Cortical subarachnoid haemorrhage (cSAH) has multiple aetiologies. No prospective study has reported the long-term progression of the condition. The objective of this study is to describe the clinical and aetiological characteristics of patients with cSAH and to gain insight into prognosis.
MethodsWe performed a prospective, observational, multi-centre study. Data on clinical and radiological variables were collected; during a one-year follow-up period, we recorded data on mortality, dependence, rebleeding, and the appearance of dementia.
ResultsThe study included 34 patients (mean age, 68.3 years; range, 27–89). The most frequent symptoms were headache and focal neurological deficits, which were frequently transient and recurrent. CT scans returned pathological findings in 28 patients (85%). Brain MRI scans were performed in 30 patients (88%), revealing acute ischaemia in 10 (29%), old haemorrhage in 7 (21%), and superficial siderosis in 2 (6%). Aetiology was identified in 26 patients (76.5%): causes were cerebral amyloid angiopathy in 8, ischaemic stroke in 5, vasculitis in 4, reversible posterior encephalopathy in 2, venous thrombosis in 2, reversible cerebral vasoconstriction syndrome in 2, carotid occlusion in 1, Marfan syndrome in 1, and meningeal carcinomatosis in 1. Three patients died during follow-up (2 due to causes related to the cause of cSAH). Three patients developed dementia, 3 had lobar haemorrhages, and one had a second cSAH.
ConclusionsThe most frequent causes of cSAH in our series were cerebral amyloid angiopathy, ischaemic stroke, and vasculitis. This type of haemorrhage has a worse prognosis than other non-aneurysmal cSAH. There are numerous possible causes, and prognosis depends on the aetiology. In elderly patients, intracranial haemorrhage is frequently associated with cognitive impairment.
Las hemorragias subaracnoideas corticales (HSAc) tienen numerosas etiologías. No hay estudios prospectivos que indiquen su evolución a largo plazo. El objetivo de este trabajo es describir las características clínicas y etiológicas de los pacientes con HSAc y conocer su pronóstico.
MétodosEstudio observacional, prospectivo y multicéntrico. Se recogieron variables clínicas y radiológicas, y se siguió la evolución al año, observando la mortalidad, dependencia, tasa de resangrado y aparición de demencia.
ResultadosSe incluyeron 34 pacientes (edad media 68.3 años, rango 27–89). Los síntomas más frecuentes fueron el déficit neurológico focal, con frecuencia transitorio y de repetición, y la cefalea. El TAC fue patológico en 28 pacientes (85%). Se realizó RM cerebral en 30 pacientes (88%), con isquemia aguda en 10 (29%), sangrados antiguos en 7 (21%) y siderosis superficial en otros 2 (6%). Se encontró etiología en 26 pacientes (76.5%): angiopatía amiloide (n=8), ictus isquémico (n=5), vasculitis (n=4), encefalopatía posterior reversible (n=2), trombosis venosa (n=2), síndrome de vasoconstricción cerebral reversible (n=2), oclusión carotidea (n=1), síndrome de Marfan (n=1) y carcinomatosis meníngea (n=1). Durante el seguimiento fallecieron tres pacientes (en dos de ellos relacionado con la causa de la HSAc). Tres pacientes desarrollaron una demencia, tres presentaron un hematoma lobar y otro una nueva HSAc.
ConclusionesEn nuestra serie las causas más frecuentes de HSAc fueron la angiopatía amiloide, el ictus isquémico y la vasculitis. La HSAc tiene peor pronóstico que otras HSA no aneurismáticas. Puede tener numerosas causas y su pronóstico depende de la etiología subyacente. En el anciano existe una frecuente asociación con hemorragia intracraneal y deterioro cognitivo.
The most frequent cause of non-traumatic subarachnoid haemorrhage (SAH) is the rupture of an intracranial aneurysm, which is recorded in 85% of cases. These patients present familial predisposition, as well as well-known risk factors such as smoking, arterial hypertension, or excessive alcohol consumption. Prognosis is poor, with a case fatality rate of approximately 50%; one-third of survivors remain dependent.1
The second most frequent cause is perimesencephalic haemorrhage (10% of all cases), in which brain angiography findings are almost always normal and progression is usually satisfactory.1
The remaining 5% of cases are mainly cortical SAHs (cSAH), which are characterised by the presence of blood in one or several sulci of the brain convexity. Several causes of cSAH are reported2: cerebral amyloid angiopathy (CAA),3–6 vaculitis,7,8 reversible cerebral vasoconstriction syndrome (RCVS),9 posterior reversible encephalopathy syndrome (PRES),10 vascular malformations,11 mycotic aneurysms,12 ischaemic stroke,13 arterial dissection or stenosis,14 and cerebral venous thrombosis (CVT).15–17
The prognosis of patients with cSAH is not well established, with data available only from small clinical series.18–23 Furthermore, no prospective studies providing information on long-term progression have been published.
The aim of this study is to describe the clinical and aetiological characteristics of patients with cSAH, and to determine their progression and prognosis.
Patients and methodsThis is an observational, prospective, multi-centre study, including patients from 6 hospitals from the Valencian Community (Spain). The study was authorised by the Research Committee at Hospital de Sagunto. Informed consent forms were signed in all cases. Patients were recruited between June 2011 and April 2016, with follow-up consultations held at 3 months and one year. Progression was assessed using the modified Rankin Scale, and was considered favourable with a score ≤2 and unfavourable with a score >2.
All patients were admitted to their reference hospitals. We excluded cases of traumatic haemorrhages and haemorrhages with associated intraparenchymal haematoma or bleeding in the interhemispheric fissure, basal cisterns, or ventricles. Clinical, demographic, electrophysiological, laboratory, and imaging data were collected.
All patients underwent a head CT scan. Whether to perform other imaging studies during admission was considered on an individual basis; these studies included CT angiography, brain MRI with vascular study, and conventional cerebral angiography. We also collected data on the presence of superficial siderosis, old haemorrhage, and acute ischaemia on diffusion sequences. We used the modified Boston criteria to diagnose CAA24 and the Calabrese criteria for RCVS.25 During follow-up, clinically stable patients did not undergo neuroimaging studies.
A cerebrospinal fluid analysis and/or electroencephalography was performed in some cases, based on each centre’s criteria and the patient’s characteristics.
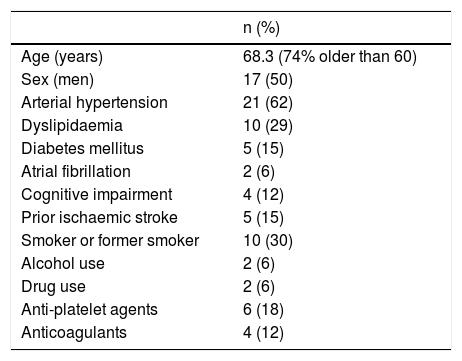
ResultsDuring the study period, we recruited a total of 34 patients, with a mean age of 68.3 years (range, 27–89). Twenty-five patients (73.5%) were older than 60. Seventeen patients were women. Patients’ demographic and clinical data are summarised in Table 1.
Patient characteristics.
| n (%) | |
|---|---|
| Age (years) | 68.3 (74% older than 60) |
| Sex (men) | 17 (50) |
| Arterial hypertension | 21 (62) |
| Dyslipidaemia | 10 (29) |
| Diabetes mellitus | 5 (15) |
| Atrial fibrillation | 2 (6) |
| Cognitive impairment | 4 (12) |
| Prior ischaemic stroke | 5 (15) |
| Smoker or former smoker | 10 (30) |
| Alcohol use | 2 (6) |
| Drug use | 2 (6) |
| Anti-platelet agents | 6 (18) |
| Anticoagulants | 4 (12) |
Several patients presented more than one symptom. Onset was acute in all cases except for one patient with meningeal carcinomatosis, who developed headache and progressive onset of focal neurological signs. The most frequent symptom was focal neurological deficit (n=21, 62%), with 10 patients (29%) manifesting repetitive stereotyped behaviours and 11 patients (33%) presenting established deficits. Eleven patients (33%) presented headache, with thunderclap headache in only 4 (12%). Three patients (9%) presented confusion, with one of these displaying symptoms resembling an episode of transient global amnesia. Three patients (9%) developed seizures as the initial symptom; these were generalised in 2 cases. Two patients (6%) reported non-specific visual alterations together with other symptoms. One patient displayed neck rigidity.
Radiological findingsHaemorrhage was identified in the CT scan in 28 cases (85%), and in the MRI study in the remaining patients. A brain MRI scan was performed in 30 patients (88%), and brain angiography in 12 patients (35%). Bleeding was mostly unilateral, with the most frequent locations being the parietal and frontal regions. Diffusion-weighted MRI sequences showed cortical hyperintensity in 10 patients (33% of all patients undergoing MRI), which was adjacent to the cSAH in most cases. Old haemorrhages were detected in 7 cases (23%), and superficial siderosis in another location was detected in 2 (7%). An intra- and extracranial vascular study using CT angiography, MRI angiography, or conventional angiography was performed in 30 cases (88%); this study revealed no aneurysm or arteriovenous malformation except in one patient, who presented a small aneurysm measuring 1.2mm in diameter, located away from the bleeding site. Five patients presented carotid artery involvement ipsilateral to the bleeding (2 significant stenoses, 2 complete occlusions, and one intrastent thrombus). Multiple intracranial stenoses were detected in 2 patients; one patient presented ipsilateral middle cerebral artery occlusion.
Other studiesTen patients (29%) underwent electroencephalography studies, revealing epileptiform activity in only 3 cases. Fourteen patients underwent lumbar puncture. Cerebrospinal fluid analysis revealed no abnormalities. Xanthochromia was not detected in any case. We detected lymphocytic pleocytosis in 3 patients, a slightly increased protein level in 2, and oligoclonal bands in one. The cytological study detected malignant cells in one patient. We calculated the tau/Abeta42 ratio in 7 patients; results were pathological in 3.
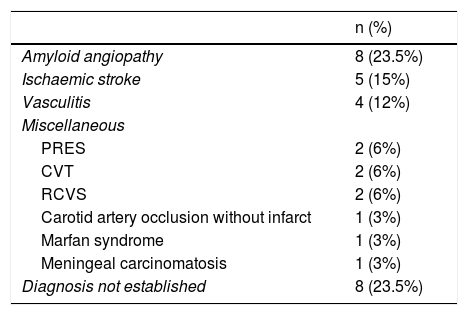
AetiologyTable 2 includes the causes of bleeding, which we were able to establish in 26 patients (76.5%).
Aetiologies of cSAH.
| n (%) | |
|---|---|
| Amyloid angiopathy | 8 (23.5%) |
| Ischaemic stroke | 5 (15%) |
| Vasculitis | 4 (12%) |
| Miscellaneous | |
| PRES | 2 (6%) |
| CVT | 2 (6%) |
| RCVS | 2 (6%) |
| Carotid artery occlusion without infarct | 1 (3%) |
| Marfan syndrome | 1 (3%) |
| Meningeal carcinomatosis | 1 (3%) |
| Diagnosis not established | 8 (23.5%) |
cSAH: cortical subarachnoid haemorrhage; CVT: cerebral venous thrombosis; PRES: posterior reversible encephalopathy syndrome; RCVS: reversible cerebral vasoconstriction syndrome.
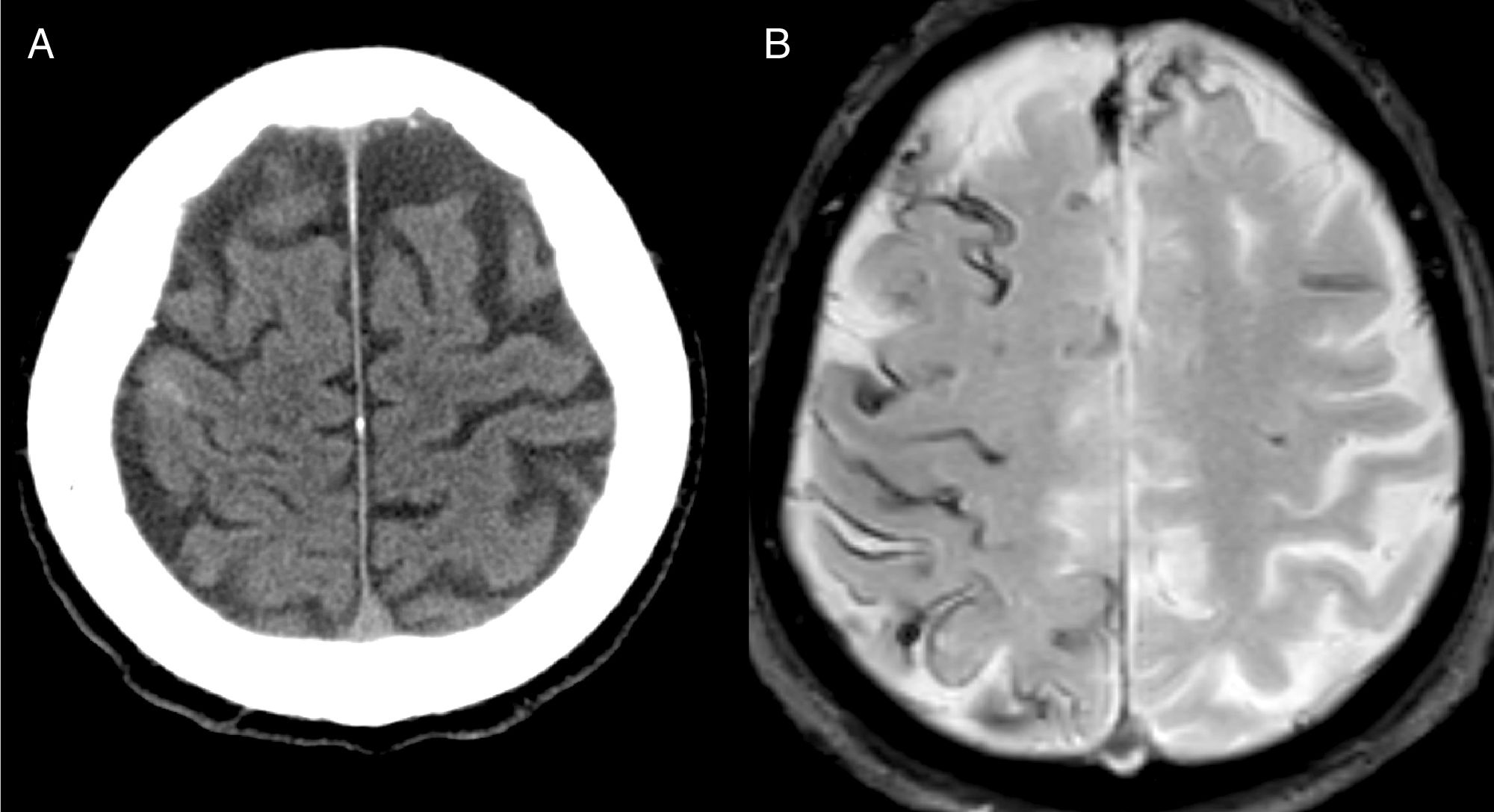
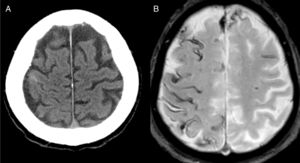
The most frequent diagnosis was CAA (Fig. 1), accounting for a total of 8 cases. Three patients were diagnosed during the one-year follow-up period: one due to recurrence of cSAH and presence of superficial siderosis on MRI, and the other 2 due to lobar haemorrhage.
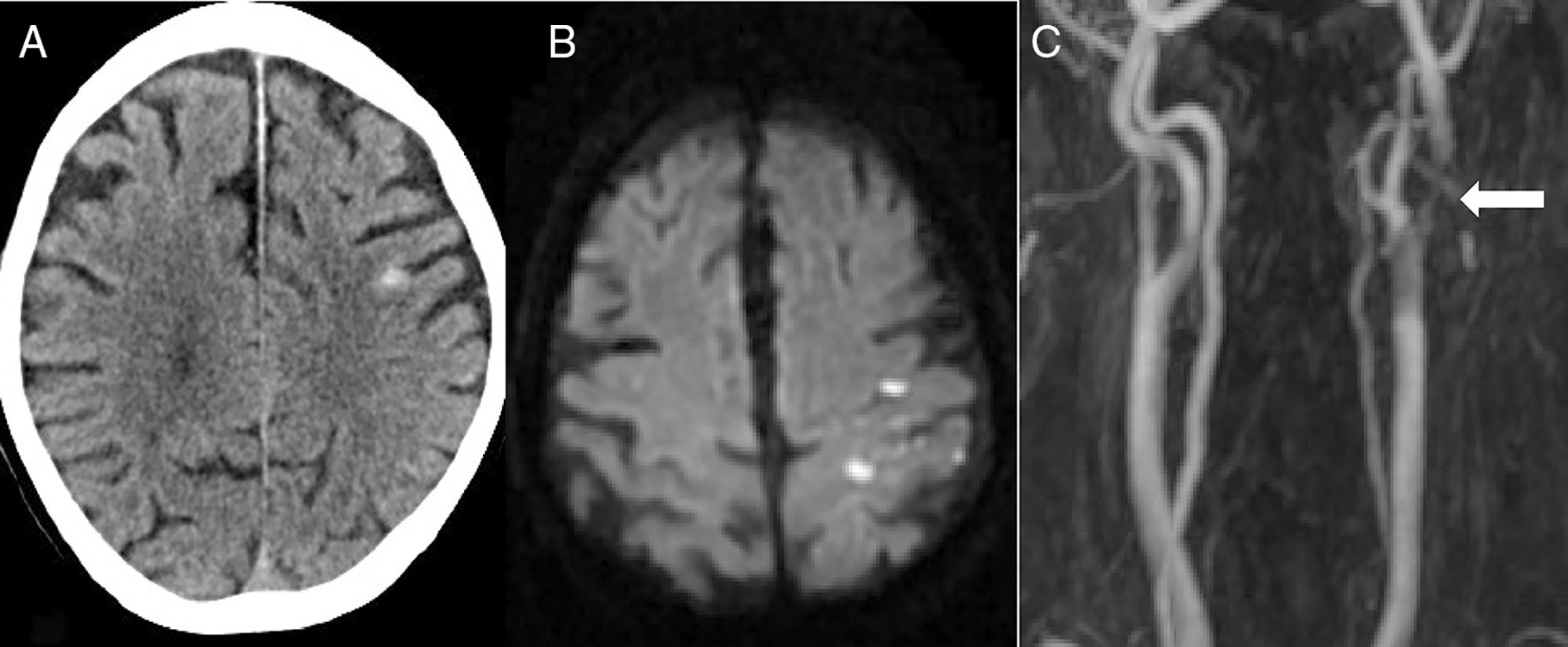
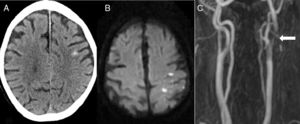
The second most frequent cause was ischaemic stroke (n=5): 2 cardioembolic strokes, one atherothrombotic infarction, one stroke secondary to carotid artery occlusion, and one case of carotid stent thrombosis (Fig. 2).
Eighty-two-year-old patient presenting sudden-onset loss of strength and sensitivity in the left arm 6 days after implantation of a left carotid stent. A) Head CT scan showing cSAH of the left convexity. B) Diffusion-weighted MRI scan revealing acute infarction adjacent to the bleeding. C) MRI-angiography showing stent thrombosis (arrow).
The third most frequent cause was vasculitis (n=4). Three cases were secondary (Sjögren syndrome, Churg-Strauss syndrome, neurosyphilis), with the fourth patient presenting primary angiitis of the central nervous system. The remaining aetiologies included: PRES (n=2), CVT (n=2), RCVS (n=2), carotid artery occlusion with no associated infarction (n=1), Marfan syndome (n=1), and meningeal carcinomatosis (n=1).
ProgressionOne patient diagnosed with CVT and prostate cancer died during admission due to a massive cerebral haematoma. Two other patients died during the follow-up period, one due to meningeal carcinomatosis originated by cSAH, and another who was diagnosed with CAA due to an unrelated cause. Overall mortality amounted to 9% (Table 3).
We were able to follow up 32 of 33 patients (97%). One-year outcomes were favourable in 24 patients (73%) and unfavourable in 9 (27%).
Twelve percent of patients presented rebleeding within one year of follow-up (3 lobar haematomas and one new cSAH). Three patients developed dementia.
DiscussionIn addition to presenting one of the largest series of patients with cSAH (34 cases), this study is to our knowledge the first prognostic study with prospective follow-up and data progression at one year.
Our results show several differences between cSAH and aneurysmal SAH. Firstly, the most frequent initial manifestation was not headache but focal neurological signs, which manifested in approximately two-thirds of patients in our series. Seventeen patients (50%) presented recurrent, stereotyped (especially paresthesia affecting one side of the body), short-lasting transient neurological deficits; in these cases, CAA was the most frequent underlying cause. One-third of patients presented headache as the initial symptom, with thunderclap headache in some cases. Neck rigidity, a typical sign of aneurysmal SAH, was exceptional. We should also underscore that there was no case of bleeding due to aneurysmal rupture, that patients had no family history of SAH or intracranial aneurysm, and that the percentage of smokers and alcohol users (well-established risk factors for aneurysmal SAH) was low.
Our findings show that cSAH may be due to various causes, going beyond other authors’ dichotomy of RCVS in young patients and CAA in elderly patients.19,23 The 3 most frequently diagnosed causes were CAA, ischaemic stroke, and vasculitis.
A possible explanation for CAA being the most frequent cause of cSAH is that most of patients were older than 60 years, as is reported in previous studies.20,22,26 Almost all patients presented recurrent, stereotyped transient neurological deficits, also known as amyloid spells. It has been suggested that cSAH may precede the appearance of a lobar haematoma by several weeks.3,4,27 In fact, 4 of our patients presented rebleeding in the months following admission. Therefore, antithrombotics should be prescribed with caution. When initial diagnosis is uncertain, a deferred brain MRI study may help to establish a diagnosis of CAA if rebleeding or superficial siderosis are observed.4,5,24
Regarding ischaemic stroke, a retrospective review of almost 5000 patients with acute stroke or transient ischaemic attack showed low incidence of cSAH (8 cases; 0.14%).13 We believe copresence of CAA to be the most probable underlying mechanism. However, another study reports an unusually high incidence of cSAH associated with brain ischaemia among patients with heart disease and implanted left ventricular assist devices.28 It should be noted that the mean age in that study was 39 years, making CAA highly improbable. In these cases, the most probable mechanism is haemorrhagic transformation of a cardioembolic stroke, which is also favoured by the fact that many patients were receiving anticoagulants. Some cases have been reported of cSAH associated with carotid artery occlusion or stenosis. The mechanism could again be haemorrhagic transformation of a small ischaemic stroke or rupture of fragile collateral vessels, even in the absence of infarction,14 as occurred in one of our patients. Carotid artery stenosis may be underdiagnosed, as the extracranial carotid artery is frequently not explored.
Cortical SAH is a frequent manifestation of primary cerebral vasculitis or vasculitis secondary to autoimmune systemic diseases.7,29,30 A study by Boulouis et al.,8 including 60 patients diagnosed with primary angiitis of the central nervous system, reported vasculitis in 26% of cases. In our series, vasculitis was suspected due to the patient’s history in 3 of the 4 cases; diagnosis was supported by the presence of multiple arterial stenoses in the vascular study. Meningeal enhancement in the MRI study helped to establish a differential diagnosis with RCVS. Changes in the cerebrospinal fluid and response to immunosuppressant treatment supported diagnosis.
It is important to identify the underlying cause of bleeding, not only to orient treatment but also to establish prognosis. Diagnostic yield increases with an extensive initial aetiological study and with follow-up of the patient. Brain MRI is the most useful complementary test. FLAIR sequences are more sensitive than head CT in identifying acute bleeding, whereas echo-gradient MRI helps to identify old haemorrhages, superficial siderosis, and cortical CVT.3,17,31,32 An intra- and extracranial vascular study should be performed in every patient. Cerebral angiography would be reserved for those cases in which diagnosis is not established after performing these studies, or in which vasculitis or RCVS is suspected.
It was possible to establish a diagnosis during admission in 23 patients (68%). We should highlight the importance of follow-up after discharge, which enabled us to increase this number to 26 (76.5%).
Prognosis of patients with cSAH differs between series. Studies reporting good progression usually include younger patients diagnosed with PRES, CVT, or RCVS.9,15,16,18 Other studies report poor progression, associated especially with age.4,19,22,26,33 Only the study by Martínez-Lizana et al.4 included patients diagnosed with CAA and ambispective follow-up. Our study is the first to include prospective follow-up, which was possible in more than 95% of cases. All patients with a modified Rankin Scale score >2 at one year (27% of patients) were older than 65. Furthermore, both cognitive impairment and all episodes of rebleeding were recorded in patients diagnosed with CAA. Therefore, prognosis will depend on age and aetiology, and is poorer in older patients with CAA.
Our study has several limitations. Not all the available diagnostic tests were performed on all patients, especially very elderly patients with a very poor situation at baseline. Specifically, brain MRI and extracranial carotid artery exploration were not performed in all cases. Cerebral angiography was not performed in all cases of uncertain diagnosis.
In conclusion, cSAH is a rare cerebrovascular disease with numerous possible aetiologies. In our series, the most frequent causes were amyloid angiopathy, ischaemic stroke, and vasculitis. Prognosis is poorer than in other non-aneurysmal SAHs. In elderly patients, risk of intracranial haemorrhage and cognitive impairment is greater due to the frequent association with CAA.
Conflicts of interestThis study has received no funding of any kind. The authors have no conflicts of interest to declare.
Please cite this article as: Galiano Blancart RF, et al. Pronóstico al año de la hemorragia subaracnoidea cortical no traumática: serie prospectiva de 34 pacientes. Neurología. 2021;36:215–221.
This study was presented at the 68th Annual Meeting of the Spanish Society of Neurology.