It was with great interest that we read Alcántara Montero and González Curado's recent article “Is there scientific evidence for the use of venlafaxine to treat neuropathic pain?”1 The authors review the available evidence on the efficacy of venlafaxine for the management of different types of neuropathic pain. Their review includes 13 studies: 11 randomised clinical trials, a case-control study, and an open-label study. The authors conclude that the studies comparing the efficacy of venlafaxine against placebo demonstrate the analgesic effects of the drug. However, they do not conduct a statistical analysis of all the studies. We performed a meta-analysis to evaluate the effect size for venlafaxine vs placebo in treating neuropathic pain, as well as a P-curve analysis to evaluate the quality of the available evidence.
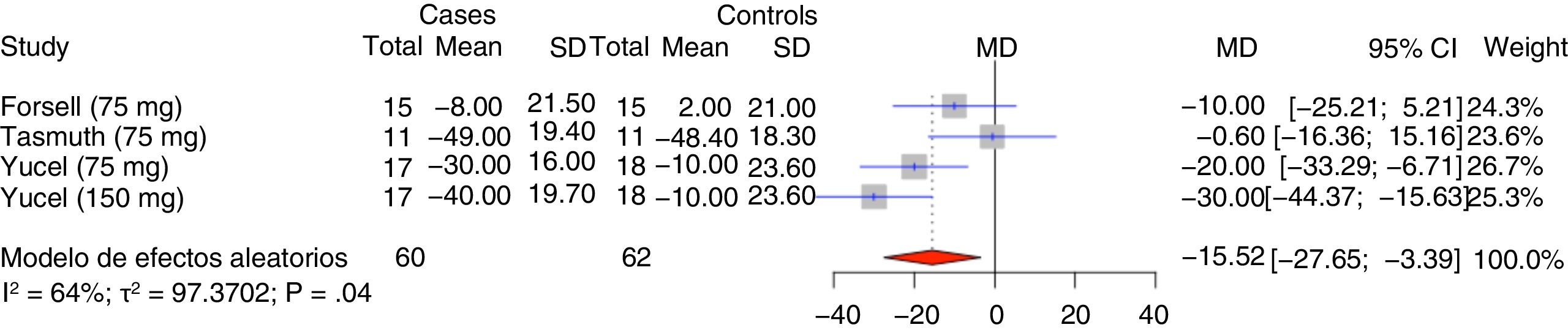
Our meta-analysis included the studies comparing the efficacy of venlafaxine for treating different types of neuropathic pain against placebo. The outcome variable analysed was pain intensity as measured with a 100-mm visual analogue scale (VAS). Three studies met the inclusion criteria: the study by Tasmuth et al.2 (post-chemotherapy neuropathic pain; increasing doses of venlafaxine up to 75mg for 8 weeks), the one by Forssell et al.3 (atypical facial pain; 37.5mg in weeks 1 and 2 and 75mg in weeks 3 and 4), and a study by Yucel et al.4 (different types of neuropathic pain; 75/150mg for 8 weeks). The meta-analysis took a random effects approach, given the heterogeneity of the data (I2). A two-tailed P value<.05 was considered statistically significant. The analysis included 122 participants (62 patients and 60 controls). The mean variation in VAS scores was −15.5 points (95% CI, −27.7 to −3.4); decreases in VAS scores were therefore statistically significant, with a small or medium effect size. Statistical analysis was performed using the R statistical software; means and standard deviations were calculated using the formula proposed by Pudar Hozo et al.5 The results of our meta-analysis are shown in Fig. 1.
To assess the integrity of the studies evaluating the efficacy of venlafaxine for neuropathic pain, we performed a P-curve analysis of the 8 double-blind trials comparing venlafaxine against placebo. Five studies were excluded to reduce the heterogeneity of our sample; these studies either did not use placebo in the control group or were non-controlled studies. The P-curve is the distribution of statistically significant P values. By analysing the distribution of P values we can infer the evidential value of the findings from independent studies. If the null hypothesis is true (the drug under study has no real effect), 5% of studies would yield a P value<.05, 4% would yield P<.04, 3% would yield P<.03, and so on. The P-curve would be flat or horizontal. In contrast, a frequency of P values showing asymmetry to the right (ie, P values are closer to .01 than to .05) indicates that findings do have evidential value. On the other hand, a majority of P values close to P=.05, indicates “P-hacking” or manipulation of the findings. P-curve analysis was performed with P-curve.com, a free-access software based on the theoretical and practical work of Simonsohn et al.6 This program can perform 2 types of statistical analysis (binomial and continuous) of a set of P values. Of the 8 studies included, only 4 reported statistically significant P values:P=.03, P=.0042, P<.001, and P<.001.7–10 The continuous test follows the Stouffer method, and yielded a Z score of 2.58 (P=.005). We may therefore conclude that the results from the studies analysed have evidential value. However, given the small number of studies included, the statistical power of the analysis is 74%, with a wide confidence interval (90% CI, 19%-97%).
We should stress that P-curve analysis is different from but complementary to a meta-analysis. Both types of statistical analysis aim to demonstrate whether the effect of a drug or medical intervention is real. A meta-analysis provides a more precise estimate of the effect size than pivotal trials. P-curve analysis, in turn, evaluates the integrity of the results from different studies, rather than the effect size. Therefore, the latter helps detect publication bias or statistical manipulation of the data.
In conclusion, venlafaxine dosed at 75mg has beneficial effects for different types of neuropathic pain as compared to placebo, improving VAS scores by 1-2 points; P-curve analysis demonstrated the integrity of the studies analysed. However, further research is needed to determine the effect size more precisely.
Please cite this article as: Roche Bueno J. Meta-análisis y análisis de curva-p sobre la eficacia de venlafaxina frente a placebo en el tratamiento del dolor neuropático. Neurología. 2020;35:597–598.