We would like to thank Sanchez-Larsen et al.1 for their letter to the editor addressing our review on the use of eslicarbazepine acetate (ESL) to treat neuropathic pain, headache, and cranial neuralgia.2 The first study to evaluate the use of ESL to treat trigeminal neuralgia (TN) in humans merits our acknowledgement, and we congratulate the authors. That study supports the hypothesis that ESL is efficacious, safe, and well tolerated in the treatment of TN.3
In their letter, the authors pose a very interesting question: why might ESL be effective for treating TN, but not other neuropathies or neuropathic pain conditions?1 At the time, we formulated this question more generically, considering whether the results observed with ESL would also be achieved with other voltage-dependent sodium channel (Nav) blockers, and specifically the subclass of carboxamides, which includes carbamazepine (CBZ) and oxcarbazepine (OXC), as well as ESL. Navs are transmembrane proteins that enable sodium ions to cross the cell membrane. This transport is passive, and depends only on the electrochemical potential of the ion (no energy is required in the form of ATP). In excitable cells, such as neurons and cardiomyocytes, Nav proteins are responsible for the rising phase of action potentials (depolarisation). As a result of this, they contribute to pain syndromes through augmented electric current and increased density of Nav proteins in the area of the lesion; both mechanisms lead to hyperexcitability and increased pain transmission.4 Nine subtypes of Nav have been identified (Nav1.1-Nav1.9), and several have been associated with the transmission of inflammatory, nociceptive, and neuropathic pain. To gather evidence on the involvement of Nav blockers in pain, we selected the most significant studies identified in a literature search of PubMed and Google Scholar, with the following keywords: “carbamazepine,” “oxcarbazepine,” “eslicarbazepine acetate,” “neuropathic pain,” “inflammatory pain,” and “chronic pain management.”4
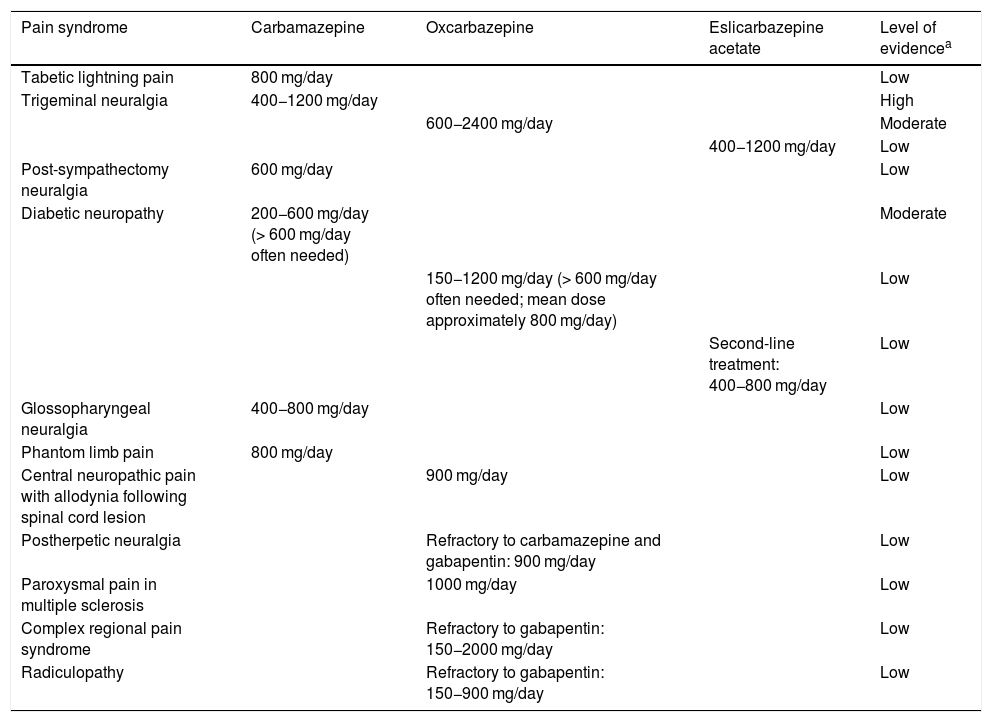
Although TN is the only pain-related indication for CBZ approved by the European Medicines Agency (EMA), some studies suggest that it may be beneficial for treating such other conditions as tabetic lightning pain, post-sympathectomy neuralgia, diabetic neuropathy, glossopharyngeal neuralgia, and phantom limb pain.4 The EMA has not approved the use of OXC to treat TN. However, some treatment guidelines for TN consider OXC a reasonable first-line treatment.5 The drug also has known benefits for treating allodynia, diabetic neuropathy, paroxysmal symptoms of multiple sclerosis, and peripheral neuropathy. Some case reports support its use for postherpetic neuralgia, complex regional pain syndrome, and radiculopathies in patients refractory to gabapentin.4
As we noted in our review article, few studies have analysed the treatment of pain with ESL. However, in addition to the study by Sanchez-Larsen et al.3 on TN, we have also found data supporting the use of the drug to treat refractory trigeminal neuralgia in multiple sclerosis, as well as diabetic neuropathy.1,2Table 1 summarises the evidence on the treatment of pain with the carboxamide subclass of Nav blockers currently available in Spain.4
Summary of the available evidence on the treatment of pain with sodium channel blockers.
| Pain syndrome | Carbamazepine | Oxcarbazepine | Eslicarbazepine acetate | Level of evidencea |
|---|---|---|---|---|
| Tabetic lightning pain | 800 mg/day | Low | ||
| Trigeminal neuralgia | 400−1200 mg/day | High | ||
| 600−2400 mg/day | Moderate | |||
| 400−1200 mg/day | Low | |||
| Post-sympathectomy neuralgia | 600 mg/day | Low | ||
| Diabetic neuropathy | 200−600 mg/day (> 600 mg/day often needed) | Moderate | ||
| 150−1200 mg/day (> 600 mg/day often needed; mean dose approximately 800 mg/day) | Low | |||
| Second-line treatment: 400−800 mg/day | Low | |||
| Glossopharyngeal neuralgia | 400−800 mg/day | Low | ||
| Phantom limb pain | 800 mg/day | Low | ||
| Central neuropathic pain with allodynia following spinal cord lesion | 900 mg/day | Low | ||
| Postherpetic neuralgia | Refractory to carbamazepine and gabapentin: 900 mg/day | Low | ||
| Paroxysmal pain in multiple sclerosis | 1000 mg/day | Low | ||
| Complex regional pain syndrome | Refractory to gabapentin: 150−2000 mg/day | Low | ||
| Radiculopathy | Refractory to gabapentin: 150−900 mg/day | Low |
The latest clinical practice guidelines agree that tricyclic and dual-action antidepressants (venlafaxine, duloxetine) and the antiepileptic drugs gabapentin and pregabalin constitute the treatment of choice for neuropathic pain, with Nav blockers representing a second-line treatment, together with many other drugs.6,7
Despite a considerable disparity between the quantity and the quality of studies supporting the use of first-line and second-line drugs for various pain conditions, a growing body of evidence continues to highlight the potential benefits of Nav blockers to treat pain; these benefits remain underestimated.4
Further research is needed to compare these drugs to gabapentinoids in the treatment of neuropathic pain; however, most studies evaluating these drugs do address neuropathic pain syndromes. It should be noted that many Nav proteins have been associated with neuropathic pain,4 and some have also been associated with inflammatory pain, demonstrating that prostaglandins increase the activity of Nav1.9 and Nav1.7.4 However, it has not been suggested that Nav blockers could substitute non-steroidal anti-inflammatory drugs in the treatment of inflammatory pain. Nonetheless, the pharmacological characteristics of these drugs indicate that a trial of Nav inhibitors may be effective in patients presenting intolerance to or contraindications for non-steroidal anti-inflammatory drugs due to gastrointestinal or cardiovascular complications. Clinical trials should be performed to fully evaluate and characterise the targeted use of Nav blockers and their role in the treatment of inflammatory pain.
Another area yet to be explored is the targeted treatment of specific Nav subtypes, which may play an important role in the development of drugs for chronic pain. Research is currently focused on designing drugs that selectively inhibit specific Nav proteins; however, this may be challenging due to the structural similarities between the different channels. Nav1.7 is mainly expressed in peripheral neurons, including those in the trigeminal nerve; findings from genetic and functional studies suggest that it may be linked to pain symptoms in humans.8 Research is currently underway into the treatment of TN with BIIB074, or vixotrigine, a selective blocker of Nav1.7. Electrophysiological studies have shown that the drug preferentially inhibits high-frequency firing of neurons, like that observed in pain episodes in trigeminal neuralgia.9 The results of phase I clinical trials suggest that vixotrigine is well tolerated in healthy individuals, and that it can be administered at therapeutic doses without prolonged up-titration. A multicentre, double blind, placebo-controlled phase IIa clinical trial including 67 patients with classical TN (with 29 randomly allocated to receive the treatment for 28 days) found no statistically significant difference (P = .0974) between vixotrigine (150 mg 3 times per day) and placebo for the primary endpoint (treatment failure), but did identify significant differences in the time to treatment failure, the number of paroxysms, average daily pain score, and assessments of overall function and quality of life. The drug was well tolerated, with headache and dizziness being the most frequent adverse events.10 Despite the negative result for the primary endpoint, the trial clearly suggests that Nav1.7 antagonists may be clinically relevant in neuropathic pain, justifying continued clinical research. For this reason, new clinical trials are currently being performed to analyse the use of vixotrigine to treat both TN and other models of neuropathic pain (eg, small-fibre neuropathy, erythromelalgia, and lumbosacral radiculopathy).9
In conclusion, the identification of Nav subtypes associated with specific pain conditions, and their potential role as therapeutic targets, offers a glimpse of the potential for truly individualised treatment and a much-needed transformation of the care provided to these patients. The available evidence suggests that Nav blockers are underused. However, current research has identified this as a promising area for additional clinical trials to better guide clinical practice.
Please cite this article as: Alcántara Montero A, Sánchez Carnerero CI. Bloqueadores de los canales de sodio dependientes de voltaje: nuevas perspectivas en el tratamiento del dolor neuropático. Neurología. 2021;36:169–171.