administer drugs only when necessary. In hereditary diseases, families should be offered genetic counselling. It is important to recognise movement disorders triggered during pregnancy, such as certain types of chorea and restless legs syndrome.
Muchas enfermedades que cursan con trastornos del movimiento hipercinético debutan o afectan a mujeres en edad fértil. Es importante conocer los riesgos que tienen las mujeres con estas enfermedades durante el embarazo así como los posibles efectos de los tratamientos sobre el feto.
ObjetivosDefinir las características clínicas y los factores que condicionan la vida de la mujer en edad fértil con distonía, corea, síndrome de Tourette, temblor y síndrome de piernas inquietas. Definir una guía de actuación y manejo del embarazo y lactancia en las pacientes con esta enfermedad.
DesarrolloEste documento de consenso se ha realizado mediante una búsqueda bibliográfica exhaustiva y discusión de los contenidos llevadas a cabo por un grupo de expertos en trastornos del movimiento de la Sociedad Española de Neurología (SEN).
ConclusionesEn todas las mujeres que padecen o debutan con trastornos del movimiento hipercinéticos se debe valorar el riesgo-beneficio de los tratamientos, reducir al máximo la dosis eficaz o administrarlo de forma puntual en los casos en que sea posible. En aquellas patologías de causa hereditaria es importante un consejo genético para las familias. Es importante reconocer los trastornos del movimiento desencadenados durante el embarazo como determinadas coreas y el síndrome de piernas inquietas.
Hyperkinetic movement disorders are a heterogeneous group of syndromes and diseases, with age of onset ranging from childhood to old age. As is the case with Parkinson’s disease (PD), many women develop these disorders during childbearing age; it is therefore important to understand and know how to manage these diseases in this stage of life.
A panel of experts belonging to the Spanish Society of Neurology’s Movement Disorders Study Group has prepared a consensus statement aiming to improve the diagnosis and treatment of movement disorders in women of childbearing age, and particularly during pregnancy and breastfeeding, given the risks and responsibilities associated with these situations. In this second part of the statement, we address the management of hyperkinetic movement disorders, such as dystonia, Tourette syndrome, tremor, and restless legs syndrome (RLS), which are the most prevalent and have the greatest impact on patients.
The document is based on a comprehensive review of articles published on the Brain, PubMed, Web of Science, PEDro, Scopus, CINAHL, and ScienceDirect databases, as well as the authors’ clinical experience. Based on the findings of the review, a series of recommendations were drafted to assist in the management of movement disorders during pregnancy and breastfeeding.
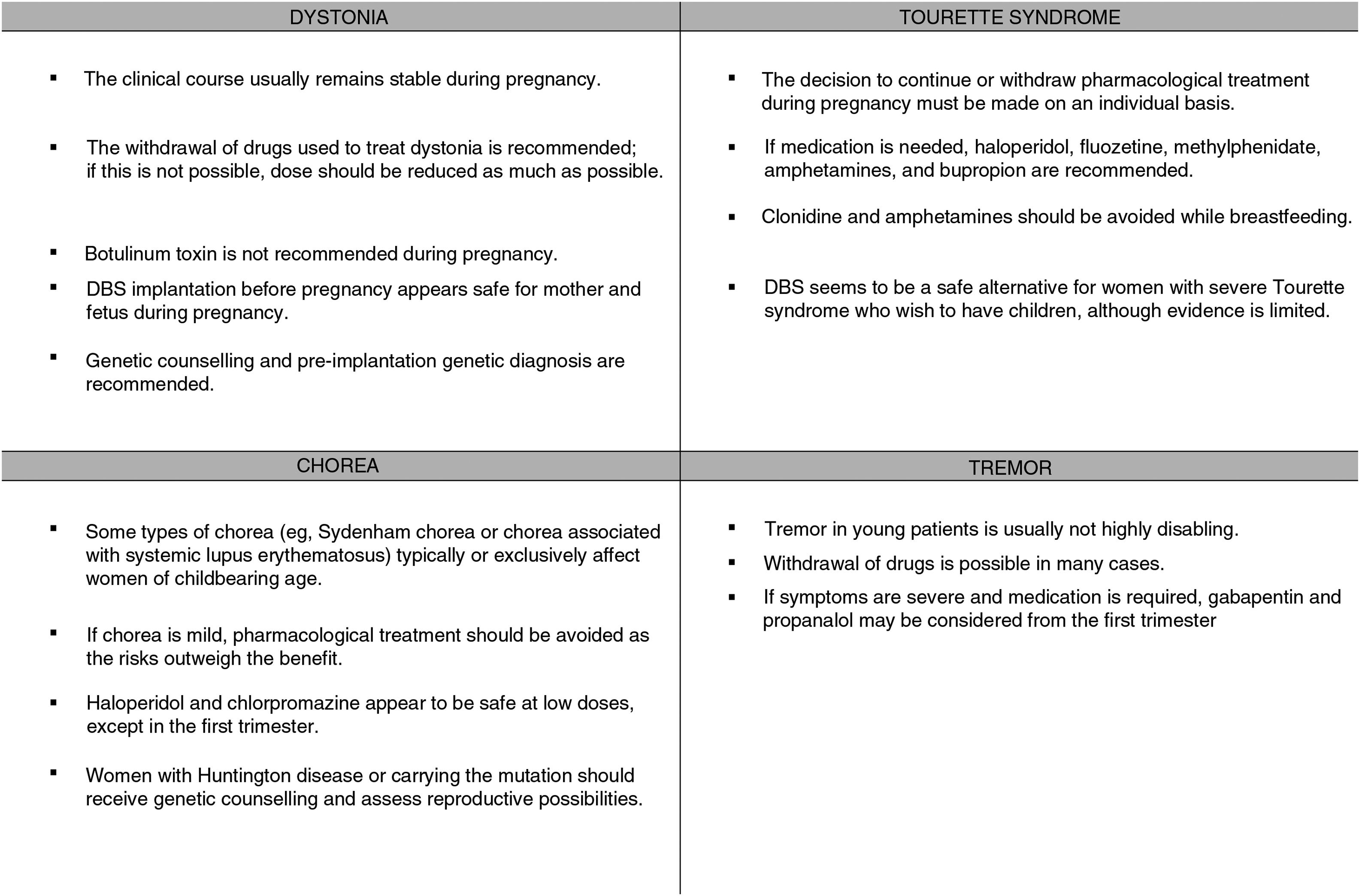
Dystonia in women of childbearing ageAs the onset of dystonia occurs in early adulthood in the majority of cases (and occasionally in childhood), we should expect to encounter the disease in pregnant women or those who wish to become pregnant. Management of these patients requires a multidisciplinary approach.
Cases have been reported of dystonia with onset during pregnancy that resolved before or after delivery. Given the resemblance to chorea gravidarum, this entity is known as dystonia gravidarum.1,2 Acute dystonic reactions to drugs including metoclopramide can also occur in pregnant women.3
The clinical course of existing dystonia usually remains stable during pregnancy, although symptoms may improve or worsen in some patients.4,5
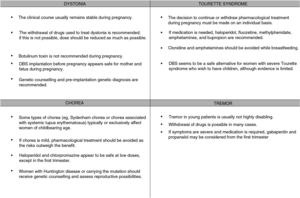
The available treatments for dystonia are symptomatic and require long-term use; therefore, these drugs cannot always be completely withdrawn. As a rule, pregnancy always requires careful balancing of the risks and benefits of treatment, and drugs should be administered at the lowest possible doses for acceptable symptom control. Therefore, we should attempt to gradually reduce dosage before pregnancy (for planned pregnancies) or during pregnancy (for unplanned pregnancies) (Fig. 1).
Treatments used in pregnant patients with dystoniaOral drugsThe oral drugs typically used to treat dystonia are:
Trihexyphenidyl: anticholinergic drug. No studies have assessed the risk of teratogenicity or embryotoxicity in animals, and no conclusive data are available for humans. Eclamptic seizures have been reported in patients using the drug.6
Baclofen: GABA receptor agonist. Studies with rodents have shown increased incidence of spina bifida,7 and a case has been reported of neonatal seizures after intrauterine baclofen exposure.8 The drug should be withdrawn gradually due to the risk of withdrawal syndrome (seizures). Reintroduction of the drug is advised in patients presenting this symptom.7 Several cases have been reported of intrathecal baclofen use to treat spasticity and dystonia, with good safety for mother and fetus. In most cases, caesarean delivery is scheduled, although some cases have also been described of vaginal delivery without complications.9
Benzodiazepines: these drugs appear not to be associated with fetal malformations, although neonates may present withdrawal syndrome, with a risk of seizures, respiratory depression, and hypotonia.10 If the drug is continued, it should be administered at low doses and the patient should be well informed of the potential risks.
Levodopa: levodopa is the treatment of choice in patients who respond to the drug.
Botulinum toxinBotulinum toxin infiltrations are usually the treatment of choice for focal dystonia.
No clear recommendations have been made on their use during pregnancy. Few studies have been performed in humans, and animal studies have shown adverse effects for the fetus. Botulinum toxin is a large molecule, and probably cannot cross the placenta passively. However, active transport of the drug cannot be ruled out.11 Cases have been reported over the years of botulinum toxin use in pregnant patients, with no effects on the fetus12; while as a general rule the treatment is not advised during pregnancy,13 a growing body of evidence supports its use in selected cases.
Therefore, it is advisable to suspend use of the drug wherever possible. Where suspension of the drug poses an unacceptable risk to the mother, we may consider continuing treatment at the minimum effective dose, ensuring proper monitoring of the fetus.
Deep brain stimulationDeep brain stimulation (DBS) of the internal globus pallidus is an effective treatment for generalised dystonia and some forms of focal dystonia.14 With DBS, conventional medication can be reduced or even suspended, which without doubt will benefit future pregnancies.
In a series of 11 patients who became pregnant after undergoing DBS to treat movement disorders (dystonia in 5 cases), no complications were reported during pregnancy or delivery, and no paediatric problems were observed. In one patient, the subclavicular location of the pulse generator hindered breastfeeding due to local pain; another patient with the generator placed in the abdominal wall experienced discomfort due to stretching of the extension cable as pregnancy progressed. Stimulation parameters may require adjustment due to potential exacerbation of dystonia during pregnancy. Delivery was vaginal in 3 cases and caesarean in the remaining patients. Most caesarean sections were performed as a precaution due to lack of understanding of the risks associated with vaginal delivery in patients with implanted nerve stimulation systems.15
In another series,16 including 6 patients treated with DBS of the globus pallidus, all pregnancies and deliveries developed without complication, with the exception of one premature birth (35 weeks). Four patients delivered vaginally; caesarean section was scheduled in the remaining cases.
In the event of caesarean delivery, the nerve stimulator must be switched off if monopolar electrocoagulation is used; diathermy is contraindicated. Proper antibiotic prophylaxis is also advisable to prevent bacteraemia or infection of the DBS system.
Follow-up of pregnancy and deliveryIdeally, pregnancy should be planned; this enables us to discuss in advance the implications with our patients: progression of dystonia, pharmacological management, delivery, and breastfeeding.
Patients receiving potentially teratogenic treatments should consult a prenatal diagnosis unit for assessment of the teratogenic risk and the relevant follow-up for high-risk pregnancies.
BreastfeedingFew or no data are available on the excretion of the drugs used to treat dystonia in breast milk. Anticholinergics may cause antimuscarinic effects in the infant, and benzodiazepines and baclofen may cause sedation. No studies have been conducted into the use of botulinum toxin in breastfeeding. Given the lack of data, its use is not recommended, although it seems unlikely that the drug may be excreted in the breast milk. DBS appears to be safe during breastfeeding. However, subclavicular placement of the pulse generator can cause a degree of discomfort.
Genetic counsellingPatients diagnosed with dystonia of genetic aetiology should receive genetic counselling and be offered the possibility of pre-implantation or prenatal diagnosis.
In selected cases, it is possible to perform pre-implantation diagnosis to select unaffected embryos; prenatal diagnosis is also possible, either through amniocentesis or with a novel, non-invasive technique that detects fetal DNA in the maternal blood.
ChoreaChorea may present in women of childbearing age since many causes of secondary chorea are hereditary. However, some hereditary degenerative causes of chorea present specific characteristics in these patients. In Huntington disease (HD), women of childbearing age are reported to present faster progression, greater disease severity according to motor and functional scales, and greater prevalence of depression.17
Patients with ataxia telangiectasia present increased risk of breast cancer (odds ratio: 2.9), with a risk of death of up to 25%; therefore, annual mammogram studies are recommended, especially after the age of 40 years.18 Wilson disease is associated with increased risk of fetal hepatotoxicity, fetal death, and decreased liver function and haemolytic anaemia in the mother (Fig. 1).
Some types of chorea typically or exclusively affect women of childbearing age; examples are Sydenham chorea and chorea associated with systemic lupus erythematosus.19 Chorea with onset during pregnancy is usually of immune aetiology or secondary to other conditions (vascular causes; thyrotoxicosis; non-ketotic hyperglycaemia in patients with diabetes mellitus; or drug-induced; or such hereditary degenerative diseases as Wilson disease or HD).3
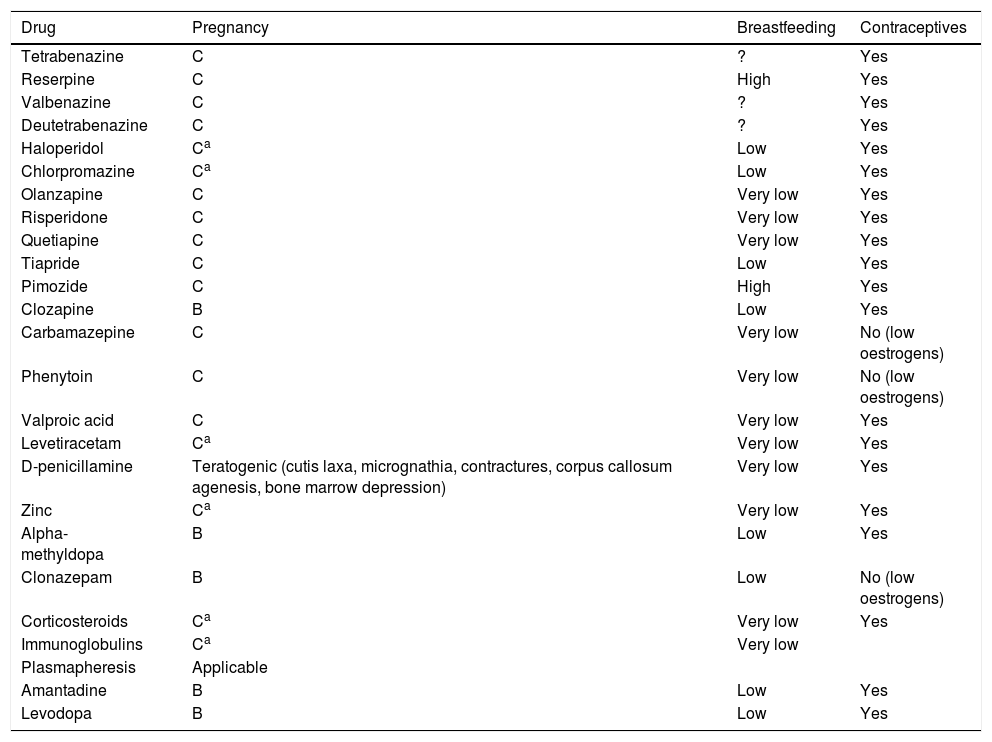
If symptoms are mild, the risks associated with drugs to treat chorea outweigh the benefits; however, more severe cases may be associated with morbidity and mortality in mother and fetus, and must be treated. Haloperidol and chlorpromazine appear to be safe at low doses, except in the first trimester; the most frequent complications are transient dystonic phenomena. Cases have been reported of pregnancies carried to term (one case of a minor ventricular defect) after exposure to tetrabenazine from the second trimester. A case of chorea gravidarum secondary to moyamoya disease was successfully treated with alpha-methyldopa, which has a better safety profile during pregnancy (United States Food and Drug Administration [FDA] pregnancy risk category B).20 In patients with Wilson disease in whom chelating treatment must be continued, zinc is a safer option than penicillamine (Table 1).3
Summary of treatments for chorea during pregnancy, breastfeeding, and hormonal contraceptive use.
| Drug | Pregnancy | Breastfeeding | Contraceptives |
|---|---|---|---|
| Tetrabenazine | C | ? | Yes |
| Reserpine | C | High | Yes |
| Valbenazine | C | ? | Yes |
| Deutetrabenazine | C | ? | Yes |
| Haloperidol | Ca | Low | Yes |
| Chlorpromazine | Ca | Low | Yes |
| Olanzapine | C | Very low | Yes |
| Risperidone | C | Very low | Yes |
| Quetiapine | C | Very low | Yes |
| Tiapride | C | Low | Yes |
| Pimozide | C | High | Yes |
| Clozapine | B | Low | Yes |
| Carbamazepine | C | Very low | No (low oestrogens) |
| Phenytoin | C | Very low | No (low oestrogens) |
| Valproic acid | C | Very low | Yes |
| Levetiracetam | Ca | Very low | Yes |
| D-penicillamine | Teratogenic (cutis laxa, micrognathia, contractures, corpus callosum agenesis, bone marrow depression) | Very low | Yes |
| Zinc | Ca | Very low | Yes |
| Alpha-methyldopa | B | Low | Yes |
| Clonazepam | B | Low | No (low oestrogens) |
| Corticosteroids | Ca | Very low | Yes |
| Immunoglobulins | Ca | Very low | |
| Plasmapheresis | Applicable | ||
| Amantadine | B | Low | Yes |
| Levodopa | B | Low | Yes |
Adapted from Seier and Hiller.45
Huntington disease (OMIM #143100) follows an autosomal dominant inheritance pattern and is caused by a CAG trinucleotide repeat expansion in exon 1 of the gene encoding huntingtin (HTT). According to the number of CAG repeats, alleles can be characterised as being within the normal (up to 26 repeats), intermediate (27-35), or pathological range (36 or more). Penetrance is considered to be incomplete in patients with 36-39 repeats and complete in those with 40 or more; the latter group of patients will invariably develop the disease at some point in their lifetimes. Individuals with 27 or more CAG repeats may transmit the expansion to their children, who may even present expansions in the pathological range, depending on the number of repeats.
HD is the most frequent hereditary cause of chorea. As clinical onset usually occurs between the ages of 30 and 50 years, the condition has a significant impact on the female reproductive period and causes considerable stress both for patients at risk and for neurologists, who must make complex decisions and inform their patients. We may be faced with different scenarios: young, asymptomatic patients with family history of the condition and a desire to have children, or symptomatic patients who wish to become parents.
Genetic counselling should include detailed discussion of the available reproductive options: pre-implantation genetic diagnosis, prenatal diagnosis, continuing pregnancy without genetic testing, donation from a healthy individual, or adoption. We must also ensure that the future parents are aware of the possible outcomes and implications: mutations with complete or incomplete penetrance, or expansions in the normal or intermediate ranges.
The predictive test should always be performed in patients of legal age, after psychological and psychiatric assessment, and results should be communicated by a multidisciplinary team. The protocol for genetic counselling is included in the international recommendations published in 2013.21
Pre-implantation genetic diagnosis is permitted in Spain for severe diseases with early onset and no curative treatment; HD meets these conditions. In prenatal diagnosis, genetic testing is performed after the patient becomes pregnant; if the fetus is affected, the parents must decide whether to continue the pregnancy. If the future parents are certain that they will not terminate the pregnancy, then prenatal diagnosis is not indicated. Some researchers have recently proposed genetic testing of both parents, and not only the one at risk of HD, based on studies demonstrating that the CAG expansion is more frequent in the general population than previously estimated22; this option should be carefully studied in the future.
The same protocol should be followed for other hereditary causes of chorea.
Tourette syndromeAs the tics characterising Tourette syndrome usually begin during childhood or adolescence, it is not unusual to observe the syndrome in women of childbearing age.23
While evidence is limited, the clinical course seems to differ between adult men and women.19,24 In women, tics are more likely to extend to other parts of the body and to worsen after adolescence (whereas they improve in men), and the condition is usually more severe24 and has a greater functional impact in social domains.19
No consistent association has been shown between tic severity and levels of sex hormones. In a survey on the effects of hormonal changes on tics in 47 women of reproductive age, 26% of respondents reported worsening of tics in the premenstrual phase. However, they did not report changes associated with other causes of hormonal changes, such as pregnancy, oral contraceptive use, or menopause.25 A prospective study of 8 patients with regular menstrual cycles found no association between oestrogen levels and severity of tics or obsessive compulsive disorder symptoms.26 Tourette syndrome seems not to have a detrimental effect on pregnancy (Fig. 1).25
Pharmacological treatment during pregnancyTreatment decisions should be made on an individual basis and take into account risks and benefits and the possibility of teratogenicity, and drugs should be administered at the lowest possible dose achieving symptom control in the mother.
Tics are not disabling in most patients, and suspension of medication during pregnancy is well tolerated.3 However, it may be necessary to maintain treatment in severe cases with violent tics in order to minimise physical risk during anaesthesia and delivery.
Antipsychotics: antipsychotics are considered safe for use during pregnancy, although limited safety data are available (particularly for new generation drugs). Haloperidol is preferred over other antipsychotics during pregnancy, as it has minimal anticholinergic, antihistaminic, and hypotensive effects compared to other drugs. Slow-release (depot) drugs should be avoided to limit the duration of adverse reactions in the neonate.26
Antidepressants: antidepressants are frequently used to treat obsessive-compulsive disorder associated with Tourette syndrome. Serotonin-norepinephrine reuptake inhibitors (SNRI) are considered safe for use during pregnancy.48 If pharmacological treatment is necessary, fluoxetine is recommended.26 Fluvoxamine, paroxetine, and sertraline are considered acceptable alternatives, despite a lower level of evidence. The neonate should be monitored to rule out any effect of the drug or withdrawal syndrome.26
Alpha-adrenergic drugs: the available data on the use of methylphenidate and amphetamines in pregnant patients with attention deficit-hyperactivity disorder suggest that they do not increase the rate of major congenital anomalies.27 Very few data are available on the use of atomoxetine and guanfacine. Clonidine (antihypertensive) and bupropion (antidepressant) seem not to increase the rate of congenital anomalies. If treatment is essential, methylphenidate, amphetamines, or bupropion are recommended.27,28 While most of these drugs are excreted in the breast milk, concentrations are low in neonates, with the exception of clonidine and amphetamines, which should be avoided while breastfeeding.
Deep brain stimulationDBS may play a role in the treatment of women with disabling Tourette syndrome who wish to have children.9 In a retrospective study of 11 pregnant women with a range of movement disorders under treatment with DBS, it was possible to fully withdraw medication after surgery in 2 patients with Tourette syndrome. Stimulation parameters were modified during pregnancy, as voltage needed to be increased, and were returned to the original settings 3 months after delivery. Both mothers carried the pregnancies to term and were able to breastfeed.15
TremorEssential tremor is one of the most common movement disorders, affecting approximately 1% of the global population. Incidence increases in line with age, and prevalence is similar in men and women. Onset can be as early as childhood, and distribution is bimodal, with peaks in the second and sixth decades of life.29 As a result, essential tremor is common in women of childbearing age.
During pregnancy, it is highly important to rule out such causes of secondary tremor as drugs, metabolic alterations (thyroid dysfunction, glycaemia), stress, or onset of such neurological diseases as parkinsonism or dystonia.19
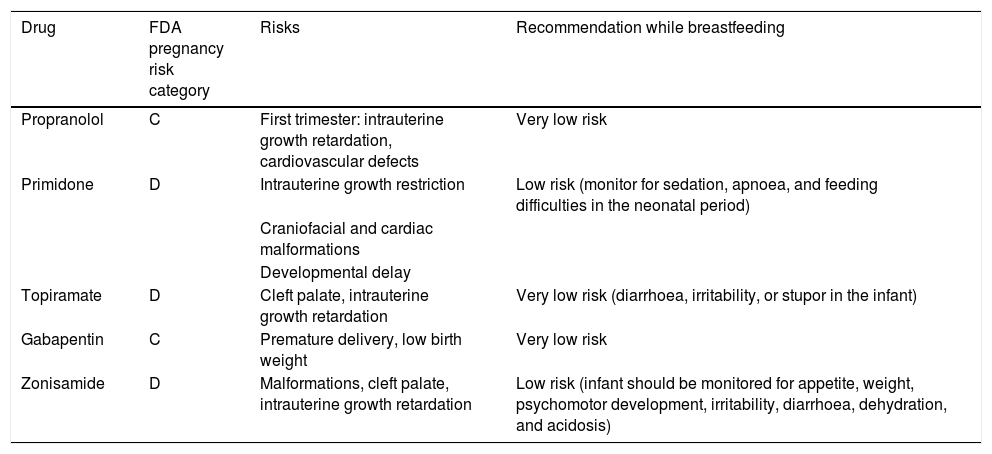
During pregnancy, treatment is only indicated for disabling tremor.30 The risks and benefits of treatment must be assessed; it is advisable to withdraw drugs in most cases. Treatments should be withdrawn prior to planned pregnancies, except in highly disabled patients, and the risk of teratogenicity should always be taken into account. In the case of patients who become pregnant while under treatment, we must evaluate the risks and benefits and withdraw treatment wherever possible. The most frequently used drugs for tremor are propranolol, primidone, topiramate, gabapentin, and zonisamide. Table 2 shows the FDA pregnancy risk categories for these drugs. DBS of the thalamus or high-intensity focused ultrasound are indicated in severe cases; these treatments should be performed before or after pregnancy (Fig. 1).
Recommendations on the use of the most frequently used treatments for tremor during pregnancy and breastfeeding.
| Drug | FDA pregnancy risk category | Risks | Recommendation while breastfeeding |
|---|---|---|---|
| Propranolol | C | First trimester: intrauterine growth retardation, cardiovascular defects | Very low risk |
| Primidone | D | Intrauterine growth restriction | Low risk (monitor for sedation, apnoea, and feeding difficulties in the neonatal period) |
| Craniofacial and cardiac malformations | |||
| Developmental delay | |||
| Topiramate | D | Cleft palate, intrauterine growth retardation | Very low risk (diarrhoea, irritability, or stupor in the infant) |
| Gabapentin | C | Premature delivery, low birth weight | Very low risk |
| Zonisamide | D | Malformations, cleft palate, intrauterine growth retardation | Low risk (infant should be monitored for appetite, weight, psychomotor development, irritability, diarrhoea, dehydration, and acidosis) |
RLS is very common, affecting 2%-10% of the population.31 It affects twice as many women as men.32 Pregnancy is considered a risk factor as RLS is more prevalent in women who have been pregnant than in men or nulliparous women.33
The prevalence of RLS during pregnancy is estimated between 15% and 25%, according to published studies. Onset usually occurs in the second trimester, with the most severe symptoms presenting in the third trimester, and symptoms resolve after delivery. In general, RLS presenting for the first time during pregnancy seems to have better prognosis, usually resolving within a month of delivery,34 although symptoms persist as idiopathic restless legs syndrome in some cases. Independent risk factors for restless legs syndrome during pregnancy include family history of RLS, personal history of RLS during other pregnancies, presence of RLS prior to pregnancy, and haemoglobin levels < 1 g/dL before pregnancy.35
RLS may alter the course of the pregnancy, as these symptoms can be associated with considerable stress or sleep alterations, negatively affecting the patient and the development of the fetus. It has also been associated with such complications as hypertension, cardiovascular disease, preeclampsia, and premature delivery.36
The pathophysiology of RLS with onset during pregnancy is complex. Dietary factors (iron, vitamin D, and folic acid deficiency), hormonal factors (increased oestrogen levels), physiological changes (venous insufficiency, weight gain), and genetic predisposition are known to play a role. It is important to take these factors into account to ensure proper, individualised management.37
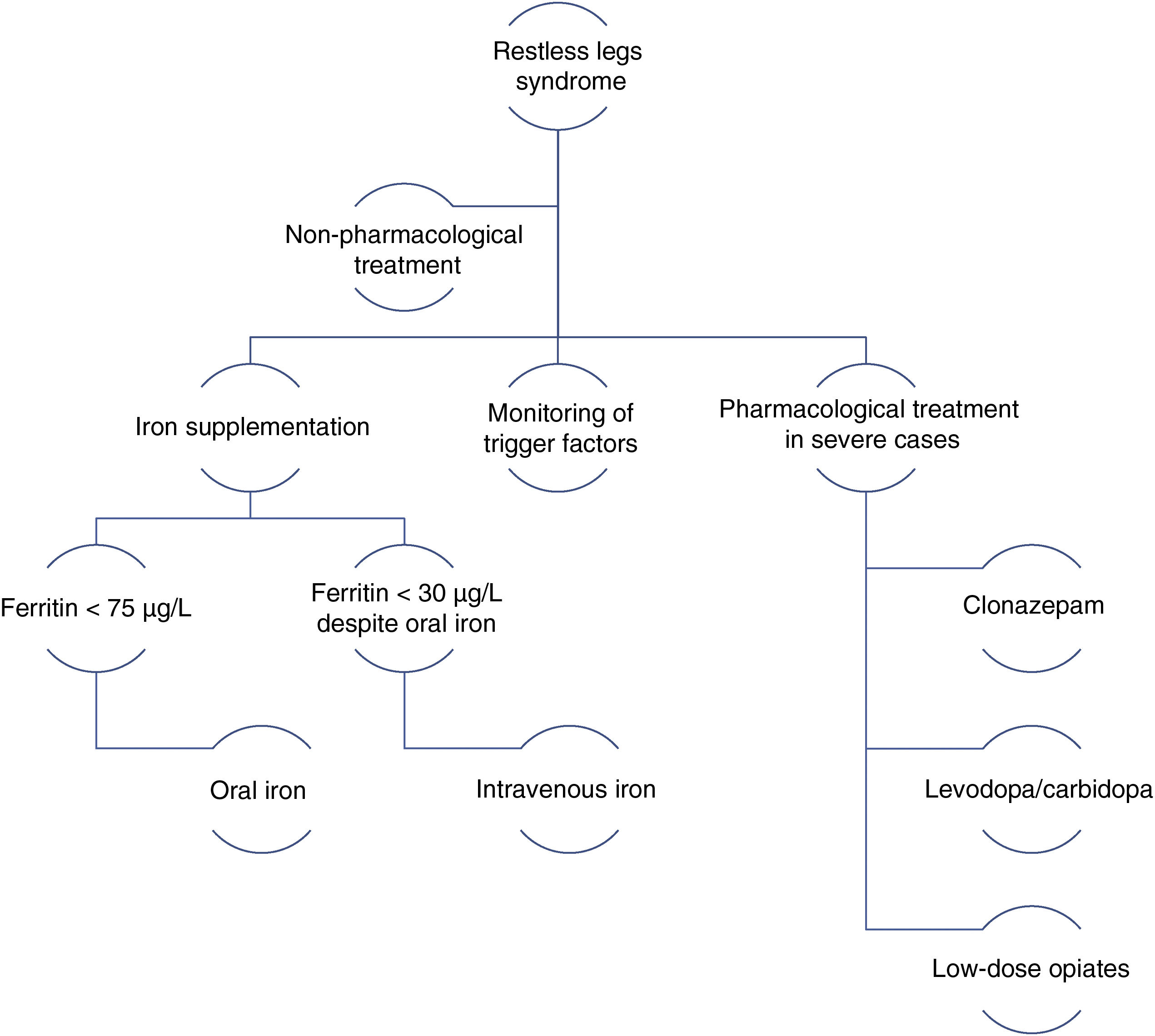
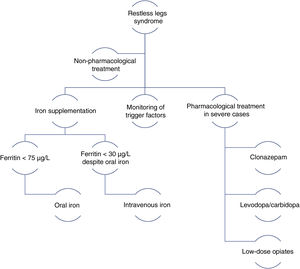
In 2014, a panel of experts published a series of recommendations for the management of RLS during pregnancy.38 The main recommendations include the following general rules: therapeutic decision-making must be based on symptom severity, the risks and benefits of drugs, and the circumstances of the patient; non-pharmacological treatments should be the first choice; pharmacological treatments should be administered at the minimum effective dose with the shortest possible duration; and iron supplementation should be prescribed for all patients (Fig. 2).
Management algorithm for restless legs syndrome in pregnancy.
Modified from Picchietti et al.38
Moderate-intensity physical exercise, such as yoga, is recommended. Measures favouring venous return, such as massages, compression stockings, or pneumatic compression may also be effective. It is also important to review the patient’s medications and to withdraw those that may be having a negative influence. RLS symptoms may improve with treatment of sleep apnoea–hypopnoea syndrome, in patients with the condition.38
Pharmacological treatmentIron: iron supplementation is recommended for pregnant patients with RLS and ferritin levels < 75 µg/L. Oral iron should be recommended first. For patients with severe symptoms in the third trimester and ferritin levels < 30 µg/L despite oral iron administration, intravenous iron should be considered.39
Dopaminergic agonists: these drugs are not recommended during pregnancy due to their poor safety profile (FDA pregnancy risk category C). They may inhibit prolactin during breastfeeding.40,45
Benzodiazepines: clonazepam is the treatment of choice for RLS. Low doses (0.5 mg or 1 mg), administered at night, are recommended for patients in the second or third trimester of pregnancy. The neonate must be monitored for signs of withdrawal syndrome.41
Levodopa: low doses of levodopa/carbidopa, administered in the evening or night, may be prescribed for patients with severe symptoms. The associated risks and benefits must always be assessed.42,45
Opiates: low doses of tramadol may be recommended during the second and third trimesters in the event of very severe symptoms. This treatment may cause opiate withdrawal syndrome in the neonate.43
Other drugs: low doses of gabapentin (300-900 mg/day) may be considered during breastfeeding.44,47
ConclusionsWe must evaluate the risks and benefits of treatment in all women with hyperkinetic movement disorders, whether pre-existing or with onset during pregnancy, aiming to reduce effective doses as much as possible or to administer drugs only when necessary. In hereditary diseases, families should be offered genetic counselling. It is important to recognise movement disorders triggered during pregnancy, such as certain types of dystonia, chorea, tremor, and restless legs syndrome.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Ramos R, Santos-García D, Alonso-Cánovas A, Álvarez-Sauco M, Ares B, Ávila A, et al. Manejo de la enfermedad de Parkinson y otros trastornos del movimiento en mujeres en edad fertil: parte 2. Neurología. 2021;36:159–168.